Back to Journals » International Journal of Women's Health » Volume 16
Maternal Sleeping Problems Before and After Childbirth - A Systematic Review
Authors Witkowska-Zimny M , Zhyvotovska A , Isakov R, Boiko DI, Nieradko-Iwanicka B
Received 25 October 2023
Accepted for publication 22 January 2024
Published 2 March 2024 Volume 2024:16 Pages 345—371
DOI https://doi.org/10.2147/IJWH.S446490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Malgorzata Witkowska-Zimny,1,* Anastasiia Zhyvotovska,2,* Rustam Isakov,2 Dmytro I Boiko,2 Barbara Nieradko-Iwanicka3
1Department of Human Anatomy, Medical University of Warsaw, Warsaw, Poland; 2Department of Psychiatry, Narcology and Medical Psychology, Poltava State Medical University, Poltava, Ukraine; 3Chair and Department of Hygiene and Epidemiology, Medical University of Lublin, Lublin, Poland
*These authors contributed equally to this work
Correspondence: Malgorzata Witkowska-Zimny, Department of Human Anatomy, Medical University of Warsaw, 5 Chalubinskiego Str., Warsaw, 02-004, Poland, Email [email protected] Anastasiia Zhyvotovska, Department of Psychiatry, Narcology and Medical Psychology, Poltava State Medical University, 1 Medychna Str., Poltava, 36013, Ukraine, Email [email protected]
Abstract: The perinatal and postpartum period is of great significance for women due to physiological changes, shifts in circadian rhythms, social setting, and psychological well-being, all of which affect the quality and quantity of their sleep. A mixed-studies systematic review was undertaken to enhance our understanding of sleep disturbances and mood disorders in women in late pregnancy and the postpartum period, their connection with breastfeeding, and the assessment of interventions for sleep disturbance. Three electronic databases (PUBMED, EMBASE and Google Scholar) were searched for qualitative, observational, and mixed-method studies from the year 2016 to June 2023. Twenty-nine articles were included in the analysis. The results were synthesized into four overarching themes: (і) the sleep quality of women in the perinatal period; (ii) the relationship between sleep and breastfeeding; (iii) the relationship between sleep quality and emotional disturbance in the perinatal period; (iv) sleep interventions in the researched group. The subjective nature of the perception of sleep disturbances, along with the absence of an objective measurement tool is clearly an inconvenience. It is advisable to include the assessment of maternal sleep hygiene and family sleep patterns during postpartum healthcare provider appointments to develop strategies not only for women’s sleep quality but also for their mental well-being.
Keywords: childbirth, breastfeeding, maternal sleep, sleep quality, sleep disturbance, psycho-emotional states
Introduction
Sleep is a complex physiological process, one of the main features of which is the cyclically changing activity of the central nervous system. Proper quality and quantity of sleep are essential for good health, well-being, and good overall quality of life. The perinatal and postpartum period is of great significance for women due to physiological changes, shifts in circadian rhythms, social setting, and psychological well-being, all of which affect the quality and quantity of their sleep. Sleep patterns may be altered by major hormonal, physiological, and behavioral changes experienced during pregnancy and after childbirth.1 Various studies have shown that 70% of pregnant women report sleep disorders which increase in the third trimester with fetal growth and movements, the occurrence of back pain, nocturia, and gastroesophageal reflux.2,3 Sleep deficiency is common in postpartum mothers who feed and care for their babies. Sleep deficiency, sleep interruption, and altered sleep patterns cause young mothers to be vulnerable to sleep disorders that sometimes progress to severe insomnia. Sleep disorders in the perinatal and postnatal period may contribute to apathy, somnolence, tiredness, fatigue, low mood, anxiety, and a higher risk of depressive symptoms in the final stage of pregnancy and after childbirth.4,5 Sleep disturbances are a very sensitive indicator which allows to identify various mental illnesses, especially depression. There is a significant relationship between sleep disturbance, insomnia, and perinatal depression (antenatal and postpartum), which left untreated may have devastating effects on women, infants, and families, or even lead to maternal or newborn mortality.5 As circadian rhythm disorders frequently co-occur with sleep disorders and depression, many studies have investigated this cause-and-effect relationship.6 Is it enough for people predisposed to depression to experience long-term poor sleep quality and insomnia for their effects to add to the set of depressive symptoms and for depression itself to reveal? Or does the occurrence of depressed mood, loss of vital energy, and cognitive disorders impair circadian rhythms to such an extent that the manifestation of insomnia will be a natural consequence?
In the era when scientists investigate the intricacies of the architecture of sleep, the mechanisms that govern sleep and wakefulness, and seek to explain the relationship between sleep disorders, mood deterioration and the onset of depression, patients expect effective interventions or resolution of their sleep disorders.
During pregnancy, women often report reduced sleep duration, poorer sleep quality, and more napping during the day. After delivery, the mother’s circadian rhythm changes and depends on hormonal changes in her body and the needs of the newborn.3 Overall, following delivery, the quality of sleep continues to deteriorate, with worsened sleep efficiency and more sleep loss at night; however, some studies show a relatively stable total sleep time with the inclusion of scattered daytime naps.7 Several studies have shown the potential of non-pharmacological interventions such as aromatherapy, reflexology, physical activity, or cognitive behavioral therapy to relieve sleeplessness and improve sleep quality in the perinatal period.8–10
Breastfeeding falls exclusively to the mother. The link between breastfeeding and maternal sleep is poorly understood. A few studies have investigated the relationship between sleep duration and breastfeeding; however, research results are inconclusive in this regard.11–15 It is not entirely known, how breastfeeding affects sleep, does sleep deficiency hinder breastfeeding and breastfeeding patterns, or is difficulty breastfeeding the reason for maternal sleep disturbances.
Sleep problems are prevalent during pregnancy and in the postnatal period, but there remains a lack of cohort studies and longitudinal population-based studies assessing the quality and quantity of sleep as well as sleep patterns before pregnancy. The most frequently performed and published studies assessing the amount and quality of sleep are based on a subjective assessment reported when completing a questionnaire.
General sleep scales, such as the most common Pittsburgh Sleep Quality Index (PSQI), used most frequently to assess general sleep status, are inadequate for pregnancy and postpartum sleep pattern changes. Only a few studies have used the Postpartum Sleep Quality Scale (PSQS) developed by Yang et al, which is a tool for rapid assessment of sleep quality in postpartum women and includes two factors: sleep inefficiency associated with physical symptoms and daytime dysfunction associated with nighttime care of the infant; yet this tool is not widespread in assessing the quality and quantity of maternal sleep.16
Given the increasing number of studies on sleep disturbances in pregnant women and young mothers, we believe it is important to compare the research results obtained with the use of standardized tools assessing sleep quality. This review was conducted to better understand sleep patterns and mood disorders in women in late pregnancy and the postpartum period, their relationship with breastfeeding, and to prevent sleep disorders through non-pharmacological interventions.
