Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Lactic Acid Chemical Peeling in Skin Disorders
Authors Feng X , Shang J , Gu Z, Luo X, Chen Y, Liu Y
Received 20 December 2023
Accepted for publication 13 April 2024
Published 23 April 2024 Volume 2024:17 Pages 901—909
DOI https://doi.org/10.2147/CCID.S455700
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xiaoyue Feng,* Jianli Shang,* Zhengping Gu, Xingyi Luo, Yong Chen, Youting Liu
Product Research & Development Center, Beijing Underproved Medical Technology Co., LTD, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Youting Liu, Product Research & Development Center, Beijing Underproved Medical Technology Co., LTD, Beijing, 102699, People’s Republic of China, Tel +86 1 851 025 6963, Email [email protected]
Abstract: Lactic acid is the most widely occurring natural organic acid in nature. It not only exhibits mild and safe properties but also possesses multiple physiological activities, such as antibacterial effects, immune regulation, and promotion of wound healing, making it one of the most popular chemical peeling agents. Chemical peels are commonly used in the field of aesthetic dermatology as a non-invasive therapeutic approach. This research aims to provide valuable references for clinical dermatologists by summarizing the characteristics of lactic acid, elucidating its mechanism of action in peeling, and investigating the clinical applications of this compound. Furthermore, it anticipates the potential for lactic acid to be the most suitable chemical peeling agent for Chinese skin.
Keywords: lactic acid, physiological activities, clinical applications, Chinese skin
Introduction
Lactic acid (LA) is the most widely found natural organic acid. The historical use of lactic acid in chemical peels can be traced back to ancient Egypt, where Queen Cleopatra was known to use yogurt for bathing, resulting in brighter and smoother skin.1 However, it was not until 1780 that the Swedish chemist Carl Wilhelm Scheele first extracted lactic acid from milk, identifying it as the crucial effective component in yogurt skincare.2 The first documented application of lactic acid for addressing skin conditions dates back to 1943 when Stern et al utilized lactic acid to treat ichthyosis, achieving significant results.3 Since then, an increasing number of scholars have conducted research on the moisturizing effects of lactic acid and its ability to alleviate skin dryness.4,5 Today, with continued research into the application of lactic acid in skincare, it is widely applied in chemical peels.
Chemical peel refers to the application of chemical agents on the skin to induce varying degrees of controlled damage in the epidermis or dermis, resulting in exfoliation, the removal of superficial lesions, and subsequent regeneration of both the epidermis and dermis.6 In the past few years, chemical peels have gained widespread use in dermatology and related cosmetic departments due to their simple and cost-effective procedures and minimal side effects, consistently ranking high among minimally invasive cosmetic treatment techniques.7
As chemical peels continue to evolve and improve, an increasing number of chemical agents are being applied in clinical treatment. Lactic acid, as a natural alpha-hydroxy acid, not only exhibits gentle and safe properties but also possesses multiple physiological activities, making it one of the most popular chemical peeling agents.8 Therefore, we provide an overview of the characteristics of lactic acid, its mechanism of action in chemical peels, and its clinical applications. This review anticipates the possibility that lactic acid may be the most suitable chemical peeling agent for Chinese skin, aiming to offer valuable references for clinical dermatologists.
Characteristics of Lactic Acid
Lactic acid, CH3CHOHCOOH, is a natural α-hydroxy acid (AHA) with a molecular weight of 90.08, slightly higher than that of glycolic acid (76.05). The lactic acid molecule contains one hydroxyl group and one carboxyl group and can ionize as a weak acid in aqueous solutions with a pKa value of 3.86. Lactic acid has two isomers: L-lactic acid and D-lactic acid, with L-lactic acid being the common form found in the skin and biological organisms. It exhibits some hygroscopic and moisturizing properties.9 Due to its mild, safe, and effective nature, lactic acid can promote cell renewal and skin hydration, making it widely used in chemical peels.
