Back to Journals » Research and Reports in Neonatology » Volume 13
Magnitude of Neonatal Admission Diagnosis and Associated Factors at Selected Hospitals in Wollo, Northeast Ethiopia
Authors Asmamaw SD , Getachew S, Demeke T , Hankarso H, Alemnew B , Wedajo S , Molla A
Received 6 May 2023
Accepted for publication 30 October 2023
Published 8 November 2023 Volume 2023:13 Pages 29—44
DOI https://doi.org/10.2147/RRN.S418964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Shambel Dessale Asmamaw,1,2 Shiferaw Getachew,2,3 Tamrat Demeke,4 Hailu Hankarso,2,5 Birhan Alemnew,6 Shambel Wedajo,2 Asressie Molla2
1Department of Preventive Medicine, Kobo Primary Hospital, Kobo, North Wollo, Amhara Regional State, Ethiopia; 2Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia; 3Department of Preventive Medicine, Meda Weremo Health office, Meda Weremo, North Showa, Amhara Regional State, Ethiopia; 4Department of Preventive Medicine, Jama Primary Hospital, Jama, South Wollo, Amhara Regional State, Ethiopia; 5Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 6Department of Medical Laboratory Sciences, College of Health Sciences, Woldia University, Woldia, Ethiopia
Correspondence: Shambel Dessale Asmamaw, Email [email protected]
Background: Neonates are commonly admitted to neonatal intensive care units, and the type(s) of admission determine the outcome of the neonate. Therefore, we sought to assess the magnitude of neonatal admission and associated factors at selected hospitals in Wollo, northeast Ethiopia in 2022.
Methods: A cross-sectional study on 422 admitted mother–neonate pairs was conducted. Data were collected by face-to-face interviews and reviewing patient records and then entered and analyzed using EpiData version 3.1 and Stata version 14, respectively. Binary logistic regression analyses were employed, and P< 0.05 was considered statistically significant on multivariate analysis.
Results: The prevalence of neonatal sepsis was 53.4% (95% CI 48.5%– 58.2%), low birth weight 36.9% (95% CI 32.3%– 41.7%), prematurity 24.2% (95% CI 20.3%– 28.5%), and hypoglycemia 9.7% (95% CI 7.2%– 13%). Urinary tract infection (AOR 2.22, 95% CI 1.13– 4.34), history of abortion (AOR 1.95, 95% CI 1.002– 3.78), and twin pregnancy (AOR 6.34, 95% CI 1.84– 11.83) were associated with low birth weight. Premature rupture of membrane (AOR 2.87 95% CI 1.31– 6.28), history of abortion (AOR 2.36, 95% CI 1.20– 4.61), and instrumental delivery (AOR 5.25, 95% CI 1.65– 16.71) were associated with neonatal sepsis. Male sex (AOR 2.78, 95% CI 1.45– 5.34), pregnancy-induced hypertension (AOR 2.73, 95% CI 1.13– 6.60), antepartum hemorrhage (AOR 3.24, 95% CI 1.03– 10.20), and premature rupture of membrane (AOR 2.77, 95% CI 1.23– 6.24) were associated with prematurity.
Conclusion: The prevalence of low birth weight, prematurity, and neonatal sepsis was high, but neonatal hypoglycemia was low. Urinary tract infection, history of abortion, and twin pregnancy were associated with low birth weight. Premature rupture of membrane, history of abortion, and instrumental delivery were associated with neonatal sepsis. Male sex, pregnancy-induced hypertension, antepartum hemorrhage, and premature ruptures of membrane were associated with prematurity.
Keywords: admission diagnosis, discriminant analysis, Ethiopia, neonatal admission, neonate
Background
Millions of babies are born each year, and a significant number of them are admitted to hospitals for various reasons.1 Neonatal admission generally refers to the admission of a newborn <29 days old to a health facility for medical care.2 Neonatal intensive care units (NICUs) provide life support to newborns; however, admission to an NICU entails risk for families and their admitted infants, including high costs.3,4 Admission to the NICU interrupts the mother–infant bonding and establishment of breastfeeding.5,6 Neonatal mortality and newborn compromise is a major concern in sub-Saharan Africa, and their rates reflect a nation’s socioeconomic status, efficiency, and effectiveness of health-care services.7 Ethiopia is one of the 10 countries where newborn mortality and morbidity are the highest.1
The reasons for entry into NICUs are the result of different morbidities. Neonatal morbidities include hypothermia, sepsis, prematurity, polycythemia hypoglycemia, meconium aspiration syndrome, perinatal asphyxia, congenital anomaly, and others.8–10 Developmental delay following neonatal morbidity leads to high health-care costs and intense use of medical resources.11
Many of the conditions that lead to early neonatal mortality and morbidity in low-income countries can be avoided with relatively simple and cost-effective interventions like contraception, vaccination of pregnant women, hygienic delivery in a hospital, training health-care workers in resuscitation practices, simplified algorithms that allow for early detection of perinatal infections, and early initiation of breastfeeding and skin-to-skin care.12
If not recognized and managed quickly, morbidities can escalate to serious complications, such as impaired vision and movement, learning, or behavior problems, which all have devastating effects on long-term developmental outcome and at severe levels lead to mortality.11,13 Several strategies have been launched to enhance neonatal survival and promotion of health and well-being, such as essential newborn-care services and reproductive, maternal, newborn, and child health in universal health coverage.14,15
Neonatal admission can be influenced by numerous factors: maternal sociodemographic characteristics (age, marital status, residence, educational status, monthly income),16–18 neonatal characteristics (sex, appearance, pulse, grimace, activity, and respiration [APGAR]),19–21 Maternal behavioral and medical related conditions (HIV/AIDS, diabetes mellitus, malaria, anemia, alcohol intake status),22–26 and gynecological and obstetric factors (premature rupture of membrane, prolonged labor, mode of delivery, urinary tract infection, antenatal care).27–31
While our country has implemented different policies to mitigate neonatal mortality, it remains tilted. According to the Ethiopian Demographic and Health Survey 2016, neonatal mortality was 29 deaths per 1000 live births, but after 3 years it had declined to 30.32,33 Ethiopia has undertaken to reduce the number of neonatal deaths from its present level to 12 per 1000 live births. Therefore, this study aimed to assess the magnitude of neonatal admission diagnosis and associated factors at selected hospitals in Wollo, northeast Ethiopia in 2022.