Methods
To the extent possible, this study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Search Strategy
During this review, we conducted a literature search in electronic databases PUBMED, EMBASE and Google Scholar using key word combinations: insomnia, sleep disorder, sleep quality, mental health, anxiety, postpartum period, breastfeeding, breast milk, lactation, circadian rhythms. The study was limited to original scientific papers written within the last eight years, involving humans, and written exclusively in English. After removing duplicates, articles were selected according to the algorithm shown in the PRISMA diagram in Figure 1.
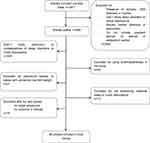 |
Figure 1 PRISMA flow diagram. |
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (a) studies involving healthy women aged 18 years or older in the third trimester of pregnancy or in the postpartum period that assessed sleep quality; (b) studies in which the relationship between the mother’s sleep and breastfeeding was evaluated; (c) studies in which sleep quality was assessed with tools such as the Pittsburgh Sleep Quality Index, Postpartum Sleep Quality Scale, Actigraphy, and mood disturbance by means of a standardized scales.
Exclusion criteria: (a) meta-analyses, systematic and non-systematic reviews, case studies; and (b) studies that included mothers who had mental health disorders and/or received medical treatment; (c) studies that included mothers with pregnancy and postpartum complications regarding physical and mental conditions that affect the health of the pregnant or postpartum person, their baby, or both, and other than a singleton pregnancy; (d) research focused on the COVID-19 pandemic.
It is important to note that there are factors that could significantly change the quality of sleep in women, such as infant feeding patterns, mother-infant co-sleeping, culture-bound views, other concomitant conditions (sleep education, postpartum depression), socioeconomic status, non-pharmacological interventions (psychotherapy, phytotherapy, psychoeducation, etc.). In this review, they were not among the exclusion criteria but were considered as modifiable factors, and were used and included in the discussion of the results where possible.
To assess methodological quality and minimize the risk of bias, research papers were distributed between three investigators for data processing. If there was any doubt during the review, the article was discussed by the researchers and a consensus was reached on it. To ensure as large a sample as possible, we contacted the authors if the full text of the article was not available in databases.
For each study, we identified a number of key elements: authors; name of the journal; year of publication; country of origin; the aim of the study; sociodemographic data; sample size; design; group number and group description; tools used to assess sleep quality or mood disturbance; research results.
Results
Of the 3971 literature references, 29 research papers were identified that involved the quality of sleep or mood disturbances in mothers during the last trimester of pregnancy and in the postpartum period. Eleven of them are clinical randomized trials regarding interventions on sleep quality and psycho-emotional states in women, such as postpartum depression, anxiety and mood changes. Only three papers evaluated the relationship between the quality of the mother’s sleep and infant feeding.8,17,18
The majority of studies were conducted in Taiwan (7 out of 29) and the United States of America (7 out of 29). Other studies were conducted in Belgium, Egypt, Iran, Azerbaijan, Nepal (1 out of 29), Canada, Turkey (2 of 29), and Australia, China (3 of 29). Most of them were cohort (9 out of 29) or cross-sectional studies (9 out of 29), although the main group also included 10 randomized controlled trials and one quasi-experimental study.
The main methods for measuring sleep quality were: PSQI (Pittsburgh Sleep Quality Index), PSQS (Postpartum Sleep Quality Scale), and Actigraphy.
The PSQS is a new scale developed in 2013 that focuses on sleep problems in postpartum women. The PSQS includes 14 self-report questions designed to assess a postpartum woman’s sleep quality in the previous 2 weeks, with items rated on a 5-point Likert scale (0 = never, 1 = little, 2 = sometimes, 3 = often, 4 = almost always). PSQS items include quantitative aspects (eg sleep duration, sleep latency, number of awakenings), subjective aspects (eg rest, daytime functioning), and factors affecting postpartum women’s sleep quality.15 The PSQI developed by Buysse et al (1989), uses a 4-point Likert scale questionnaire ranging from 0 (no difficulty) to 3 (significant difficulty) to measure subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of hypnotics and daytime dysfunction. The sum of the scores for the seven components gives a total PSQI score. Higher values indicate a worsening of sleep quality.19
EPDS (Edinburgh Postnatal Depression Scale), PHQ-9 (Patient Health Questionnaire-9), DASS (Depression, Anxiety, and Stress Scales), HADS (Hamilton Depression Scale) were used to assess the emotional and mental state of women.
The EPDS is a 10-item self-rated questionnaire with each item scored from 0 to 3, with a total possible scale score ranging from 0 to 30 points. The PHQ-9 is used to assess depressive symptoms. This scale assesses nine depressive symptoms based on the DSM-V (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), and each item is scored on a Likert scale ranging from 0 to 3. Specific items include: decreased pleasure in daily activities, depressed mood, difficulty sleeping, low energy, appetite/eating changes, self-deprecation, difficulty concentrating, psychomotor changes and suicidal thoughts. DASS is a 42-item questionnaire, with a set of three self-report scales to measure psychological and physiological signs of depression, anxiety and stress. HADS includes fourteen items, divided into anxiety and depression subscales. Each item scores on a Likert scale from 0 to 3.
Other questionnaires were used less often: ISI (Insomnia Severity Index) and GSDS (General Sleep Disturbance Scale) to assess insomnia and sleep disturbances; BSES-SF (The Breastfeeding Self-Efficacy Scale-Short Form), PHQ-2 (Patient Health Questionnaire-2), HAMA (Hamilton Anxiety Rating Scale), HAMD (Hamilton Depression Rating Scale), GAD-7 (Generalised Anxiety Disorder Assessment), BRUMS (The Brunel Mood Scale), SEES (Subjective Exercise Experience Scale), SDS (Self-Rating Depression Scale), PSS (Perceived Stress Scale), PBQ (Postpartum Bonding Questionnaire), PASS (Perinatal Anxiety Screening Scale), SAS (Self-Rating Anxiety Scale) and OASIS (Overall Anxiety Severity and Impairment Scale) to assess the level of different emotional disturbances; ESS (Epworth Sleepiness Scale), PFS (Postpartum Fatigue Scale), MAF (Multidimensional assessment of fatigue) and CIS (Checklist Individual Strength) to examine the level of fatigue.