Gentleness
While ensuring the effectiveness of chemical peels, one important characteristic of lactic acid is its low level of irritation. The effects of various alpha-hydroxy acids, including glycolic acid, L-lactic acid, D-lactic acid, citric acid, hydroxybutyric acid, and malic acid, were assessed on different properties such as cell renewal rate, anti-wrinkle ability, and irritation. This evaluation was conducted under the same molar concentration and pH conditions.10 The results showed that, in terms of efficacy, L-lactic acid and glycolic acid had the best effects. In terms of gentleness, L-lactic acid exhibited significantly lower irritation compared to other acids, indicating that lactic acid is a chemical peeling agent that balances efficacy with gentleness. It’s worth noting that the mildness of lactic acid may be related to its self-esterification properties. Research has shown that lactic acid solutions with concentrations exceeding 18% undergo self-esterification reactions, forming dimers (including lactide, propyl lactate, methyl lactate) or polymers, and the conversion between them is reversible.11 This might be a significant reason why lactic acid can slowly penetrate the skin and reduce skin irritation.
Safety
Lactic acid is a highly safe chemical peeling agent, and its safety is mainly evident in the following aspects: ①Lactic acid has low skin irritation, and it does not lead to severe adverse reactions or persistent erythema. Stinging tests with a 10% lactic acid solution have demonstrated its excellent safety for sensitive skin.12 ②Lactic acid is a crucial natural moisturizing factor in the skin, being an endogenous substance, which makes it highly skin-compatible and unlikely to cause allergic reactions.13 ③Lactic acid is a major recyclable carbohydrate and serves as a universal fuel for the tricarboxylic acid cycle. Its end metabolic products are carbon dioxide and water, which are safely excreted from the body through urine and sweat, ensuring safety and non-toxicity.14 As a result, lactic acid can be safely used on sensitive skin and patients with rosacea, making it an ideal choice for individuals undergoing their first chemical peel.8
Highly Effective
Lactic acid serves as an energy substrate for muscle contraction, initially considered an inert product of glycolysis under anaerobic conditions. However, increasing evidence indicates that lactic acid, derived from glycolysis, is not only an inert energy donor but also a signaling molecule that plays a crucial role in various cellular processes through its transporters (MCTs) and receptors (GPR81). It possesses multiple physiological activities, including antibacterial effects, immune regulation, wound repair, and barrier protection.15,16
Bacteriostatic
Lactic acid is a widely utilized antibacterial agent that inhibits the growth of crucial microbial pathogens. The antibacterial mechanism of lactic acid was investigated, revealing that 0.5% lactic acid could completely inhibit the growth of Salmonella Enteritidis, Escherichia coli, and Listeria monocytogenes.15 The final results indicated that the antibacterial mechanism of lactic acid primarily involves damaging the bacterial cell membrane, resulting in damage to the intracellular structure and inhibiting the normal growth of the bacteria. In a separate study, lactic acid can penetrate the periplasmic space through bacterial outer membrane channels.17 It protonates the carboxyl and phosphate groups of lipopolysaccharide, thereby weakening the interactions among various components in the outer membrane. This process leads to the release of lipopolysaccharide and glycerophospholipids from the bacterial outer membrane, disrupting cell membrane integrity. Consequently, the leakage of internal substances induces sublethal damage to the bacteria, thereby achieving the antibacterial effect.
Immune Regulation
Lactic acid possesses anti-inflammatory and immune-regulating effects, contributing to the reduction of skin inflammation and sensitivity issues. High lactate concentrations with low pH can decrease the production of tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) by macrophages/monocytes.18 Moreover, studies have reported that lactic acid may inhibit the secretion of TNF by interfering with the glycolysis of human monocytes and suppress the differentiation of monocytes into dendritic cells, as well as the activation of T cells.19–22 For a more in-depth exploration of the immunomodulatory mechanisms of lactic acid, Peter K et al conducted a comprehensive analysis of its impact on gene expression during monocyte activation.23 The results revealed that lactic acid significantly delays the LPS-induced gene expression of monocyte effector proteins, including cytokines (eg TNF and IL-23) and chemokines (eg CCL2 and CCL7). Further analysis showed that lactic acid attenuates the phosphorylation of Protein Kinase B (PKB) and the degradation of IκBα, decreasing the expression of Nuclear Factor-Kappa B (NF-κB), thus exerting immune regulatory effects.
