Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Late Onset Diaphragmatic Hernia: A Forgotten Etiology of Recurrent Vomiting in the Adolescent Population
Authors Kwag KH, Habibi Zoham M, Brown B, Sohn A, Harrison S, Brandwein A
Received 6 March 2023
Accepted for publication 1 May 2023
Published 10 May 2023 Volume 2023:14 Pages 141—146
DOI https://doi.org/10.2147/PHMT.S406010
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Koren Hyogene Kwag,1 Mojdeh Habibi Zoham,1 Brande Brown,1 Andy Sohn,2 Sebron Harrison,3 Ariel Brandwein1
1Department of Pediatrics, New York-Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, USA; 2Department of Surgery, New York-Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, USA; 3Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York, NY, USA
Correspondence: Koren Hyogene Kwag, Department of Pediatrics, New York-Presbyterian Brooklyn Methodist Hospital, 506 6th Street, Brooklyn, NY, 11215, USA, Tel +1 718 780-5970, Fax +1 718 780-3266, Email [email protected]
Abstract: Diaphragmatic hernia is a rare disorder in adolescents with oftentimes delayed diagnosis due to late-onset and non-specific clinical manifestations. In this report, we present a case of diaphragmatic hernia in an 18-year-old male, where initial diagnosis was complicated by confounding factors of type 1 diabetes mellitus and cannabinoid hyperemesis syndrome. This case highlights the importance of having a high index of suspicion for diaphragmatic hernia in patients with nonspecific gastrointestinal symptoms to ensure timely recognition and surgical intervention.
Keywords: diaphragmatic hernia, Bochdalek hernia, Morgagni hernia, type 1 diabetes mellitus, cannabinoid hyperemesis syndrome, vomiting, gastrointestinal symptoms
Introduction
Diaphragmatic hernia (DH) involves the herniation of abdominal contents into the thoracic cavity due to either a congenital malformation of the diaphragm or a tear or rupture of the diaphragm after trauma. The overall incidence of congenital diaphragmatic hernia (CDH) may be as high as 1 in 2000 to 1 in 5000 live births.1 CDHs occur due to the failure of canal closure between the transversum and esophagus during the eighth week of embryogenesis.2,3 Conversely, 3–5% of trauma patients are affected by a DH4 with an incidence of 0.8–8% following blunt trauma and an incidence of 25–75% after penetrating trauma.5 Most cases of CDHs occur in the neonatal period, with only 25% of cases identified after 1 month of age, and are further classified by their position of occurrence. Bochdalek hernias occur in the posterolateral portion of the diaphragm and account for 85% of cases. The overall incidence of Bochdalek hernias in adults, however, is 0.17%.6 Morgagni hernias affect the anterior portion of the diaphragm and constitute about 2% of cases. Given the earlier closure of the right pleuroperitoneal canal and the anatomic position of the liver, right-sided hernias are far less common.7 A significant mortality of 38–62% has been reported in the newborn period8 with symptom presentation dependent on hernia size. Smaller hernias, often resulting from posterolateral defects, may remain asymptomatic and undiagnosed until even adulthood.9
While the exact incidence of DH in the adolescent population is unknown, many cases report trauma as the primary cause.2,4,5,10 Trauma patients should be closely evaluated for a DH as the presentation may be asymptomatic in up to 53% of blunt traumas (eg, motor vehicle accidents) and 44% of penetrating traumas (eg, gunshot or stab wounds). Additionally, studies have shown that only 33% of hernias are detected by chest X-ray (CXR) on initial evaluation by a trauma team leader.4 In adults, most cases are caused by blunt trauma with left-sided rupture due to the protective effect of the liver and increased strength of the right hemi-diaphragm. In children, however, due to the laxity of liver attachments, there is an equal rate of rupture on both hemi-diaphragms.4
Case Presentation
An 18-year-old male presented to the emergency department with a one-day history of intractable vomiting, mild diarrhea, and generalized abdominal pain. He denied any chest pain, fever, rashes, dysuria, or other symptoms. His past medical history was significant for poorly controlled type 1 diabetes mellitus (T1DM) diagnosed at 7 years old and well-controlled mild intermittent asthma. The patient also reported chronic intermittent left shoulder pain caused by a motor vehicle accident (MVA) 3-years prior where he sustained a femur fracture that was surgically repaired. No abdominal or chest trauma was reported at the time of the MVA.