Methods
Study Area, Study Design, and Participants
A hospital-based cross-sectional study was conducted in selected South Wollo and North Wollo hospitals in Amhara Regional State from May 7 to August 10, 2022. Four governmental hospitals were selected according to their case flow after reviewing their 45 days of registration: Dessie Comprehensive Specialized Hospital (DCSH), Woldia Comprehensive Specialized Hospital (WCSH), Kombolcha General Hospital (KGH), and Kobo Primary Hospital (KPH).
Inclusion and Exclusion Criteria
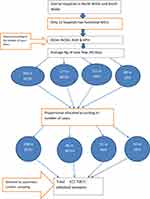
Mother–neonate pairs that were selected by systematic random sampling were included in the study. Mother–neonate pairs who were admitted to NICUs in the selected hospitals were included All neonates who were admitted to NICUs whose mothers were not available were excluded, as shown in Figure 1.
 |
Figure 1 Geographical areas of selected study hospitals. |
Sample Size and Sampling Procedure
The sample size was calculated by a single-population proportion formula based on the prevalence of neonatal sepsis in Nepal (50%),34 95% confidence level, and 5% margin of error. After factoring in a 10% nonresponse rate, the final sample for this study was 422. Among governmental hospitals in North Wollo and South Wollo, four hospitals were selected — DCSH, WCSH, KGH, and KPH — based on case flows after reviewing 45 days of admission. The calculated sample size was proportionately allocated based on the previous number of neonates admitted per 45 days in each hospital. Again, the sampling interval was determined by dividing the average number of neonates admitted per 45 days by the number of cases that were included in the study for each hospital, and then the study participants were selected by systematic random sampling after deciding on the random start randomly for each selected hospital, as illustrated in Figure 2.
Data-Collection Procedures and Measurements
Data were collected through an interviewer-administered structured Amharic version questionnaire and data-extraction checklist to extract data from charts developed from previous literature and guidelines. Maternal characteristics were asked about, ensuring the mothers’ privacy and confidentiality, after they had finished their contact with the health professional. Some of the questionnaires were also filled by referring to neonate folders/cards, eg, birth weight and APGAR score. Two BSc public health officer supervisors and four BSc nurses who are currently employed in the same health facility were employed as data collectors. Every incomplete questionnaire was sent back to the corresponding data collector for checkup.
Neonatal admission refers to the admission of the newborn <29 days old to a health facility for medical care.2 Neonates were considered to have sepsis if they had a clinical syndrome of bacteremia with systemic signs and symptoms of infection in the first 4 weeks of life. The neonate may present with any of the systemic manifestations of danger signs: not feeding well, convulsions, drowsy or unconscious, movement only when stimulated or no movement at all, fast breathing [60 breaths/min), grunting, severe inward drawing of chest, raised temperature >38°C, hypothermia <35.5°C, central cyanosis, severe jaundice, severe abdominal distension, or localizing signs of infection. Signs of pneumonia include many or severe skin pustules, bulging fontanel, painful joints, joint swelling, and reduced movement.35 A neonate is said to be hypoglycemic when blood glucose is <47 mg/dL.35 A newborn is considered premature when he/she was delivered before a gestational age of 37 weeks (259 days) starting from the last normal menstrual period. For those mothers who did not know their last normal menstrual period, early ultrasound and new Ballard score were employed.36,37 A neonate is considered to have low birth weight (LBW) at <2500 grams, regardless of gestational age.36,38
Statistical Analysis
The data were entered into EpiData version 3.1 and analyzed using Stata version 14. Descriptive analyses, such as frequencies and percentages, were detemined and discriminant analyses employed to identify the predictors of neonatal mortality. Then, after bivariate binary logistic regression analysis, those variables with P<0.25 were included in a multivariate logistic regression model to control for all possible confounders and to identify predictors of the outcome variable. Hosmer and Lemeshow goodness of fit and standard error were used to check the model goodness of the test and multicollinearity, respectively. In the final model, those variables with P<0.05 were considered statistically significant. AORs and 95% CI were estimated to measure the strength and direction of association.