Wound Repair
Lactic acid or lactate not only serves as a signal for collagen synthesis and regeneration but also as a pro-angiogenic agent to accelerate wound healing.24 As early as 1964, observations have indicated that lactate stimulates collagen synthesis in fibroblasts.25 Subsequent research has clarified that this stimulation occurs through the activation of prolyl hydroxylase, an enzyme critical for collagen hydroxylation and maturation in fibroblasts.26 Some evidence suggests that lactate not only stimulates the production and release of various cytokines (eg vascular endothelial growth factor, basic fibroblast growth factor, interleukin-8) but also promotes endothelial cell migration and the recruitment of vascular precursor cells. These actions collectively promote angiogenesis, facilitating wound repair.27,28 Furthermore, Experiments confirmed that lactate is indeed a pro-angiogenic agent, enhancing the deposition of extracellular matrix in the skin and thereby promoting the healing process of ischemic and excisional wounds in mice.24
Barrier Protection
Lactic acid can stimulate the biosynthesis and increase the levels of ceramide in the stratum corneum, thereby enhancing the lipid barrier. The impact of different lactic acid isomers on ceramide synthesis in keratinocytes, as well as stratum corneum lipid levels and barrier function, was explored both in vitro and vivo.29 The results of cell experiments in vitro indicated that L-lactic acid significantly increased the levels of ceramide in keratinocytes (a 300% increase). D-lactic acid showed no statistically significant effect. Radiolabeling experiments confirmed that lactic acid can be metabolized and then employed as a carbon source for lipid biosynthesis. Additionally, it was also demonstrated that lactic acid elevated ceramide levels in the stratum corneum in vivo (L-lactic acid treatment resulted in a 38% increase and D, L-lactic acid resulted in a 25% increase, while D-lactic acid showed no statistically significant effect). Furthermore, the topical application of 4% lactic acid for one month not only significantly alleviated the damage caused by sodium lauryl sulfate (SLS) to the skin barrier but also provided additional evidence that lactic acid effectively improves the skin barrier, enhances barrier protection, and boosts skin resilience.
The Mechanism of Lactic Acid Peeling
The mechanism of lactic acid peeling is similar to that of glycolic acid and all Alpha-Hydroxy Acids (AHAs). Although the exact mechanism is not entirely clear, it has been established that AHAs improve skin conditions by exfoliating the stratum corneum, promoting epidermal desquamation, dispersing basal layer melanin, and increasing collagen synthesis in the dermal layer.30 Through the study of the structure and properties of AHAs, we further summarize and refine their possible mechanisms, as illustrated in Figure 1.
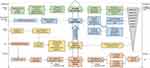 |
Figure 1 Mechanism of Action of AHAs peel. |
Promoting Desquamation
The acidic properties of AHAs reduce the pH of the epidermis, increase the activity of desquamation-related enzymes, accelerate desmosomal degradation, and promote desquamation. The effects of AHAs on desmosomal proteins in the stratum corneum were investigated to understand their mechanisms of action.31 They found that AHAs act in at least two different ways in the stratum corneum: ①The acidic properties of AHAs induce acidification of the stratum corneum, leading to the immediate activation of Cathepsin D-like (CD), a desmosomal protein with an optimal pH of approximately 2.8. This enhances desmosomal degradation, thereby weakening of cell-cell adhesion in the stratum corneum. ②In the two weeks following AHA treatment, the expression of CD in the stratum corneum gradually increases, which may be associated with the long-term promotion of stratum corneum desquamation. It was suggested that the chelating ability of AHAs directly interferes with the calcium ion concentration gradient in the epidermis, disrupting the conjugate structure of desmosomes (calcium ions can form conjugate structures with desmoglein, shielding it from degradation), making desmosomes more prone to degradation.32
Pigment Lightening
The antioxidant properties of AHAs can directly inhibit the activity of tyrosinase, resulting in a reduction in melanin synthesis and, consequently, lightening of pigmentation. The effects of AHAs on melanin formation were studied, revealing that both glycolic acid and lactic acid directly inhibit tyrosinase activity in a dose-dependent manner.33 This effect was shown to be independent of their acidic properties. Furthermore, AHAs promote epidermal desquamation, dispersing melanin in the basal layer, which is another crucial mechanism for lightening pigmentation. This is achieved through the chelation of calcium ions by AHAs, which not only affects the structure and function of desmosomes but also disrupts the tight junctions between epidermal cells. This disruption decreases cell adhesion and facilitates epidermal desquamation, thereby dispersing melanin accumulated in the basal layer of the epidermis.32
Shortening the Epidermal Renewal Cycle
The acidic properties of AHAs induce acidification within epidermal cells, speeding up the processes of cell keratinization and apoptosis, thereby shortening the epidermal renewal cycle. The investigation into their impact on epidermal keratinization unveiled that AHAs acidify intracellular environments, dependent on concentration and extracellular pH.34 This process effectively triggers the activation of transient receptor potential vanilloid 3 (TRPV3) channels in keratinocytes, leading to an influx of calcium ions and accelerating keratinization and apoptosis processes. This mechanism may contribute to explaining the role of mild acidity in promoting skin renewal. Additionally, calcium ions in the skin act as second messengers and play a crucial role in regulating cell proliferation and differentiation processes. Exploring the effects of different calcium ion concentrations on keratinocytes revealed that reduced levels of calcium ions could accelerate cell proliferation.35 Importantly, AHAs possess chelating properties that effectively reduce the calcium ion concentration in the epidermis, thereby promoting the proliferation of basal layer cells.