The results of the research were synthesized into four overarching themes: (і) the sleep quality of women in the perinatal period; (ii) the relationship between sleep and breastfeeding; (iii) the relationship between sleep quality and emotional disturbance in the perinatal period; (iv) sleep interventions in the researched group.
Sleep Quality
Nine studies evaluated maternal sleep quality in the perinatal period (Table 1). They identified key factors related to the quality of maternal sleep: the course of labor, the male gender of the newborn, maternal age, the burden of household duties, the presence of children at home, and the subjective perception of sleep quality.
 |
Table 1 Characteristics of the Studies Included in the Evaluation of Maternal Sleep Quality in the Perinatal Period |
While investigating certain demographic and obstetric factors related to the subjective quality of sleep of women in the postpartum period (n=202), Taiwanese scientists found that the mean PSQS score was 22.82, indicating that, on average, mothers had mild sleep disturbances. The main problems they highlighted were the inability to sleep more than 7 hours a day and caring for the baby at night. As one of the first studies to establish a relationship between obstetric factors and subjective sleep quality, it revealed that women who were dissatisfied with their childbirth experience (n=43) reported poorer sleep quality compared to those who were satisfied with it. The main indicators of dissatisfaction were: pain during childbirth (81.4%), pain associated with vaginal examination (9.3%), and induction of labor (9.3%). For women who had a C-section performed (n=10), the main criteria for dissatisfaction were: anesthesia (50%) and fear (30%). A positive correlation was also found between sleep quality and daily household workload. A higher daily workload at home was positively correlated with a lower quality of sleep.20
Another study on sociodemographic indicators was conducted by Doering et al in the United States, on a sample of 183 women from low income families. Among the study participants, 65%, mostly African-American, had short sleep duration, that is, they slept six hours or less per day. Compared to existing data that postpartum middle-class women sleep just over 7 hours per night, with the longest uninterrupted period of sleep of 4.1 hours, the study participants had significantly worse indicators. The total duration of their sleep was 5.5 hours, and the longest uninterrupted period of sleep was 2.8 hours.21 Christian et al studied subjective sleep quality among nulliparous and multiparous women, African American and European American, during each trimester of pregnancy and 4 to 11 weeks postpartum. In early/mid pregnancy, multiparous women repeatedly reported shorter sleep time, increased sleep latency, and higher prevalence of poor sleep (PSQI > 5) compared to nulliparous women. In the third semester and after childbirth, sleep differences related to the number of births were the smallest. Race also did not emerge as a significant factor affecting the prevalence and indicators of subjective sleep quality.22
A study on 380 women in Nepal, found about 28% of the respondents to have poor sleep quality and 19% to demonstrate symptoms of postpartum depression. Having children at home, male sex of the newborn, medical complications after delivery, and postpartum depression were factors positively associated with poor sleep quality. This also applied to women of lower socio-economic status and those who continued to work in the postpartum period. This study did not establish a significant relationship between poor sleep quality and mode of delivery or time of introduction of complementary foods.23 Wen et al found that women over 35 years of age were three times more likely to demonstrate poor sleep quality than younger women at 3 months postpartum, after adjusting for severity of postpartum somatic symptoms, sociodemographics, obstetric history, and health habits. An increased risk of poor sleep quality may be associated with natural aging. Older mothers had significantly higher PSQI scores for sleep efficiency and sleep disturbance, but did not differ in other parameters compared to younger mothers. Fragmented sleep and difficulty falling asleep after interrupted sleep were also more common in mothers over 35 years of age. Using a PSQI threshold score of 5, the study found that more than 65% of women over the age of 35 and 41.3% of women under the age of 35 showed poor sleep quality. The severity of postpartum somatic symptoms was significantly and positively associated with poor sleep quality. Although the severity of postpartum somatic symptoms did not differ significantly by age; cesarean delivery and perineal pain were more pronounced in older women. Mothers who exercised were found to have better sleep quality than mothers who did not exercise.24
In their study on the dynamics of indicators of objective, performed using actigraphy, and subjective sleep quality, Coo et al found that the quality of sleep in the third trimester of pregnancy mainly worsened due to sleep fragmentation. In the first 15 days after childbirth, this indicator increased by two-fold, which was accompanied by a decrease in the duration and efficiency of sleep, and was restored after 10–12 weeks. Impaired nighttime sleep also disrupted sleep and wake habits, as daytime sleep time and daytime napping increased as objective measures of sleep quality, and its quantity declined. Instead, subjective measures of sleep were more closely related to mood than objective measures, although the latter had a significant effect on stress levels, when significant changes in sleep were observed during the first 15 days after delivery.25
In another study, Canadian scientists evaluated subjective and objective indicators of the quality of sleep in mothers and fathers. Maternal sleep was found to be more fragmented and had a significantly shorter duration of continuous night sleep and more night awakenings than paternal sleep, both at 1 month and 3 months postpartum. The results of the study showed that the subjective and objective fragmentation of sleep in women persisted for 6 months in the postpartum period. The researchers also analyzed the relationship between sleep quality and family factors, and found that older mothers reported shorter subjective duration of uninterrupted sleep and fewer nighttime awakenings. They also demonstrated great variability in actigraphy of nighttime sleep duration. Mothers who had more than one child, had shorter subjective nighttime sleep duration, shorter uninterrupted sleep duration, and a lower percentage of nights with 6 consecutive hours of sleep. In addition, mothers who were on maternity leave reported more night awakenings than mothers who were actively working. While mothers on maternity leave cared for their infants during the nighttime period, it is also likely that they slept during the day to compensate for disturbed nighttime sleep. Thus, the increase in time spent in bed during the day could affect maternal sleep at night. No relationship was found between maternal sleep parameters (subjective and objective) and the level of education or the method of feeding the child.26
An American study by King et al investigated the relationship between maternal sleep disturbances and mothers’ emotional sensitivity to their infants, and found that none of the symptoms of sleep disturbances or insomnia were significantly associated with the general level of maternal sensitivity to the child, nor with the ability to sustain sensitivity during interaction with the infant. Using multilevel modeling, the researchers found that mothers with worse objective sleep duration showed decreased responsiveness to their infants during free play interactions.27
A study by Stremler demonstrated a strong relationship between subjective measures of sleep quality at 6 weeks postpartum and symptoms of postpartum depression at 6 or 12 weeks. Women who subjectively rated sleep quality as a “little problem” were three times more likely to demonstrate symptoms of postpartum depression, compared to women who reported a “big problem” with sleep and were eight times more likely to do so. The authors conclude that the subjective perception of sleep quality may be an important clinical indicator of bad mood in women in the postpartum period.29
Psycho-Emotional States and Poor Maternal Sleep Quality
This section discusses the experience of sleep quality related to mood disturbance in women during late pregnancy and after postpartum (Table 2). Nine papers were included in the analysis; however, two of them were previously presented in the section on sleep quality because they relate to both sections: maternal sleep quality and mood disturbance.23,25 Insufficient professional postpartum care, subjective sleep quality, low enthusiasm for daily housework, breastfeeding difficulties, lack of support from family and relatives, and the gender of the newborn (male) were identified as factors associated with sleep deterioration and predictors of postpartum anxiety and depression.