The patient’s vital signs on presentation were temperature 36.6°C, heart rate 144 beats per minute, respiratory rate 22 breaths per minute, blood pressure 124/80 mm Hg, and oxygen saturation 100% on room air. On physical exam, the patient showed signs of dehydration, including decreased skin turgor and a dry oral mucosa. He was alert and oriented without any focal neurologic deficits. The patient’s abdomen was soft and non-tender without any palpable masses. Pulmonary and cardiac examinations were also normal.
Given the patient’s history of T1DM, laboratory workup for diabetic ketoacidosis was performed. A comprehensive metabolic panel was unremarkable except for elevated glucose (286 mg/dL, N: 100–180 mg/dL) and borderline anion gap metabolic acidosis (bicarbonate 17 mmol/L). Complete blood count and pancreatic enzymes were within normal limits and a respiratory viral panel was negative. The patient was admitted to the pediatric unit for the management of vomiting and dehydration in the setting of hyperglycemia. He was given long-acting insulin at bedtime with a short-acting insulin sliding scale.
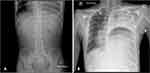
On days of admission 1 and 2, the patient complained of new onset chest pain with persistence of nausea and vomiting. Vital signs at the time were as follows: temperature 37°C, heart rate 116, respiratory rate 19, blood pressure 127/87 and oxygen saturation 97% on room air. His blood glucose levels remained elevated (>200mg/dL) despite insulin therapy. ECG and cardiac enzyme levels were within normal range. Nausea and vomiting continued on day 3, but with minimal abdominal pain. Of note, he reported relief from his symptoms following hot showers, leading to a consideration of cannabinoid hyperemesis syndrome (CHS) due to his history of prolonged cannabis use and positive urine toxicology for cannabinoids and benzodiazepines. Due to persistent nausea and vomiting, an abdominal X-ray (AXR) was performed showing a non-obstructive bowel gas pattern with haziness in the left lung base (Figure 1A).
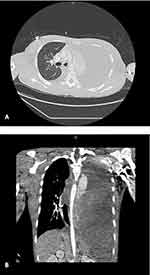
The following day, the patient became persistently tachycardic to 140 and experienced mild respiratory distress. Due to these abnormal findings, he was subsequently transferred to the pediatric intensive care unit, where vital signs at admission were temperature 36°C, heart rate 143, respiratory rate 35, blood pressure 115/76, and oxygen saturation 98% on room air. Physical exam at this point was notable for dry oral mucosa and diminished breath sounds at the left mid- and lower-lung fields. Fluid resuscitation was initiated, and laboratory tests were repeated. A CXR demonstrated diffuse opacification of the left hemithorax suspicious for a left-sided DH (Figure 1B). CT scan confirmed the presence of a large left-sided DH with pleural effusion (Figure 2).
A nasogastric tube was placed to decompress the stomach, draining dark brown fluid. The patient was taken for an emergency exploratory laparotomy. An incarcerated, strangulated stomach was reduced after extensive adhesiolysis within the left chest. There was no frank soilage of the thoracic cavity, although a large serous effusion was drained through the diaphragm defect. A wedge gastrectomy was then performed for a necrotic segment along the greater curvature, and the diaphragmatic defect was repaired primarily. A left chest tube was placed given the size of the pleural effusion. He tolerated the procedure well and had an uneventful post-operative course. The patient was discharged on post-operative day 18, ambulating and tolerating a regular diet. He was prescribed oral antibiotics and antiemetics for intermittent nausea and vomiting with instructions for outpatient follow-up with gastroenterology.
Two months after hospital discharge, the patient reported a 2-pound weight loss with abdominal fullness, constipation, and decreased oral intake without nausea and vomiting. A nuclear medicine gastric emptying study confirmed gastroparesis. Antiemetics and promotility agents were deferred, with the recommendation of strict glycemic control to address gastroparesis along with dietary changes and increased physical activity. Serial imaging has not shown a recurrence of the hernia.
On retrospective review of this patient’s imaging, a CXR performed 8 months prior to hospital presentation was remarkable for a 4 cm oval-shaped density in the left lung base, likely due to a primary small undiagnosed DH. The picture was consistent with the patient’s AXR in the hospital, which showed haziness in the left lung base.