Ethics Approval
This research was carried out in line with the Declaration of Helsinki. Data collection was carried out after obtaining ethics approval from the ethics review committee of Wollo University College of Medicine and Health Sciences (reference CMHS1473/2014). Participants <18 years of age were approved by the ethics committee to provide informed consent on their own behalf. In addition, official letters of cooperation were submitted to each selected hospital. Verbal informed consent was obtained from each respondent prior to enrollment and was considered acceptable and approved by the ethics committee. Each participant was informed about the aim and importance of the study. Anyone who was not willing to participate in the study was not forced to do so and had the full right to refuse or even withdraw from the study. Privacy of the participants and confidentiality of the information they gave was secured at all levels. The information was used only for the purposes of the study.
Results
Sociodemographic Characteristics of Mothers
In sum, 422 mother–neonate pairs participated in this study for a response rate of 100%. The mean age of the mothers was 27±4.76 years, 396 (93.84%) were married, 62.3% (263) resided in an urban setting, 128 (30.33%) had had no formal education, and 206 (48.82%) were housewives, as indicated in Table 1. Of the 422 admitted neonates, 54.74% (231) were female with a mean age of four days, 43.27% (90) had an APGAR score <7 at 1 minute after birth and 70.67% (147) had an APGAR score ≥7 at five minutes after birth. More than half (58.25%, 240) had normal birth weight, as shown in Table 2.
 |
Table 1 Sociodemographic characteristics of mothers (n=422) |
 |
Table 2 Characteristics of admitted neonates (n=422) |
Medical and Behavioral Factors of Mothers
Of the participants, 49 (11.61%) of the mothers were anemic during their pregnancy period while they were pregnant, five mothers were HIV/AIDS-positive, and six were known diabetes mellitus clients. Nineteen (4.5%) drank alcohol and 26 (6.16%) chewed khat during their pregnancy. A total of 406 (96.21%) did not read a newspaper at least once per week, 357 (84.6%) did not listen to the radio at least once per week, and 253 (59.95%) watched TV at least once per week, as illustrated in Table 3.
 |
Table 3 Medical and behavioral data of mothers (n=422) |
Obstetric and Gynecological Factors
Of all study participants, 238 (56.4%) mothers were multiparous, 403 (95.5%) mothers had had antenatal care visits at least once, 30 (7.11%) were diagnosed with antepartum hemorrhage (APH), and 70 (17.3%) had premature rupture of membrane. In total, 403 (95.5%) mothers delivered in a health institution and 275 (65.17%) of the neonates were delivered through SVD, 53 (12.05%) via instrumental means (either vacuum or forceps), and the remaining 94 (22.27%) by cesarean section, as indicated in Table 4.
 |
Table 4 Obstetric and gynecological factors (n=422) |
Reasons for NICU Admission
The most common reasons for neonatal admission to the NICU were neonatal sepsis (53.8%), LBW (36.9%), hypothermia (32.3%), respiratory distress (26.9%), prematurity (24.2%), asphyxia (20.1%), congenital malformation (12.9%), anemia (12.4%), hypoglycemia (9.7%), jaundice (9.5%), meconium aspiration syndrome (9.2%), and hypoxic–ischemic encephalopathy/encephalopathy (3.2%). Based on discriminant analysis results, LBW (P=0.001), prematurity (P=0.001), neonatal sepsis (P=0.001), and hypoglycemia (P=0.008) were predictors of neonatal mortality.
Factors Associated with NICU Admission
Low Birth Weight
Following bivariate logistic regression, 11 variables with P<0.25 were considered in multivariate analysis. The results showed that urinary tract infection, history of abortion, and birth type were had statistically significant associations with LBW. Those mothers who had had a UTI during pregnancy period were 2.22 times as likely to deliver an LBW neonate as mothers who had not. Mothers with a history of abortion were 1.95 times as likely to deliver an LBW neonate as mothers with no history of abortion. Mothers who had a twin gestation were 6.34 times as likely to deliver an LBW neonate as mothers who had a single gestation, as shown in Table 5.
 |
Table 5 Bivariate and multivariate binary logistic regression for mothers of low-birth-weight neonates |
Neonatal Sepsis
Following bivariate logistic regression, 11 variables with P<0.25 were considered in multivariatle analysis. The results showed that PROM, history of abortion, and mode of delivery have statistically significant associations with neonatal sepsis. Neonates delivered from PROM mothers were 2.87 times as likely to develop sepsis as their counterparts. Neonates delivered from mothers who had had at least one abortion were 2.36 times as likely to develop sepsis as their counterparts. The odds of developing sepsis for neonates who were delivered via instrumental delivery were 5.25 times those of neonates who were delivered by spontaneous vaginal delivery, as illustrated in Table 6.