Injury Reconstruction of Dermal Tissues
AHAs can stimulate epidermal cells to release cytokines that mediate collagen synthesis by dermal fibroblasts, resulting in the reshaping of dermal tissues. Investigating the effects of AHAs on keratinocytes and the metabolism of the dermal matrix by fibroblasts revealed that AHAs not only directly accelerate collagen synthesis in fibroblasts but also indirectly regulate the degradation of the extracellular matrix and collagen synthesis.36 This indirect regulation occurs through the release of cell cytokines, such as interleukin-1α (IL-1α), by activating keratinocytes. Furthermore, AHAs may increase collagen synthesis by functional activation or proliferation of fibroblasts through the regulation of the transforming growth factor-β (TGF-β) signaling pathway, while simultaneously increasing the biosynthesis and secretion of extracellular matrix components, such as hyaluronic acid and glycosaminoglycans.37
Clinical Applications of Lactic Acid Peels
Lactic acid, a natural acidic substance, gently exfoliates dead skin cells from the skin surface, fostering cell turnover and collagen production. This process contributes to smoother, softer, and more elastic skin. The numerous benefits of lactic acid include skin whitening, moisturization, and pigmentation reduction. Consequently, lactic acid peels find common application in clinical practice for both cosmetic purposes and skin disorders.
Acne
Lin Cong conducted a clinical study to evaluate the effectiveness and adverse reactions of 40% lactic acid in the treatment of mild-moderate acne.38 Using a self-control method, 30 patients were observed, with treatment administered every two weeks for three peels. Before and after each treatment, the researchers recorded the number of inflammatory and non-inflammatory acne lesions and observed the occurrence of adverse reactions. The results indicated a significant decrease in both non-inflammatory and inflammatory acne lesions after each treatment compared to before treatment (P < 0.01). No severe adverse reactions were observed during the treatment, indicating that lactic acid treatment for mild-moderate acne is safe and effective.
Another study assessed the efficacy and safety of 88% lactic acid in the treatment of acne vulgaris and post-acne scarring.39 It involved ten patients with acne vulgaris and 15 patients with acne scars were included in the study. Lactic acid peeling was performed three times at two-week intervals, and the severity of acne and the degree of scarring were evaluated before and after each patient’s treatment. The results demonstrated a remarkable 87.2% reduction in the number of papules after three sessions of lactic acid peeling (P = 0.0001), with a simultaneous significant improvement observed in post-acne scarring (P = 0.002). All patients expressed complete satisfaction with the effects of lactic acid peeling, indicating that it is an effective method for treating acne vulgaris and post-acne scarring.
Additionally, research has shown that the combined use of plant preparations, 5% lactic acid, and ultrasound is more effective in reducing skin eruptions and sebum levels in acne patients compared to ultrasound alone.40 This suggests that the combined application of plant preparations and lactic acid has a positive effect on acne. The efficacy of lactic acid relies on its various properties including antibacterial action and promotion of proliferation.