 |
Table 2 Characteristics of the Studies Included in the Evaluation of Maternal Mood Disturbance in the Perinatal Period |
An American study of women at increased risk of postpartum depression found a marked improvement in sleep between the sixth and seventh week postpartum. At the same time, linear regression analysis showed that deterioration or only minimal improvement in sleep quality was associated with greater severity of depressive symptoms 7 months after the birth of a child. When studying individual components of sleep quality, a higher level of depressive symptoms led to changes in the indicators of sleep latent period and daytime dysfunction.30
Australian researchers found that subjective sleep quality, and therefore the mother’s personal perception of sleep and low enthusiasm for daily activities, was more closely related to mood than objective sleep quality. The researchers noted that the improvement in mood during the first 15 days after childbirth depended precisely on subjective night sleep, and not on daytime sleep and the severity of daytime dysfunction.25 When assessing the relationship between subjective sleep quality and symptoms of anxiety and depression, Okun et al found a significant effect of poor sleep quality on postpartum depression, even after taking into account the strong correlation between prenatal and postpartum mood scores.28 A similar study was conducted in Nepal. The sample included 380 women, among whom 18.7% developed postpartum depression at 2 to 12 months after childbirth. A significant correlation was found between postpartum depression and the following subcomponents of the PSQI: sleep quality, efficiency, sleep latency, use of sleep medication, and daytime dysfunction. Among the sociodemographic indicators of the development of depression, separate living was noted, which deprived young mothers of support from their mothers or other relatives. The lack of professional postpartum care, the presence of medical complications in oneself or the child, and the birth of a male child were also factors associated with depression.23
In a study of the factors that cause postpartum depression (PPD), Howard et al identified a number of modifying factors, including body mass index (BMI), household income, fatigue, sleep, breastfeeding status, diet, and physical activity. Correlation analysis revealed a positive association of PPD with BMI and fatigue, and a negative association of postpartum depression with household income and sleep quality. Women who breastfed also had lower rates of PPD.31
Another study on postpartum depression found that breastfeeding women who switched from a pro-inflammatory to an anti-inflammatory diet had a reduced risk of PPD. Moreover, this protective effect persisted after adjusting for mothers’ level of employment, sleep quality, and social support.32 A study by Turkish scientists involving 245 pregnant women revealed possible psychosocial risk factors that may contribute to developing symptoms of postpartum depression in the period 6–8 weeks after childbirth. Using a threshold score of ≥13 for the EPDS scale, the prevalence of postpartum PPD was determined to be 18%. Among women suffering from postpartum depression, 25% had an unplanned pregnancy or one that their partner did not want, 34.1% had marital problems, and 22.7% experienced family distress during pregnancy. Women who confirmed episodes of abuse and had high levels of anxiety or stress were more likely to suffer from symptoms of postpartum depression. Young mothers with PPD also had low psychosocial health scores.33
Clout et al found no association between sociodemographic factors, including age, education, and income, and emotional states such as depression, anxiety, and postpartum stress. This could be due to a limitation of the study, which mainly involved upper-middle-income women, which was a factor of low financial stress. Pregnancy factors such as planned pregnancy, repeated births, and use of assisted reproductive technology were also not associated with anxiety, depression, and postpartum stress. Among the obstetric variables, only c-section was associated with increased levels of stress and anxiety in the period up to 6 months after delivery. The established association between children’s sleep and health problems and maternal anxiety and depression was no longer significant after adjusting for antenatal distress.34
Predictors of postpartum depression and postpartum anxiety (PPA) were identified by Chinese scientists in a large cross-sectional study involving 1204 women. A multivariate logistic regression analysis showed that mothers with low levels of support from the family, colleagues, or friends were more likely to experience symptoms of postpartum depression six weeks after childbirth. Risk factors for PPD also include smoking, mother-child separation, and breastfeeding difficulties. Women who were satisfied with their birth experience and received high family support were less likely to report symptoms of PPA. The presence of PPA symptoms concurrently increased the risk of developing PPD symptoms. Parturients who experienced severe fatigue reported more anxiety and stress symptoms. They also reported more depressive symptoms than parturients without severe fatigue.9
Interventions for Improving Maternal Sleep Quality
The need for sleep is individual and variable, but if improving sleep hygiene does not yield the expected results, there are cheap and easy ways to improve temporary sleep problems before seeking professional help. Sleep-promoting interventions include pharmacological and non-pharmacological treatment, but since many hypnotics are not recommended for pregnant or lactating women, non-pharmacological interventions such as aromatherapy, acupressure, physical activity, cognitive behavioral psychotherapy to improve sleep are being extensively studied in this population. Table 3 contains a summary of the 11 publications selected during the analysis of sleep interventions in women’s sleep disturbance in the perinatal period.