Discussion
Although most CDHs are diagnosed in early childhood and addressed by pediatric surgeons, on rare occasions, small hernias may remain undiagnosed into adolescence or early adulthood. Similarly, minor tears to the diaphragm consequent of trauma may remain asymptomatic. In both etiologies, even if patients become symptomatic later in life, the diagnosis is often delayed or missed due to the non-specific nature of symptoms or subtle radiographic findings. Few studies have addressed DHs diagnosed in adolescents and adults.11 In those that have, clinical presentation typically involved nonspecific respiratory or gastrointestinal symptoms. Respiratory symptoms included dyspnea, cough, cyanosis, and chest pain. Gastrointestinal symptoms ranged from nausea and vomiting, such as seen in our patient on presentation to the emergency department, to dyspepsia, abdominal pain (diffuse or localized), diarrhea, obstipation, subcostal pain, and failure to thrive.12 There is suggestion of an association between neonatal diabetes and CDH,13 although no studies to date have addressed an association between type 2 or T1DM and DHs later in life.
As non-specific symptoms of CDH can mimic more common etiologies such as pneumothorax, pneumonia, and pleural effusion,14 diagnoses are often missed or only explored after the onset of more severe symptoms such as tachycardia or acute abdominal pain. With delayed diagnosis, morbidity and mortality significantly increase due to complications such as gastric volvulus, bowel obstruction, strangulation of herniated contents, bowel perforation, and short bowel syndrome.15 A mortality rate of up to 31% has been reported for acquired DH.16 Prompt diagnosis is of utmost importance: DH should be considered as a differential, even in patients with subtle radiographic findings such as an atypical gastric bubble position on an erect CXR or a rightward shift in the cardiac silhouette.14 Moreover, unless radiographic signs include air or an air-fluid level within a hollow viscus overlying the thorax, or with the additional coiling of the supra-diaphragmatic nasogastric tube on insertion, CXR is rarely useful as a diagnostic tool for DH. Due to the low sensitivity of CXR, other imaging modalities may be useful in the diagnosis of CDH. Chest CT is the current gold standard for diagnosis of DH as it can detect discontinuity of the diaphragm with high sensitivity.4 Although of value in the diagnosis of other conditions in a trauma setting, FAST exams have limited sensitivity in the diagnosis of traumatic diaphragmatic injury.5
The exact underlying etiology of DH in the presenting case remains unknown. Based on the history of MVA and suggestive findings on CXR taken 8 months prior to admission, the patient’s DH was most likely precipitated by blunt trauma. However, an undiagnosed CHD cannot be ruled out. Vomiting in the setting of hyperglycemia and CHS most likely exacerbated the diaphragmatic injury, resulting in the patient’s severe, symptomatic DH presentation.
Once a diagnosis is confirmed, surgical management is the gold standard of care with non-absorbable suture repair of the diaphragm.5 Both laparotomy and laparoscopy are reasonable surgical approaches depending on the severity of the hernia and organ involvement. Most patients tolerate the surgery, although complications have been seen in up to 4.55% of adults after CDH repair, requiring return to the operating room.11
Conclusion
The authors present a rare case of DH in an adolescent male, whose diagnosis was complicated due to nonspecific gastrointestinal presenting symptoms initially attributed to hyperglycemia in the setting of known T1DM. Although the exact cause of DH remains unknown, the patient’s history of a MVA and retrospective CXR suggesting a diaphragmatic injury point to trauma as the most likely etiology. We cannot rule out, however, the possibility of a CDH that was undiagnosed in the newborn period. Due to nonspecific radiologic as well as clinical presentation of DH that closely resemble more common respiratory, gastrointestinal, and in our case, even endocrine etiologies, diagnosis of DH can be missed or delayed, resulting in significant morbidity and mortality. Providers should, therefore, maintain a high index of suspicion when encountering adolescent patients with such symptoms.
Abbreviations
DH, diaphragmatic hernia; CDH, congenital diaphragmatic hernia; CXR, chest X-ray; T1DM, type 1 diabetes mellitus; MVA, motor vehicle accident; CHS, cannabinoid hyperemesis syndrome; AXR, abdominal X-ray.
Ethics Statement
The case report was reviewed and approved for exemption by the New York-Presbyterian Brooklyn Methodist Hospital Institutional Review Committee. A written informed consent was obtained from the patient to have the case details and any accompanying images published. Documentation is available upon request. The study follows the guidelines outlined in the Declaration of Helsinki.
Author Contributions
All authors:
Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
Have drafted or written, or substantially revised or critically reviewed the article.
Have agreed on the journal to which the article will be submitted.
Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
Agree to take responsibility and be accountable for the contents of the article.