Photoaging
A study compared the effectiveness of a combination of lactic acid and Ferulic acid with the application of Ferulic acid alone in addressing the effects of photoaging.41 The study involved two groups, each with 30 patients. The first group received treatment with 30% lactic acid in combination with 12% Ferulic acid, while the second group received treatment with 12% Ferulic acid alone. Treatments were administered every 15 days, and the assessment of clinical improvement was conducted before and after six sessions using the ASRS and AFLS scoring. The results demonstrated a significant improvement in photoaging with the combination of lactic acid and Ferulic acid (42%), surpassing the efficacy of Ferulic acid alone (27%), and no complications were reported. Although this study did not exclusively focus on lactic acid treatment alone, its findings still suggest the beneficial effects of lactic acid on skin photoaging. Another report investigated the clinical efficacy of 85% lactic acid in improving periorbital fine lines.42 The experimental group comprised 9 patients who underwent lactic acid peels in conjunction with sunscreen, while the control group consisted of 9 patients who solely used sunscreen, administered once a month for a total of 3 sessions. The clinical efficacy was assessed by digitizing images before and after treatment using a CCD color microscope. The results revealed a notable enhancement in the experimental group, particularly following the third session on the lateral sides of the right eye (P < 0.05). This suggests the efficacy of lactic acid peels in treating skin photoaging. Moreover, the clinical and histological improvements observed following lactic acid peel treatment for photoaging, as revealed by immunohistochemistry and electron microscopy studies, may be associated with mast cell degranulation.43 This, in turn, triggers dendritic cell activation in the dermis and an increased expression of transglutaminase (TGase).
Melasma
A comparative investigation assessing the efficacy and safety of lactic acid and glycolic acid peels in treating melasma was conducted, involving 112 patients aged 18–40.44 Group A, comprising 56 patients, received 60% lactic acid peels, while Group B, with 56 patients, received 40% glycolic acid peels. Treatments were administered every four weeks for a total of six sessions. The Melasma Area and Severity Index (MASI) were employed to assess clinical improvement before and after treatments. The results indicated a higher treatment effective rate in Group A (80.4%) compared to Group B (67.5%). Group A also exhibited a lower incidence of adverse reactions, suggesting that 60% lactic acid was more effective and safer than 40% glycolic acid. Sandin and Singh, in their evaluations of 82% lactic acid peels for facial melasma, affirmed lactic acid peels as a significant approach for improving treatment-resistant melasma with good tolerability.45,46 Notably, while assessing the safety of lactic acid and glycolic acid peels for melasma, it was discovered that 92% lactic acid resulted in fewer side effects than 50% glycolic acid peels.47 This not only enhanced patient compliance but also underscored the potential of lactic acid in melasma treatment.
Other Skin Disorders
Periorbital melanosis (POM), commonly referred to as dark circles, can impart a fatigued and lackluster appearance, thereby significantly impacting an individual’s quality of life and emerging as a major cosmetic concern. Among the various treatment modalities available, chemical peeling stands out as an effective and safe approach to reduce pigmentation issues around the eyes while enhancing skin texture and mitigating wrinkles. In a study, the clinical effectiveness and safety of lactic acid and glycolic acid peels in treating POM were compared.48 The study encompassed 56 participants, with 27 individuals undergoing 20% glycolic acid treatment and 29 subjects opting for 30% lactic acid treatment. Treatments were administered every three weeks for three sessions. Assessment parameters included clinical improvement using POM grading, overall patient evaluation and satisfaction, as well as overall physician satisfaction.
The results showed that both lactic acid and glycolic acid demonstrated significant improvement (p < 0.0001), with lactic acid showing better improvement (p = 0.008) and higher patient satisfaction, coupled with good tolerability.
Ichthyosis is a genetic skin disease characterized by skin dryness and scaling resembling fish scales. The current management of this condition involves alleviating symptoms by maintaining skin moisture through the application of topical emollients. A study explored the clinical effectiveness of lactic acid and vaseline (petroleum jelly) in the treatment of Ichthyosis.49 The research comprised 30 patients with moderate-to-severe Ichthyosis, divided into two groups. One group applied 12% lactic acid, while the other used vaseline. The treatment protocol involved applying the treatment twice daily for four weeks, with weekly evaluations during treatment and two weeks post-treatment cessation. The findings indicated that 12% lactic acid exhibited significantly greater efficacy compared to vaseline. These findings suggest that the biological effects of lactic acid on the skin, including moisturizing and exfoliation, play a crucial role in its effectiveness for Ichthyosis treatment.