 |
Table 3 Characteristics of the Studies Included in the Evaluation of Sleep Interventions in Women’s Sleep Disturbance in the Perinatal Period |
Randomized controlled trials have found enhancement in sleep inefficiencies associated with physical symptoms and reductions in depressive symptoms after drinking German chamomile tea.36 A longer-term effect on sleep quality was observed with the use of lavender cream and foot baths.37 Another study using aromatherapy, namely bergamot essential oil, found no improvement in sleep quality between experimental and placebo groups. However, a significant difference in depressive mood between groups was found (p < 0.001).38
Auricular acupressure treatment also showed positive effects on PSQI measures such as sleep quality, sleep latency, sleep duration, and sleep disturbance. A parallel analysis conducted in this study did not show any significant relationship between sleep quality and demographics in postpartum women.8
Performing aerobic exercise at least three times a day (15 minutes per session) has been shown to significantly reduce fatigue, stress, and sleep inefficiency associated with physical symptoms in postpartum women.10 In contrast, 20–30 minutes of walking exercise showed significant improvement only in physical symptoms related to sleep inefficiency and had no significant effect on fatigue or depression.39
Cognitive behavioral psychotherapy also had a positive effect on anxiety and depression in nulliparous women. In a Chinese study, post-intervention HAMA, HAMD, EPDS and PSQI scores were significantly lower than baseline scores in the experimental group.8 However, in an American study conducted by Felder et al, which involved digital cognitive behavioral therapy aimed at treating insomnia, there was no significant improvement in the severity of insomnia symptoms compared to participants in standard treatment, although there were higher rates of insomnia remission and lower rates cases of insomnia 6 months after childbirth. Participants in this study also had significant improvements in depression and anxiety symptoms.39
Behavioral sleep intervention (BSI) on infant sleep patterns, as shown in a study by Rouzafzoon et al, also significantly affects maternal sleep quality and depressive symptoms (p < 0.05).40 The impact of the skin-to-skin intervention (Kangaroo mother care) on the mother’s health had a positive effect: there was an improvement in the quality of sleep, a decrease in the intensity of postoperative pain and proper healing of wounds, a decrease in anxiety and the frequency of depression. In addition, it promoted a better bond between the mother and the child, and had a positive effect on weight gain and increased sleep hours of infants at 12 weeks postpartum.41
On the other hand, prenatal psychological sleep education did not have a lasting effect on the mother’s sleep in the postpartum period, nor was there a significant reduction in depressive symptoms.42
Sleep and Breastfeeding
The connection between infant feeding behavior and maternal sleep patterns requires clarification. Hence, we paid particular attention to research on the relationship between maternal sleep quality and breastfeeding, and surprisingly, only three papers explored this connection (Table 4). There was no significant difference in overall sleep quality scores between breastfeeding and mixed-feeding women. Among women who breastfed, mothers who got up more than twice a night had slightly worse sleep quality indicators.8 A similar study was conducted by Belgian scientists who compared the indicators of individual components of the PSQI between women who breastfed and those who use a bottle to feed their baby. Breastfeeding mothers demonstrated better subjective sleep quality (p=0.008) but lower sleep efficiency (p=0.003). Since these components balanced each other, no significant differences in the overall quality of the PSQI were found depending on the method of feeding. Scores for other components did not differ between the groups.17 In a study conducted by Aksu and Yilmaz on Turkish mothers, the sleep quality of mothers emerges as an important element in breastfeeding self-efficacy. It was found that there is a strong negative relationship between total means scores for the PSQS and the Breastfeeding Self-efficacy Scale (p<0.01).18 Therefore, it is important to teach and support the mothers on how they can improve their sleep quality.
 |
Table 4 Characteristics of the Studies Included in the Analysis on the Relationship Between Maternal Sleep Quality and Breastfeeding |
Discussion
Pregnancy and the postpartum period bring about numerous changes since women undergo adaptation not only at the physiological level but they also adapt to a new lifestyle, which involves physical, social, and emotional changes as well as adjusting to a newborn child’s sleep/wake cycles. This review was conducted to better understand sleep disruption and mood disorders in late pregnancy and postpartum women, their association with breastfeeding, and the prevention of sleep disturbances.
Sleep has a critical role in promoting the health of both the woman and the fetus. There is a growing recognition of the role of maternal circadian rhythm and sleep hygiene, which entrain the fetus and then the newborn’s circadian rhythms. In young mothers at 6 weeks of the postpartum period, a shift in the circadian sleep phase to an earlier, compared to late pregnancy period was observed, as an adaptation mechanism for caring for infants during the night.43 At the same time mothers who had a later sleep onset time and a shorter duration of night sleep had an increased level of melatonin, which was shown by backward stepwise regression as a predictor of more severe depressive symptoms.44 Taking into account socio-economic and demographic factors, certain patterns of their influence on women’s sleep and mood disorders were found.
Studies have reported that the most common determinants of poor sleep quality among healthy pregnant women were: advanced age, higher gestational age, being unmarried or living in a nuclear family, multiparity, multigravida, watching television in the bedroom, anxiety, and fatigue.21,23,45–47 Depressive symptoms have been found to be more common in the third trimester of pregnancy48 which increases women’s sensitivity to stress and, in turn, to anxiety. Conversely, increased symptoms of antenatal anxiety are a risk factor for postpartum depression.49 The severity of obsessive-compulsive disorder (OCD) symptoms also increases the risk of the exacerbation of anxiety symptoms. OCD symptoms have demonstrated tend to be affected by stress during pregnancy, childbirth, and the postpartum period, when women may experience anxiety and obsessive thoughts about preparing for childbirth and providing a safe environment for their newborns. Maternal sleep did not appear to be a factor in increasing perinatal anxiety in women with pre-existing anxiety disorders.50 Higher levels of anxiety and depression were found in women during pregnancy than in the postpartum period. Researchers suggest that this may be related to the course of pregnancy, the pregnant woman’s concern about her health and the health of the child, as well as her fears about the pregnancy. A higher level of prenatal commitment was found in highly educated and actively working pregnant women, and a lower level in women with a larger number of live births. The difference between the levels of postpartum attachment, depending on the birth of a child of the desired sex, was established. Difficulty with breastfeeding after birth, intestinal colic or the child’s crying that does not stop cause mothers to have negative feelings towards the child, experience anxiety and depression. Mothers who have a high level of prenatal attachment to their unborn children experience more positive bonding in the postpartum period as well.51,52 As indicated by our systematic review, insufficient professional postpartum care, labor difficulties, the male gender of the newborn, lack support from the family and relatives, too many domestic chores, and the subjective perception of sleep are strongly associated with maternal poor sleep and mood disturbance. The gender of the child as a prognostic factor of an attachment disorder can be taken into account only while also considering social parameters, such as, for example, giving preference to the birth of boys in some Asian countries, which have been confirmed by research.53