 |
Table 6 Bivariate and multivariate binary logistic regression for neonatal sepsis |
Prematurity
Multivariate analysis result showed that sex, pregnancy-induced hypertension (PIH), PROM, and APH, were found to have statistically significant associations with prematurity. Male neonates were 2.78 times as likely to be premature as female neonates. Mothers who were hypertensive during pregnancy were 2.73 times as likely to deliver a premature baby as their counterparts. Mothers who encountered APH were 3.2 times as likely to have premature neonates as mothers who had no APH. Mothers who had PROM were 2.8 times as likely to deliver premature neonates as their counterparts, as indicated in Table 7.
 |
Table 7 Bivariate and multivariate binary logistic regression for premature birth |
Discussion
Our study aimed to identify neonatal admission diagnoses and associated factors at selected hospitals in Wollo, northeast Ethiopia. We identified LBW, prematurity, sepsis, and hypoglycemia as predictors of neonatal mortality by discriminant analysis.
Magnitude of Hypoglycemia
Our study identified hypoglycemia as the admission diagnosis in 9.7% of patients. This is consistent with the reported incidence in Jerusalem, Israel of 12.1%,39 but is higher than that found in a Ugandan study of 2.2%.40 This rate is less than that identified in a study conducted at St Paul’s Hospital Millennium Medical College, Ethiopia of 25%.41 These differences may be due to study design, diagnostic modality, patient populations, lactation education and support, and effectiveness in breastfeeding initiation.
Magnitude of Prematurity and Factors Influencing It
Prematurity accounted for 24.2% of admissions in the current study. Male sex, PIH, PROM and APH were significantly associated with prematurity. Our study’s prevalence of preterm birth is consistent with a previous study conducted in Pakistan42 of 27.9%, but is higher than studies conducted in Nigeria (19%)43 and Jimma (0.2%).19 It is lower than a previous study conducted in an Addis Ababa tertiary care hospital, St Paul’s Hospital Millennium Medical College, which was found to be 36.6%.44 Differences in reported rates of hospital admissions due to prematurity are likely due to hospital setting and patient population served, as well as newborn assessment using standardized tools, such as Ballard score.45
In the current study, we found that male newborns were 2.8 times as likely to be born prematurely as females. This is consistent with studies conducted in France and the Netherlands,20,21 but differs from a study conducted at Jimma University Specialized Hospital, Ethiopia.19 The etiopathogenesis of higher rates of prematurity in male fetuses is multifactorial and could be related to intrauterine growth rates or hormonal differences between sexes.46,47 Birthing parents who were hypertensive during the pregnancy were 2.7 times as likely to deliver prematurely as those who were not hypertensive. This was also found in a study conducted in China.48 This may be related to PIH-associated uteroplacental insufficiency and higher rates of placental abruption.49
Antepartum hemorrhage was associated with a 3.2-fold increased risk of prematurity. This finding is consistent with studies from Israel and Japan.50,51 This association is plausible, since profuse, life-threatening vaginal bleeding is an indication for immediate delivery.52 Premature rupture of membranes in our study was associated with a 2.7-fold increased risk of prematurity, a finding consistent with previous studies conducted in Kenya, Nigeria, and India.53–55 Whether this is the result of an inflammatory or infectious process is beyond the scope of the present study, but has been reviewed previously.
Magnitude of Low Birth Weight and Factors Influencing It
LBW in our study population was 36.9%. Maternal factors including urinary tract infection and previous abortion as well as mode of delivery were significantly associated with LBW. Rates of LBW were lower than in previous Ethiopian studies conducted in the northwest and southwestern regions: 14.6% and 11.0%, respectively.56,57 These differences are multifactorial, but may be largely explained by referral patterns to the specialty hospital.
In the current study, the odds of delivering an LBW newborn were significantly higher in twin versus singleton gestation. This finding is in line with studies conducted in sub-Saharan Africa and Nigeria.58,59 In addition, we found that maternal urinary tract infection during pregnancy was associated with LBW, a finding also noted in studies conducted in the USA, Dumlupinar University in Turkey, and Israel.60–63Gravidas with at least one previous abortion doubles the risk risk of LBW. This finding is supported by studies conducted in Denmark, Hawassa, and a systematic review.64–66 There are many contributing factors that may be related to prior cervical or uterine injury67 and/or psychological stress, depression, and stigmatization. Those factors could contribute to decreased dietary diversity and higher risk of LBW delivery.68,69
Magnitude of Neonatal Sepsis and Factors Influencing It
The incidence of neonatal sepsis in our study population was 53.8%, with PROM, prior abortion, and mode of delivery found to be significantly associated. The prevalence of sepsis correlates with a study conducted in Nepal,34 but is higher than that found in a Pakistan study42 and a South African study — 20%, 21%, respectively9 — and lower than the incidence of 68% found in Gondar.8 The variance in reported prevalence of neonatal sepsis could be explained by patient characteristics, referral patterns, and infection-control practices.