Acanthosis nigricans (AN) is a dermatological condition characterized by pigment deposition and excessive skin keratinization. To date, no satisfactory local treatment method has been identified. Nevertheless, it was discovered that a combined therapy involving 12% ammonium lactate and 0.05% tretinoin leads to a significant improvement in Acanthosis nigricans symptoms. The exfoliating effect and desmosomal degradation of lactic acid are deemed crucial for mitigating excessive skin keratinization and treating Acanthosis nigricans.50
Conclusion
The modern era of chemical peels began with dermatologist Mackee G, who pioneered the use of phenol to address facial scars, achieving notable therapeutic outcomes.51 Essentially, the initial adoption of chemical peels in China drew inspiration from foreign practices and was subsequently refined and customized to align with the unique characteristics of Chinese skin. Research indicates that Chinese skin, characterized by a thinner stratum corneum, higher sweat gland density, and a weaker mechanical barrier function, tends to be more sensitive to external chemical substances.52,53 Considering these factors, the meticulous selection of clinical peeling agents becomes crucial to ensure both safety and efficacy in chemical peel procedures. Lactic acid emerges as an exemplary choice for a gentle, safe, and efficient chemical peeling agent, well-suited to the particularities of Chinese skin. Furthermore, lactic acid, as a chemical peeling agent suitable for Chinese skin, offers several advantages: ①Suitable for various skin types: Lactic acid has minimal skin irritation and side effects, making it suitable for various skin types, including dry and sensitive skin. ②Applicable to different body areas: Lactic acid exhibits good adaptability and safety, allowing it to be used for chemical peels on various body areas such as the face, neck, chest, arms, and legs, improving skin texture and overall appearance. ③Short recovery period after peeling: Lactic acid’s progressive skin improvement does not cause severe damage to the skin barrier and even promotes the recovery of the lipid barrier. As a result, the recovery period after peeling is relatively short, and patients can typically resume their normal daily skincare routine as soon as the second day following the chemical peel.
In summary, lactic acid, as a mild, safe, and effective chemical peeling agent, not only has general applicability but is also highly suitable for Chinese skin. Therefore, lactic acid peels have become an essential tool in the field of chemical peeling in China and are gradually gaining recognition from clinicians and patients alike.
Abbreviations
LA, Lactic acid; AHA, α-hydroxy acid; MCTs, monocarboxylate transporters; GPR81, G protein-coupled receptor 81; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1β; CCL, chemokine (C-C motif) ligand; PKB, Protein Kinase B; NF-κB, Nuclear Factor-Kappa B; SLS, sodium lauryl sulfate; CD, Cathepsin D-like; TRPV3, transient receptor potential vanilloid 3; IL-1α, interleukin-1α; TGF-β, transforming growth factor-β; TGase, transglutaminase; MASI, Melasma Area and Severity Index; POM, Periorbital melanosis; AN, Acanthosis nigricans.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rajanala S, Vashi NA. Cleopatra and sour milk-the ancient practice of chemical peeling. JAMA Dermatol. 2017;153(10):1006. doi:10.1001/jamadermatol.2017.3393
2. Müller J, Radej J, Horak J, et al. Lactate: the fallacy of oversimplification. Biomedicines. 2023;11(12):3192. doi:10.3390/biomedicines11123192
3. Grunebaum LD, Baumann LS. Nonprescription topical treatments for skin rejuvenation. Facial Plast Surg. 2014;30(1):3–11. doi:10.1055/s-0033-1363755
4. Dahl MV, Dahl AC. 12% lactate lotion for the treatment of xerosis. A double-blind clinical evaluation. Arch Dermatol. 1983;119(1):27–30.
5. Van Scott EJ, Yu RJ. Hyperkeratinization, corneocyte cohesion, and alpha hydroxy acids. J Am Acad Dermatol. 1984;11(5 Pt 1):867–879. doi:10.1016/s0190-9622(84)80466-1
6. Kouris A, Platsidaki E, Christodoulou C, Efstathiou V. Patients’ self-esteem before and after chemical peeling procedure. J Cosmet Laser Ther. 2018;20(4):220–222. doi:10.1080/14764172.2017.1400168