By the first weeks after childbirth, women go through a period of enormous adaptation. Postpartum women also experience altered sleep patterns that may lead to sleep disturbance. One of the most common reasons for maternal sleep disturbance is related to newborn sleep and feeding patterns. Among selected manuscripts, there were many, which noted sleep disturbances in women in the postpartum period. During this period, the mother’s bond with her baby is of particular importance. Better indicators of sleep quality had a positive effect on social interactions, although individual symptoms of sleep disorders or insomnia were not related to the general level of maternal sensitivity.27 Lillis et al have found that mothers were better able to cope with daytime problems and negative social interaction (NSI) if they had a good night’s sleep the night before.54 A comparable effect may explain the association of maternal sleep with postpartum depression, where poor sleep increases mood reactivity and increases perception of NSI, as well as hinders recovery from a bad day. When studying different factors of social interaction in young mothers, playing or cuddling with the baby was rated as the most positive social interaction (PSI) of all. At the same time, if the baby’s crying at night disturbed the mother’s sleep, it led to difficulties for the mother to cope with negative social interaction. If we take into account socio-economic and demographic factors, specific patterns emerge regarding their impact on sleep and mood disorders. Women with postpartum poor sleep quality had significantly higher depression scores adjusted for age, employment, BMI, marital status, and education. Postpartum fatigue also had a negative impact on the mother-child bond. Based on the findings reported by Henderson et al, women with postpartum fatigue (PPF) used significantly more negative adjectives to describe their child and themselves, and perceived their caregiving to be much worse than average. They also slower to bond with the child, and often reported that their child belonged to them “only recently” or “not quite yet”. Interestingly, women experiencing fatigue were also more inclined to report not receiving enough assistance and guidance, although more hands-on help from a partner in postpartum care had a negative effect on the development of PPF at 3 months.55
According to the mothers themselves, there are quite a few factors that affect their postpartum functioning. The negative impact of excessive fatigue and the importance of sleep were reported by 73% of mothers. They noted that lack of sleep leads to less satisfaction with time spent with the child and is associated with impaired emotion regulation. All women indicated support as the main factor of maternal well-being, the main aspects of which were: emotional support, encouragement to take care of themselves, involvement in social relationships, practical help with childcare, and household management.56
Low socioeconomic status may be an additional factor in sleep disturbance and anxiety, as it limits a woman’s ability to obtain resources. One month after birth, the level of women’s depressive symptoms was the same in low and high socioeconomic groups. This may be related both to the mother’s adaptation to the new role and routine with her child, and to additional financial support from the state and emotional support from friends and relatives in the first weeks after childbirth. After 2–3 months, the low-income women again reported significantly higher anxiety and a greater number of depressive symptoms. The risk of PPD was also high in unmarried women, unemployed women, and those who did not have a high level of education.57
There are also many environmental and health-related factors that are responsible for sleep quantity and quality, eg diet, sleeping arrangements, or using electronic devices before bedtime, which have not been taken into consideration in research.58,59
Diet is equally important for a woman’s physical and emotional functioning. Previous studies found that both a low BMI31 and the use of anti-inflammatory products significantly reduced the level of PPD.54
Furthermore, due to lifestyle modernization and the common use of artificial lightning, chronodisruption may pose a significant risk to the maternal circadian rhythm and make it difficult to form a circadian system in a child. One reason for lost sleep duration and quality is that the maternal circadian rhythm is disturbed by various factors such as endocrine changes, newborn feeding, and baby activity. Nevertheless, perinatal sleep disturbance must be distinguished from insomnia to avoid an overdiagnosis of the latter. A randomized controlled study conducted by American scientists confirmed that, without taking into account the criteria for the ability to sleep, insomnia rates could be 2–4 times higher than the actual reported rates.60 Preventing the misdiagnosis of insomnia and establishing the factors of “adequate sleep opportunity” is an important step to improve the prevention and treatment of sleep disturbance. It is worth considering the assessment of maternal sleep hygiene and habits as a component of identifying women at risk for postpartum depression and anxiety. Women typically attend a postpartum provider appointment and the practitioner or a midwife could ask about and asses the maternal quality and quantity of sleep and educate them about sleep hygiene and good sleep habits. The strategies have the potential to enhance not only women’s sleep but also their mental well-being.
Our review took into consideration the efficacy and incorporation of behavioral interventions, including cognitive behavioral therapy, mindfulness meditation, herbal medicine, aromatherapy, gymnastics and physical exercises, psychotherapy and relaxation, as there is growing evidence that they are helpful for sleep issues as well as mood symptoms.9,10,35–40 The positive effect of a woman’s relaxation also affects the behavior of infants.61
Our understanding of the link between maternal sleep and breastfeeding remains limited. Improving sleep, reducing symptoms of mood disorders, and reducing stress levels enhance the mother’s psychological and physiological functioning, including an increase in the volume of breast milk.62 Ruan et al investigated the relationship between sleep duration and breast milk macronutrient concentrations but did not find a significant correlation.15 A study by Astbury et al has demonstrated that breastfeeding itself is not associated with lower quality and quantity of nocturnal sleep, but a higher number of nighttime feeds is a strong predictor of shorter sleep duration and poor sleep quality.63
There is a compelling need for a thorough investigation of the factors affecting postpartum sleep quality and breastfeeding, with a focus on the relationship between them through qualitative examinations. It would be interesting to determine which genes and biological pathways mediate or moderate the association between sleep and maternal mood. It would also be interesting to compare data on sleep quality and sleeping habits of mothers and newborns in different ethnographic cultures.
Limitation
There are some limitations of this study. First, in research, sleep quality was not measured repeatedly in the same conditions or at the same time points. The authors did not collect always data on daytime naps. Future work should include repeated measurements of sleep quality before and after childbirth.
Second, a major challenge lies in the lack of an objective tool for the measurement of sleep disturbance. Many studies do not use the questionnaire, validated for use during pregnancy and the postpartum period, which is a potential limitation.
There are no replicable studies on the same population, and results may differ depending on the women’s origin, and cultural patterns of a given country or region. Future studies should include additional measures and pursue a deeper understanding of the relationships between maternal mood, sleep, and mother-infant interactions, eg collecting information on bed-sharing, breastfeeding behavior, or the structure of the family.
Conclusion
This mixed-studies systematic review was conducted to better understand sleep disturbance and mood disorders in late pregnancy and postpartum women, their association with breastfeeding, and the efficacy of sleep disturbance interventions. The inclusion of the assessment of maternal sleep hygiene and family sleep habits during postpartum provider appointments is worth considering. This may lead the improvement of not only women’s sleep but also their overall mental well-being.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015;16(4):483–488. doi:10.1016/j.sleep.2014.12.006
2. Sleep Foundation. Pregnancy and sleep [Internet]; 2023. Available from: http://sleepfoundation.org/sleep-topics/pregnancy-and-sleep.