Premature rupture of membranes was associated with threefold increased odds of developing sepsis, a finding consistent with studies in Jordan, Pakistan, and some studies in Ethiopia.66,70–73 This finding is plausible given the increased risk of chorioamnionitis and sepsis from ascending infection.52,74 Prior abortion was associated with double the odds of sepsis, and mode of delivery, particularly with the use of instruments (vacuum or forceps), showed five times the odds of neonatal sepsis. These findings are consistent with previous studies conducted in Sri Lanka, India, and Ethiopia,75–77 possibly related to fetal scalp/head trauma and less than optimal infection-control measures in place.78
Conclusion
Our study of patients admitted to hospitals in Wollo, northeast Ethiopia identified LBW, prematurity, sepsis, and hypoglycemia as predictors of neonatal mortality by discriminant analysis. Half of the newborns were septic on admission and a third were LBW. Future studies should examine interventions to reduce risks of sepsis and LBW.
Strengths and Limitations
Strengths
Predictors of neonatal mortality addressed after implementing discriminant analysis and this research address both maternal and neonatal factors using primary and secondary data. This will be an input for planning and guiding neonatal investments in the catchment area.
Limitations
The results may not be representative of all newborns in South Wollo and North Wollo, due to the study being hospital-based and cross-sectional. Additionally, the subjective nature of diagnosis is another limitation, and lastly it shares the drawbacks of cross-sectional design.
Abbreviations
APGAR, appearance, pulse, grimace, activity, and respiration; APH, antepartum hemorrhage; ANC, antenatal care; DCSH, Dessie Comprehensive Specialized Hospital; DM, diabetes mellitus; GA, gestational age; HIE, hypoxic–ischemic encephalopathy; KPH, Kobo Primary Hospital; KGH, Kombolcha General Hospital; LBW, low birth weight; NICU, neonatal intensive care unit; PIH, pregnancy-induced hypertension; PROM, premature rupture of membrane, SVD, spontaneous vaginal delivery; SDGs, Sustainable Development Goals; UTI, urinary tract infection; WCSH, Woldia Comprehensive Specialized Hospital.
Data Sharing
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank North Wollo and South Wollo hospitals’ human resource management officers and NICU staff, data collectors, supervisors, and study participants for their cooperation and participation during the data collection period.
Disclosure
The authors declare that they have no competing interests.
References
1. Kokeb M, Desta T. Institution Based prospective cross-sectional study on patterns of neonatal morbidity at Gondar University Hospital Neonatal Unit, North-West Ethiopia. Ethiop J Health Sci. 2016;26(1):73–79. doi:10.4314/ejhs.v26i1.12
2. Lawn JE, Cousens S, Zupan J. Team LNSS. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi:10.1016/S0140-6736(05)71048-5
3. Hynan M, Mounts K, Vanderbilt D. Screening parents of high-risk infants for emotional distress: rationale and recommendations. J Perinatol. 2013;33(10):748–753. doi:10.1038/jp.2013.72
4. Polin RA, Denson S, Brady MT, et al. Epidemiology and diagnosis of health care–associated infections in the NICU. Pediatrics. 2012;129(4):e1104–e9. doi:10.1542/peds.2012-0147
5. Crenshaw JT. Healthy birth practice# 6: keep mother and baby together—It’s best for mother, baby, and breastfeeding. J Perinat Educ. 2014;23(4):211–217. doi:10.1891/1058-1243.23.4.211
6. Dumas L, Lepage M, Bystrova K, Matthiesen A-S, Welles-Nyström B, Widström A-M. Influence of skin-to-skin contact and rooming-in on early mother–infant interaction: a randomized controlled trial. Clin Nurs Res. 2013;22(3):310–336. doi:10.1177/1054773812468316
7. Serbesa M, Oumer A, Iffa M. Assessment of Reason for Admission and Factors Associated with their Treatment outcome of Neonates in Dilchora Referral Hospital, Eastern, Ethiopia: institutional Based Cross-Sectional Record Review Study. J Neonatal Stud. 2018;1:103.
8. Demisse AG, Alemu F, Gizaw MA, Tigabu Z. Patterns of admission and factors associated with neonatal mortality among neonates admitted to the neonatal intensive care unit of University of Gondar Hospital, Northwest Ethiopia. Pediatric Health Med Therapeutics. 2017;8:57. doi:10.2147/PHMT.S130309
9. Hoque M, Haaq S, Islam R. Causes of neonatal admissions and deaths at a rural hospital in KwaZulu-Natal, South Africa. Southern Af J Epidemiol Infection. 2011;26(1):26–29. doi:10.1080/10158782.2011.11441416
10. Syed RA, Shakeel A, Heeramani L. Disease patterns and outcomes of neonatal admissions at a secondary care hospital in Pakistan. Sultan Qaboos University Medical Journal. 2013;13(1):192–193.