7. Khunger N, Chanana C. A perspective on what’s new in chemical peels. Cosmoderma. 2022;2:14. doi:10.25259/csdm_5_2022
8. Khunger N. Step by Step Chemical Peels.
9. Rajkumar J, Chandan N, Lio P, Shi V. The skin barrier and moisturization: function, disruption, and mechanisms of repair. Skin Pharmacol Physiol. 2023;36(4):174–185. doi:10.1159/000534136
10. Smith WP. Comparative effectiveness of α-hydroxy acids on skin properties. Int J Cosmet Sci. 1996;18(2):75–83. doi:10.1111/j.1467-2494.1996.tb00137.x
11. Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium, glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and tea-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1–241. doi:10.1177/109158189801700101
12. Hasan MZ, Kitamura M, Kawai M, et al. Transcriptional profiling of lactic acid treated reconstructed human epidermis reveals pathways underlying stinging and itch. Toxicol In Vitro. 2019;57:164–173. doi:10.1016/j.tiv.2019.03.005
13. Mizukoshi K. Effects of lactic acid on the flexibility of the stratum corneum. Skin Res Technol. 2020;26(4):599–607. doi:10.1111/srt.12841
14. Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2020;2(7):566–571. doi:10.1038/s42255-020-0243-4
15. Wang C, Chang T, Yang H, Cui M. Antibacterial mechanism of lactic acid on physiological and morphological properties of salmonella enteritidis, Escherichia coli and listeria monocytogenes. Food Control. 2015;47:231–236. doi:10.1016/j.foodcont.2014.06.034
16. Sun S, Li H, Chen J, Qian Q. Lactic acid: no longer an inert and end-product of glycolysis. Physiology. 2017;32(6):453–463. doi:10.1152/physiol.00016.2017
17. Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66(5):2001–2005. doi:10.1128/AEM.66.5.2001-2005.2000
18. Douvdevani A, Rapoport J, Konforti A, Zlotnik M, Chaimovitz C. The effect of peritoneal dialysis fluid on the release of IL-1 beta and TNF alpha by macrophages/monocytes. Perit Dial Int. 1993;13(2):112–117.
19. Dietl K, Renner K, Dettmer K, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184(3):1200–1209. doi:10.4049/jimmunol.0902584
20. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi:10.1182/blood-2006-07-035972
21. Gottfried E, Kunz-Schughart LA, Ebner S, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi:10.1182/blood-2005-05-1795
22. Nasi A, Fekete T, Krishnamurthy A, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191(6):3090–3099. doi:10.4049/jimmunol.1300772
23. Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochem Biophys Res Commun. 2015;457(3):412–418. doi:10.1016/j.bbrc.2015.01.005
24. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15(4):581–592. doi:10.1007/s10456-012-9282-0
25. Green H, Goldberg B. Collagen and cell protein synthesis by an established mammalian fibroblast line. Nature. 1964;204:347–349. doi:10.1038/204347a0
26. Hussain MZ, Ghani QP, Hunt TK. Inhibition of prolyl hydroxylase by poly(ADP-ribose) and phosphoribosyl-AMP. Possible role of ADP-ribosylation in intracellular prolyl hydroxylase regulation. J Biol Chem. 1989;264(14):7850–7855.
27. Sonveaux P, Copetti T, De Saedeleer CJ, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3):e33418. doi:10.1371/journal.pone.0033418
28. Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;7:71. doi:10.1158/0008-5472.CAN-10-2828
29. Rawlings AV, Davies A, Carlomusto M, et al. Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function. Arch Dermatol Res. 1996;288(7):383–390. doi:10.1007/BF02507107
30. Tung RC, Bergfeld WF, Vidimos AT, Remzi BK. alpha-Hydroxy acid-based cosmetic procedures. Guidelines for patient management. Am J Clin Dermatol. 2000;1(2):81–88. doi:10.2165/00128071-200001020-00002
31. Horikoshi T, Matsumoto M, Usuki A, et al. Effects of glycolic acid on desquamation‐regulating proteinases in human stratum corneum. Exp Dermatol. 2005;14(1):34–40. doi:10.1111/j.0906-6705.2005.00224.x
32. Patiño AC. A theory for the mechanism of action of the α-hydroxy acids applied to the skin. Med Hypotheses. 1999;53(5):380–382. doi:10.1054/mehy.1998.0788
33. Rawlings AV, Harding CR. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp Dermatol. 2004;12(s2):43–50. doi:10.1034/j.1600-0625.12.s2.7.x
34. Cao X, Yang F, Zheng J, Wang K. Intracellular proton-mediated activation of TRPV3 channels accounts for the exfoliation effect of α-hydroxyl acids on keratinocytes. J Biol Chem. 2012;287(31):25905. doi:10.1074/jbc.M112.364869
35. Adams MP, Mallet DG, Pettet GJ. Towards a quantitative theory of epidermal calcium profile formation in unwounded skin. PLoS One. 2015;10(1):e0116751. doi:10.1371/journal.pone.0116751
36. Okano Y, Abe Y, Masaki H, Santhanam U, Ichihashi M, Funasaka Y. Biological effects of glycolic acid on dermal matrix metabolism mediated by dermal fibroblasts and epidermal keratinocytes. Exp Dermatol. 2003;12(s2):57–63. doi:10.1034/j.1600-0625.12.s2.9.x
37. Kim SJ, Park JH, Kim DH, Won YH, Maibach HI. Increased in vivo collagen synthesis and in vitro cell proliferative effect of glycolic acid. Dermatol Surg. 1998;24(10):1054–1058. doi:10.1111/j.1524-4725.1998.tb04074.x
38. CONG l. Efficacy evaluation of 40% supramolecular lactic acid in the treatment of mild to moderate acne vulgaris. China Med Cosmetol. 2020;10(12):4.