3. Yang Y, Li W, Ma TJ, et al. Prevalence of poor sleep quality in perinatal and postnatal women: a comprehensive meta-analysis of observational studies. Front Psychiatry. 2020;11:161. doi:10.3389/fpsyt.2020.00161
4. Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, Giesbrecht GF. Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 2015;38(8):1237–1245. doi:10.5665/sleep.4900
5. Gueron-Sela N, Shahar G, Volkovich E, Tikotzky L. Prenatal maternal sleep and trajectories of postpartum depression and anxiety symptoms. J Sleep Res. 2021;30(4):e13258. doi:10.1111/jsr.13258
6. Zhang MM, Ma Y, Du LT, et al. Sleep disorders and non-sleep circadian disorders predict depression: a systematic review and meta-analysis of longitudinal studies. Neurosci Biobehav Rev. 2022;134:104532. doi:10.1016/j.neubiorev.2022.104532
7. Bei B, Coo Calcagni S, Milgrom J, Trinder J. Day-to-day alteration of 24-h sleep pattern immediately before and after giving birth. Sleep Biol Rhythms. 2012;10:212–221. doi:10.1111/j.1479-8425.2012.00563.x
8. Ko YL, Lin SC, Lin PC. Effect of auricular acupressure for postpartum insomnia: an uncontrolled clinical trial. J Clin Nurs. 2016;25(3–4):332–339. doi:10.1111/jocn.13053
9. Liu H, Yang Y. Effects of a psychological nursing intervention on prevention of anxiety and depression in the postpartum period: a randomized controlled trial. Ann Gen Psychiatry. 2021;20(1):2. doi:10.1186/s12991-020-00320-4
10. Yang CL, Chen CH. Effectiveness of aerobic gymnastic exercise on stress, fatigue, and sleep quality during postpartum: a pilot randomized controlled trial. Int J Nurs Stud. 2018;77:1–7. doi:10.1016/j.ijnurstu.2017.09.009
11. Rosenbaum DL, Gillen MM, Markey CH. The importance of sleep and parity in understanding changes in weight and breastfeeding behavior among postpartum women. Appetite. 2022;170:105889. doi:10.1016/j.appet.2021.105889
12. Herring SJ, Yu D, Spaeth A, et al. Influence of sleep duration on postpartum weight change in black and Hispanic women. Obesity. 2019;27(2):295–303. doi:10.1002/oby.22364
13. Cloherty M, Alexander J, Holloway I. Supplementing breast-fed babies in the UK to protect their mothers from tiredness or distress. Midwifery. 2004;20(2):194–204. doi:10.1016/j.midw.2003.09.002
14. Doan T, Gay CL, Kennedy HP, Newman J, Lee KA. Nighttime breastfeeding behavior is associated with more nocturnal sleep among first-time mothers at one month postpartum. J Clin Sleep Med. 2014;10(3):313–319. doi:10.5664/jcsm.3538
15. Ruan H, Zhang Y, Tang Q, et al. Sleep duration of lactating mothers and its relationship with feeding pattern, milk macronutrients and related serum factors: a combined longitudinal cohort and cross-sectional study. Front Nutr. 2022;9:973291. doi:10.3389/fnut.2022.973291
16. Yang CL, Yu CH, Chen CH. Development and validation of the postpartum sleep quality scale. J Nurs Res. 2013;21(2):148–154. doi:10.1097/jnr.0b013e3182921f80
17. Tobback E, Behaeghel K, Hanoulle I, et al. Comparison of subjective sleep and fatigue in breast- and bottle-feeding mothers. Midwifery. 2017;47:22–27. doi:10.1016/j.midw.2017.01.009
18. Aksu A, Vefikulucay Yilmaz D. The relationship of postpartum sleep quality and breastfeeding self-efficacy of Turkish mothers. Scand J Caring Sci. 2019;33(4):833–839. doi:10.1111/scs.12679
19. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
20. Hung HM, Chen CH. Sleep quality in postpartum women: exploring correlation with childbirth experience and household work. J Nurs Res. 2014;22(1):20–27. doi:10.1097/jnr.0000000000000015
21. Doering JJ, Szabo A, Goyal D, Babler E. Sleep quality and quantity in low-income postpartum women. MCN Am J Matern Child Nurs. 2017;42(3):166–172. doi:10.1097/NMC.0000000000000323
22. Christian LM, Carroll JE, Porter K, Hall MH. Sleep quality across pregnancy and postpartum: effects of parity and race. Sleep Health. 2019;5(4):327–334. doi:10.1016/j.sleh.2019.03.005
23. Khadka R, Hong SA, Chang YS. Prevalence and determinants of poor sleep quality and depression among postpartum women: a community-based study in Ramechhap district, Nepal. Int Health. 2020;12(2):125–131. doi:10.1093/inthealth/ihz032
24. Wen SY, Ko YL, Jou HJ, Chien LY. Sleep quality at 3 months postpartum considering maternal age: a comparative study. Women Birth. 2018;31(6):e367–e73. doi:10.1016/j.wombi.2018.02.004
25. Coo S, Milgrom J, Trinder J. Mood and objective and subjective measures of sleep during late pregnancy and the postpartum period. Behav Sleep Med. 2014;12(4):317–330. doi:10.1080/15402002.2013.801348
26. Kalogeropoulos C, Burdayron R, Laganière C, Dubois-Comtois K, Béliveau MJ, Pennestri MH. Sleep patterns and intraindividual sleep variability in mothers and fathers at 6 months postpartum: a population-based, cross-sectional study. BMJ Open. 2022;12(8):e060558. doi:10.1136/bmjopen-2021-060558
27. King LS, Rangel E, Simpson N, Tikotzky L, Manber R. Mothers’ postpartum sleep disturbance is associated with the ability to sustain sensitivity toward infants. Sleep Med. 2020;65:74–83. doi:10.1016/j.sleep.2019.07.017
28. Okun ML, Mancuso RA, Hobel CJ, Schetter CD, Coussons-Read M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J Behav Med. 2018;41(5):703–710. doi:10.1007/s10865-018-9950-7
29. Stremler R, McMurray J, Brennenstuhl S. Self-reported sleep quality and actigraphic measures of sleep in new mothers and the relationship to postpartum depressive symptoms. Behav Sleep Med. 2020;18(3):396–405. doi:10.1080/15402002.2019.1601629
30. Lewis BA, Gjerdingen D, Schuver K, Avery M, Marcus BH. The effect of sleep pattern changes on postpartum depressive symptoms. BMC Women's Health. 2018;18(1):12. doi:10.1186/s12905-017-0496-6
31. Howard K, Maples JM, Tinius RA. Modifiable maternal factors and their relationship to postpartum depression. Int J Environ Res Public Health. 2022;19(19). doi:10.3390/ijerph191912393
32. Zou H, Sun M, Liu Y, et al. Relationship between dietary inflammatory index and postpartum depression in exclusively breastfeeding women. Nutrients. 2022;14(23):5006. doi:10.3390/nu14235006
33. Çankaya S. The effect of psychosocial risk factors on postpartum depression in antenatal period: a prospective study. Arch Psychiatr Nurs. 2020;34(3):176–183. doi:10.1016/j.apnu.2020.04.007
34. Clout D, Brown R. Sociodemographic, pregnancy, obstetric, and postnatal predictors of postpartum stress, anxiety and depression in new mothers. J Affect Disord. 2015;188:60–67. doi:10.1016/j.jad.2015.08.054
35. Liu Y, Guo N, Li T, Zhuang W, Jiang H. Prevalence and associated factors of postpartum anxiety and depression symptoms among women in Shanghai, China. J Affect Disord. 2020;274:848–856. doi:10.1016/j.jad.2020.05.028
36. Chang SM, Chen CH. Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: a randomized controlled trial. J Adv Nurs. 2016;72(2):306–315. doi:10.1111/jan.12836
37. Effati-Daryani F, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Taghizadeh M, Bekhradi R, Zarei S. Effect of Lavender cream with or without footbath on sleep quality and fatigue in pregnancy and postpartum: a randomized controlled trial. Women Health. 2018;58(10):1179–1191. doi:10.1080/03630242.2017.1414101
38. Chen ML, Chen YE, Lee HF. The effect of bergamot essential oil aromatherapy on improving depressive mood and sleep quality in postpartum women: a randomized controlled trial. J Nurs Res. 2022;30(2):1.
39. Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA. Randomized controlled trial of digital cognitive behavior therapy for prenatal insomnia symptoms: effects on postpartum insomnia and mental health. Sleep. 2022;45(2). doi:10.1093/sleep/zsab280
40. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30(5):e13344. doi:10.1111/jsr.13344
41. El Sehmawy AA, Younes Abd Elaziz S, Elwahed RMA, Elsheikh AA. Skin-to-skin contact and its effect on mothers’ postpartum psychological distress and their full-term neonate in Egypt. J Trop Pediatr. 2023;69(3). doi:10.1093/tropej/fmad020
42. Kempler L, Sharpe LA, Marshall NS, Bartlett DJ. A brief sleep focused psychoeducation program for sleep-related outcomes in new mothers: a randomized controlled trial. Sleep. 2020;43(11). doi:10.1093/sleep/zsaa101
43. Sharkey KM, Pearlstein TB, Carskadon MA. Circadian phase shifts and mood across the perinatal period in women with a history of major depressive disorder: a preliminary communication. J Affective Disorders. 2013;150(3):1103–1108. doi:10.1016/j.jad.2013.04.046
44. Kudo N, Shinohara H, Kagabu S, Kodama H. Evaluation of salivary melatonin concentrations as a circadian phase maker of morning awakening and their association with depressive mood in postpartum mothers. Chronobiol Int. 2021;38(10):1409–1420. doi:10.1080/07420528.2021.1930028
45. Anbesaw T, Abebe H, Kassaw C, Bete T, Molla A. Sleep quality and associated factors among pregnant women attending antenatal care at Jimma Medical Center, Jimma, Southwest Ethiopia, 2020: cross-sectional study. BMC Psychiatry. 2021;21(1):469. doi:10.1186/s12888-021-03483-w
46. Zhang H, Li P, Fan D, et al. Prevalence of and risk factors for poor sleep during different trimesters of pregnancy among women in China: a Cross-Sectional Study. Nat Sci Sleep. 2021;13:811–820. doi:10.2147/NSS.S303763
47. Sedov ID, Cameron EE, Madigan S, Tomfohr-Madsen LM. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. doi:10.1016/j.smrv.2017.06.005
48. Truijens SEM, Spek V, van Son MJM, Guid Oei S, Pop VJM. Different patterns of depressive symptoms during pregnancy. Arch Women's Ment Health. 2017;20(4):539–546. doi:10.1007/s00737-017-0738-5
49. Mott SL, Schiller CE, Richards JG, O’Hara MW, Stuart S. Depression and anxiety among postpartum and adoptive mothers. Arch Women's Ment Health. 2011;14(4):335–343. doi:10.1007/s00737-011-0227-1
50. Furtado M, Van Lieshout RJ, Van Ameringen M, Green SM, Frey BN. Biological and psychosocial predictors of anxiety worsening in the postpartum period: a longitudinal study. J Affect Disord. 2019;250:218–225. doi:10.1016/j.jad.2019.02.064
51. Daglar G, Nur N. Level of mother-baby bonding and influencing factors during pregnancy and postpartum period. Psychiatry Danub. 2018;30(4):433–440. doi:10.24869/psyd.2018.433
52. Dubber S, Reck C, Müller M, Gawlik S. Postpartum bonding: the role of perinatal depression, anxiety and maternal-fetal bonding during pregnancy. Arch Women's Ment Health. 2015;18(2):187–195. doi:10.1007/s00737-014-0445-4
53. Edhborg M, Nasreen HE, Kabir ZN. Impact of postpartum depressive and anxiety symptoms on mothers’ emotional tie to their infants 2–3 months postpartum: a population-based study from rural Bangladesh. Arch Women's Ment Health. 2011;14(4):307–316. doi:10.1007/s00737-011-0221-7
54. Lillis TA, Hamilton NA, Pressman SD, et al. Sleep quality buffers the effects of negative social interactions on maternal mood in the 3–6 month postpartum period: a daily diary study. J Behav Med. 2018;41(5):733–746. doi:10.1007/s10865-018-9967-y
55. Henderson J, Alderdice F, Redshaw M. Factors associated with maternal postpartum fatigue: an observational study. BMJ Open. 2019;9(7):e025927. doi:10.1136/bmjopen-2018-025927
56. Albanese AM, Geller PA, Steinkamp JM, Barkin JL. In their own words: a qualitative investigation of the factors influencing maternal postpartum functioning in the United States. Int J Environ Res Public Health. 2020;17(17):6021. doi:10.3390/ijerph17176021
57. Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Women's Health Issues. 2010;20(2):96–104. doi:10.1016/j.whi.2009.11.003
58. Godos J, Grosso G, Castellano S, Galvano F, Caraci F, Ferri R. Association between diet and sleep quality: a systematic review. Sleep Med Rev. 2021;57:101430. doi:10.1016/j.smrv.2021.101430
59. Nelson KL, Davis JE, Corbett CF. Sleep quality: an evolutionary concept analysis. Nurs Forum. 2022;57(1):144–151. doi:10.1111/nuf.12659
60. Park EM, Meltzer-Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch Women's Ment Health. 2013;16(6):539–547. doi:10.1007/s00737-013-0356-9
61. Mohd Shukri NH, Wells J, Eaton S, et al. Randomized controlled trial investigating the effects of a breastfeeding relaxation intervention on maternal psychological state, breast milk outcomes, and infant behavior and growth. Am J Clin Nutr. 2019;110(1):121–130. doi:10.1093/ajcn/nqz033
62. Tucker Z, O’Malley C. Mental health benefits of breastfeeding: a literature review. Cureus. 2022;14(9):e29199. doi:10.7759/cureus.29199
63. Astbury L, Bennett C, Pinnington DM, Bei B. Does breastfeeding influence sleep? A longitudinal study across the first two postpartum years. Birth. 2022;49(3):540–548. doi:10.1111/birt.12625
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