11. Bakhuizen SE, De Haan TR, Teune MJ, et al. Meta‐analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatrica. 2014;103(12):1211–1218. doi:10.1111/apa.12764
12. Lawn JE, Blencowe H, Oza S, et al. Every Newborn: progress, priorities, and potential beyond survival. lancet. 2014;384(9938):189–205. doi:10.1016/S0140-6736(14)60496-7
13. Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135. doi:10.1097/MOP.0000000000000315
14. Organization WH. Newborns: Improving Survival and Well-Being. Geneva: World Health Organization; 2020.
15. Kieny MP, Evans DB. Universal health coverage. EMHJ-Eastern Mediterranean Health J. 2013;19(4):305–306. doi:10.26719/2013.19.4.305
16. Wudie F, Tesfamicheal F, Fisseha H, et al. Determinants of preterm delivery in the central zone of Tigray, northern Ethiopia: a case-control study. South Af J Child Health. 2019;13(3):108–114. doi:10.7196/SAJCH.2019.v13i3.1479
17. Shah PS, Zao J, Ali S. Maternal marital status and birth outcomes: a systematic review and meta-analyses. Matern Child Health J. 2011;15(7):1097–1109. doi:10.1007/s10995-010-0654-z
18. Rugaimukam JJ, Mahande MJ, Msuya SE, Philemon RN. Risk factors for preterm birth among women who delivered preterm babies at Bugando Medical Centre, Tanzania. SOJ Gynecol Obstet Womens Health. 2017;3(2):1–7. doi:10.15226/2381-2915/3/2/00124
19. Berhane M, Workineh N, Girma T, et al. Prevalence of low birth weight and prematurity and associated factors in neonates in Ethiopia: results from a hospital-based observational study. Ethiop J Health Sci. 2019;29(6):67.
20. Zeitlin J, Saurel-Cubizolles M-J, De Mouzon J, et al. Fetal sex and preterm birth: are males at greater risk? Human Reproduction. 2002;17(10):2762–2768. doi:10.1093/humrep/17.10.2762
21. Peelen MJ, Kazemier BM, Ravelli AC, et al. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet Gynecol Scand. 2016;95(9):1034–1041. doi:10.1111/aogs.12929
22. Woday A, Muluneh MD, Sherif S. Determinants of preterm birth among mothers who gave birth at public hospitals in the Amhara region, Ethiopia: a case-control study. PLoS One. 2019;14(11):e0225060. doi:10.1371/journal.pone.0225060
23. Mekonen DG, Yismaw AE, Nigussie TS, Ambaw WM. Proportion of Preterm birth and associated factors among mothers who gave birth in Debretabor town health institutions, northwest, Ethiopia. BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-018-4037-7
24. Gebreslasie K. Preterm birth and associated factors among mothers who gave birth in Gondar town health institutions. Adv Nursing. 2016;2016. doi:10.1155/2016/4703138
25. Abaraya M, Seid SS, Ibro SA. Determinants of preterm birth at Jimma University Medical Center, Southwest Ethiopia. Pediatric health, medicine and therapeutics. Pediatric Health, Medicine and Therapeutics. 2018;9(101):101–107. doi:10.2147/PHMT.S174789
26. Yalew M, Getachew S, Mohammed K, et al. Individual and contextual-level factors associated with iron-folic acid supplement intake during pregnancy in Ethiopia: a multi-level analysis. BMC Pregnancy Childbirth. 2023;23(1):260. doi:10.1186/s12884-023-05593-7
27. Babu BVA, Devi SS, Kumar BK. Birth asphyxia–Incidence and immediate outcome in relation to risk factors and complications. Int J Res Health Sci. 2014;2(4):1064–1071.
28. Ekwochi U, Asinobi NI, Osuorah CD, et al. Incidence and predictors of mortality among newborns with perinatal asphyxia: a 4-year prospective study of newborns delivered in health care facilities in Enugu, South-East Nigeria. Clin Med Insights Pediatr. 2017;11:1179556517746646. doi:10.1177/1179556517746646
29. Kiyani AN, Arshad Khushdil AE. Perinatal factors leading to birth asphyxia among term newborns in a tertiary care hospital. Iran J Pediatr. 2014;24(5):68.
30. Chiabi A, Nguefack S, Evelyne M, et al. Risk factors for birth asphyxia in an urban health facility in Cameroon. Iranian j Child Neurol. 2013;7(3):46.
31. Gane B, Bhat V, Rao R. Antenatal and intrapartum risk factors for perinatal asphyxia: a case control study. Curr Pediatric Res. 2013.
32. Csa I. Ethiopia Demographic and Health Survey. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central statistical agency (CSA)[Ethiopia] and ICF; 2016.