Disclosure
Dr Sebron Harrison reports personal fees from AstraZeneca’s ADAURA Virtual Advisory Board, outside the submitted work. Authors declare no other conflict of interest.
References
1. Zalla JM, Stoddard GJ, Yoder BA. Improved mortality rate for congenital diaphragmatic hernia in the modern era of management: 15 year experience in a single institution. J Pediatr Surg. 2015;50(4):524–527. doi:10.1016/j.jpedsurg.2014.11.002
2. Testini M, Girardi A, Isernia RM, et al. Correction to: emergency surgery due to diaphragmatic hernia: case series and review. World J Emerg Surg. 2019;14:48. PMCID: PMC6796325. doi:10.1186/s13017-019-0269-7
3. Kluth D, Keijzer R, Hertl M, Tibboel D. Embryology of congenital diaphragmatic hernia. Semin Pediatr Surg. 1996;5(4):224–233.
4. Vyas PK, Godbole C, Bindroo SK, Mathur RS, Akula B, Doctor N. Case-based discussion: an unusual manifestation of diaphragmatic hernia mimicking pneumothorax in an adult male. Int J Emerg Med. 2016;9(1):11. PMID: 26924754; PMCID: PMC4770005. doi:10.1186/s12245-016-0108-5
5. Cameron AM. Current surgical therapy: john cameron’s contribution to surgical education and training via textbook. Ann Surg. 2019;267(2S):S6–9. doi:10.1097/SLA.0000000000002518
6. Mullins ME, Stein J, Saini SS, Mueller PR. Prevalence of incidental Bochdalek’s hernia in a large adult population. Am J Roentgenol. 2001;177(2):363–366. doi:10.2214/ajr.177.2.1770363
7. Spiridakis KG, Flamourakis ME, Gkionis IG, et al. Right-sided strangulating diaphragmatic hernia in an adult without history of trauma: a case report. J Med Case Rep. 2021;15(1):372. PMID: 34256846; PMCID: PMC8278739. doi:10.1186/s13256-021-02861-y
8. Killeen K, Mirvis S, Shanmuganathan K. Helical CT of diaphragmatic rupture caused by blunt trauma. AJR Am J Roentgenol. 2000;173(6):1611–1616. doi:10.2214/ajr.173.6.10584809
9. Al-Zayer F, Aljaroof AH, Al-Marhoun M, Abualsaud B, Al-Zaher M, Meshikhes AW. Congenital right diaphragmatic hernia in an adult. J Surg Case Rep. 2019;2019(12):rjz371. PMID: 31908759; PMCID: PMC6936744. doi:10.1093/jscr/rjz371
10. Turhan K, Makay O, Cakan A, et al. Traumatic diaphragmatic rupture: look to see. Eur J Cardiothorac Surg. 2008;33(6):1082–1085. PMID: 18299201. doi:10.1016/j.ejcts.2008.01.029
11. Brungardt JG, Nix QA, Schropp KP. Congenital diaphragmatic hernia: demographics and 30-day outcomes in adults. Am Surg. 2021;87(8):1341–1346. PMID: 33342249. doi:10.1177/0003134820960061
12. Banac S, Ahel V, Rozmanić V, Gazdik M, Saina G, Mavrinac B. Prirodena dijafragmalna hernija u veće djece [Congenital diaphragmatic hernia in older children]. Acta Clin Croat. 2004;58(3):225–228.
13. Topiol ES, Minarich LA, Williams CA, Zori RT, Kays DW, Haller MJ. Neonatal diabetes mellitus and congenital diaphragmatic hernia: coincidence or concurrent etiology? Int J Pediatr Endocrinol. 2012;2012(1):21. PMID: 22781086; PMCID: PMC3408326. doi:10.1186/1687-9856-2012-21
14. Shin HB, Jeong YJ. Late presenting congenital diaphragmatic hernia misdiagnosed as a pleural effusion: a case report. Medicine. 2020;99(24):e20684. PMID: 32541516; PMCID: PMC7302620. doi:10.1097/MD.0000000000020684
15. Yang GP, Tang CN, Siu WT, Ha JP, Tai YP, Li MK. Diaphragmatic hernia: an uncommon cause of dyspepsia. JSLS. 2005;9(3):352–355. PMID: 16121887; PMCID: PMC3015605.
16. Lu J, Wang B, Che X, et al. Delayed traumatic diaphragmatic hernia: a case-series report and literature review. Medicine. 2016;95(32):e4362. doi:10.1097/MD.0000000000004362
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.