39. Sharquie KE, Noaimi AA, Al-Janabi EA. Treatment of active acne vulgaris by chemical peeling using 88% lactic acid. Our Dermatol Online. 2014;2:1.
40. Chilicka K, Rogowska AM, Rusztowicz M, et al. The effects of green tea (Camellia sinensis), bamboo extract (Bambusa vulgaris) and lactic acid on sebum production in young women with acne vulgaris using sonophoresis treatment. Healthcare. 2022;10(4). doi:10.3390/healthcare10040684
41. Chauhan A, Singh S. Comparative analysis of efficacy of lactic acid with ferulic peel (Combination Peel) Vs ferulic peel alone as a monotherapy for photoaging. Aesthetic Plast Surg. 2021;45(1):281–288. doi:10.1007/s00266-020-02004-6
42. Prestes PS, Oliveira MM, Leonardi GR. Randomized clinical efficacy of superficial peeling with 85% lactic acid versus 70% glycolic acid. An Bras Dermatol. 2013;88(6):900–905. doi:10.1590/abd1806-4841.20131888
43. Griffin TD, Murphy GF, Sueki H, Telegan B, Scott EJV. Increased factor XIIIa transglutaminase expression indermal dendrocytes after treatment with α-hydroxy acids: potential physiologic significance. J Am Acad Dermatol. 1996;34(2 Pt 1):196–203. doi:10.1016/s0190-9622(96)80111-3
44. Hafeez A, Shaukat S, Sanai M, Ahmad TJ, Aman S. Comparison of the efficacy and safety of 40% glycolic acid & 60% lactic acid chemical peel in treatment of epidermal melasma. J Pak Assoc Dermatol. 2019;29(2):176–181.
45. Sandin J, Oliveira TGD, Curi VC, Macedo ACLD, Vasconcelos RCFD. Application of lactic acid peeling in patients with melasma: a comparative study. Surg Cosmet Dermatol. 2014;6(3):255–260.
46. Singh R, Goyal S, Ahmed QR, Gupta N, Singh S. Effect of 82% Lactic Acid in Treatment of Melasma. Int Sch Res Notices. 2014;2014:407142. doi:10.1155/2014/407142
47. Raka A, Brahmbhatt VU. Comparative study of efficacy of glycolic acid (50%) peel and lactic acid (92%) peel in the treatment of melasma. Int J Res Dermatol. 2019;5(2):370–375.
48. Ahmed G, Mishra DK. Clinical efficacy and safety of 20% glycolic acid versus 30% lactic acid peel in constitutional type of periorbital melanosis: a comparative study. Int J Res Dermatol. 2019;5(3):546–553.
49. Buxman M, Hickman J, Ragsdale W, Stretcher G, Krochmal L, Wehr RF. Therapeutic activity of lactate 12% lotion in the treatment of ichthyosis. Active versus vehicle and active versus a petrolatum cream. J Am Acad Dermatol. 1986;15(6):1253–1258. doi:10.1016/s0190-9622(86)70299-5
50. Blobstein SH. Topical therapy with tretinoin and ammonium lactate for acanthosis nigricans associated with obesity. Cutis. 2003;71(1):33–34.
51. Brody HJ, Monheit GD, Resnik SS, Alt TH. A history of chemical peeling. Dermatol Surg. 2000;26(5):405–409. doi:10.1046/j.1524-4725.2000.00505.xs
52. Lee E, Kim S, Lee J, Cho SA, Shin K. Ethnic differences in objective and subjective skin irritation response: an international study. Skin Res Technol. 2014;20(3):265–269. doi:10.1111/srt.12111
53. Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28(2):79–93. doi:10.1111/j.1467-2494.2006.00302.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