33. Csace I. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
34. Shrestha S, Shah A, Prajapati R, Sharma Y. Profile of neonatal admission at Chitwan Medical College. J Chitwan Med College. 2013;3(4):13–16. doi:10.3126/jcmc.v3i4.9547
35. Protocol M. Neonatal Intensive Care Unit (NICU) Training. 2014.
36. Ababa A. Neonatal Intensive Care Unit (NICU) Training. 2014.
37. Lockwood C, Ramin S, Barss V. Overview of preterm labor and delivery. Up to Date. 2011;19(1):56.
38. Yadav D, Chaudhary U, Shrestha N. Risk factors associated with low birth weight. J Nepal Health Res Counc. 2011;9(2):159–164.
39. Bromiker R, Perry A, Kasirer Y, Einav S, Klinger G, Levy-Khademi F. Early neonatal hypoglycemia: incidence of and risk factors. A cohort study using universal point of care screening. J Maternal Fetal Neonatal Med. 2019;32(5):786–792. doi:10.1080/14767058.2017.1391781
40. Mukunya D, Odongkara B, Piloya T, et al. Prevalence and factors associated with neonatal hypoglycemia in Northern Uganda. 2020.
41. Nurussen I, Fantahun B. Prevalence and risk factors of neonatal hypoglycaemia at St. Paul’s Hospital Millennium Medical College, Ethiopia. Ethiopian J Pediatrics Child Health. 2021;16(1).
42. Ali SR, Ahmed S, Lohana H. Disease patterns and outcomes of neonatal admissions at a secondary care hospital in Pakistan. Sultan Qaboos Univ Med J. 2013;13(3):417–421. doi:10.12816/0003265
43. Ajao AE, Adeoye IA. Prevalence, risk factors and outcome of congenital anomalies among neonatal admissions in OGBOMOSO, Nigeria. BMC Pediatr. 2019;19(1):1–10. doi:10.1186/s12887-019-1471-1
44. Tekleab AM, Amaru GM, Tefera YA. Reasons for admission and neonatal outcome in the neonatal care unit of a tertiary care hospital in Addis Ababa: a prospective study. Res Rep Neonatol. 2016;6(17):41–49. doi:10.2147/RRN.S76270
45. Ballard J, Khoury J, Wedig K, Wang L, Eilers-Walsman B, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–423. doi:10.1016/S0022-3476(05)82056-6
46. Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–550. doi:10.1210/edrv.21.5.0407
47. Cooperstock MS, Bakewell J, Herman A, Schramm WF. Effects of fetal sex and race on risk of very preterm birth in twins. Am J Obstet Gynecol. 1998;179(3):762–765. doi:10.1016/S0002-9378(98)70079-1
48. Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Global Health. 2017;5(6):e624–e32. doi:10.1016/S2214-109X(17)30142-0
49. Sivakumar S, Vishnu Bhat B, Badhe BA. Effect of pregnancy induced hypertension on mothers and their babies. Indian J Pediatrics. 2007;74(7):623–625. doi:10.1007/s12098-007-0110-2
50. Klinger G, Bromiker R, Zaslavsky-Paltiel I, et al. Antepartum hemorrhage and outcome of very low birth weight, very preterm infants: a population-based study. Am J Perinatol. 2021;38(11):1134–1141. doi:10.1055/s-0040-1710353
51. Kuribayashi M, Tsuda H, Ito Y, et al. Evaluation of the risk factors for antepartum hemorrhage in cases of placenta previa: a retrospective cohort study. J Int Med Res. 2021;49(11):03000605211054706. doi:10.1177/03000605211054706
52. FMo H. Management Protocol on Selected Obstetrics Topics. Federal Democratic Republic of Ethiopia; 2010.
53. Kuppusamy N, Vidhyadevi A. Prevalence of preterm admissions and the risk factors of preterm labor in rural Medical College Hospital. Int J Sci Study. 2016;4(9):123–126.
54. Okube OT, Sambu LM. Determinants of preterm birth at the postnatal ward of Kenyatta National Hospital, Nairobi, Kenya. Open J Obstetrics Gynecol. 2017;7(09):973. doi:10.4236/ojog.2017.79099
55. Butali A, Ezeaka C, Ekhaguere O, et al. Characteristics and risk factors of preterm births in a tertiary center in Lagos, Nigeria. Pan Af Med J. 2016;24(1). doi:10.11604/pamj.2016.24.1.8382
56. Gebremedhin M, Ambaw F. Maternal associated factors of low birth weight: a hospital based cross-sectional mixed study in Tigray, Northern Ethiopia. BMC Pregnancy Childbirth. 2015;15(1):222. doi:10.1186/s12884-015-0658-1
57. Zeleke BM, Zelalem M, Mohammed N. Incidence and correlates of low birth weight at a referral hospital in Northwest Ethiopia. Pan Af Med J. 2012;12(1).
58. Tessema ZT, Tamirat KS, Teshale AB, Tesema GA. Prevalence of low birth weight and its associated factor at birth in Sub-Saharan Africa: a generalized linear mixed model. PLoS One. 2021;16(3):e0248417. doi:10.1371/journal.pone.0248417
59. Dahlui M, Azahar N, Oche OM, Aziz NA. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Glob Health Action. 2016;9(1):28822. doi:10.3402/gha.v9.28822
60. Bilgin H, Yalinbas EE, Elifoglu I, Atlanoglu S. Maternal Urinary Tract Infection: is It Associated With Neonatal Urinary Tract Infection? J Family Reproductive Health. 2021;15(1):8.
61. Sheiner E, Mazor-Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. j Maternal Fetal Neonatal Med. 2009;22(5):423–427. doi:10.1080/14767050802360783
62. McGRADY GA, Daling JR, Peterson DR. Maternal urinary tract infection and adverse fetal outcomes. Am J Epidemiol. 1985;121(3):377–381. doi:10.1093/oxfordjournals.aje.a114009
63. Mazor-Dray E, Levy A, Schlaeffer F, Sheiner E. Maternal urinary tract infection: is it independently associated with adverse pregnancy outcome? J Maternal Fetal Neonatal Med. 2009;22(2):124–128. doi:10.1080/14767050802488246
64. Desta M, Tadese M, Kassie B, Gedefaw M. Determinants and adverse perinatal outcomes of low birth weight newborns delivered in Hawassa University Comprehensive Specialized Hospital, Ethiopia: a cohort study. BMC Res Notes. 2019;12(1):1–7. doi:10.1186/s13104-019-4155-x
65. Zhou W, Sørensen HT, Olsen J. Induced abortion and low birthweight in the following pregnancy. Int J Epidemiol. 2000;29(1):100–106. doi:10.1093/ije/29.1.100
66. Shah PS, Zao J, Births KSGoDo PL. Induced termination of pregnancy and low birthweight and preterm birth: a systematic review and meta‐analyses. BJOG. 2009;116(11):1425–1442. doi:10.1111/j.1471-0528.2009.02278.x
67. Abajobir AA, Alati R, Kisely S, Najman JM. Are past adverse pregnancy outcomes associated with maternal anxiety and depressive symptoms in a sample of currently pregnant women? Ethiop J Health Sci. 2017;27(4):351–362. doi:10.4314/ejhs.v27i4.6
68. Stylianou-Riga P, Kouis P, Kinni P, et al. Maternal socioeconomic factors and the risk of premature birth and low birth weight in Cyprus: a case–control study. Reprod Health. 2018;15(1):1–8. doi:10.1186/s12978-018-0603-7
69. Van Ngo T, Gammeltoft T, Nguyen HTT, Meyrowitsch DW, Rasch V. Antenatal depressive symptoms and adverse birth outcomes in Hanoi. Vietnam PloS One. 2018;13(11):e0206650. doi:10.1371/journal.pone.0206650
70. Gebremedhin D, Berhe H, Gebrekirstos K. Risk factors for neonatal sepsis in public hospitals of Mekelle City, North Ethiopia, 2015: unmatched case control study. PLoS One. 2016;11(5):e0154798. doi:10.1371/journal.pone.0154798
71. Alemu M, Ayana M, Abiy H, Minuye B, Alebachew W, Endalamaw A. Determinants of neonatal sepsis among neonates in the northwest part of Ethiopia: case-control study. Ital J Pediatr. 2019;45(1):1–8. doi:10.1186/s13052-019-0739-2
72. Saleem A, Shaikh AF. Neonatal sepsis following prolonged rupture of membrane in a tertiary hospital in Karachi Pakistan. J Infect Dev Ctries. 2014;8(1):67–73. doi:10.3855/jidc.3136
73. Moges F, Eshetie S, Yeshitela B, Abate E. Bacterial etiologic agents causing neonatal sepsis and associated risk factors in Gondar, Northwest Ethiopia. BMC Pediatr. 2017;17(1):1–10. doi:10.1186/s12887-017-0892-y
74. Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Sys Rev. 2003;2:67.
75. Woldu M, Guta M, Lenjisa J, Tegegne G, Tesafye G, Dinsa H. Assessment of the incidence of neonatal sepsis, its risk factors, antimicrobial use and clinical outcomes in Bishoftu General Hospital. Neonatal Intensive Care Unit. 2014;4(214):2161.
76. Feleke AA, Abdella MY. Determinants and Magnitude of Neonatal Sepsis at Hiwot Fana Comprehensive Specialized University Hospital, Harar, Ethiopia: a Cross-Sectional Study. medRxiv. 2021.
77. Jayasinghe C, Abeysena C. Risk Factors for Neonatal Sepsis in Secondary and Tertiary Care Hospitals of a District in Sri Lanka: a Case–Control Study. J Pediatric Infectious Dis. 2021;16(06):269–277. doi:10.1055/s-0041-1732472
78. Akalu TY, Gebremichael B, Desta KW, Aynalem YA, Shiferaw WS, Alamneh YM. Predictors of neonatal sepsis in public referral hospitals, Northwest Ethiopia: a case control study. PLoS One. 2020;15(6):e0234472. doi:10.1371/journal.pone.0234472
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.