Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Jiawei Shengjiangsan’s Effect on Renal Injury in Diabetic Nephropathy Mice is Investigated via the PI3K/Akt/NF-κB Signaling Pathway
Authors Yang C, Huang F, Fang H, Zang Y
Received 22 December 2023
Accepted for publication 21 March 2024
Published 12 April 2024 Volume 2024:17 Pages 1687—1698
DOI https://doi.org/10.2147/DMSO.S456205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Chenhua Yang,1 Fengling Huang,2 Huiqin Fang,3 Yunhua Zang1
1General Medicine, Bao’an Authentic TCM Therapy Hospital, Shenzhen, Guangdong, People’s Republic of China; 2College of Traditional Chinese Medicine, Henan University of Chinese Medicine, Zhengzhou, Henan, People’s Republic of China; 3The First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China
Correspondence: Yunhua Zang, Bao’an Authentic TCM Therapy Hospital, No. 99, Lai’an Road, Xixiang Street, Bao’an District, Shenzhen, Guangdong, 518101, People’s Republic of China, Email [email protected]
Purpose: This study aimed to investigate the intervention mechanism of Jiawei Shengjiangsan (JWSJS) on kidney injury in diabetic nephropathy mice.
Methods: Thirty 8-week-old db/db mice were randomly divided into five groups: model group, Perindopril group, and JWSJS low-, medium-, and high-dose groups (n=6 per group) based on body weight. Additionally, a blank control group was established consisting of 6 db/m mice aged 8 weeks. The blank and model groups received daily intragastric administration of 7g/kg/d pure water. The remaining groups were assigned to JWSJS low (3.5g/kg/d), medium (7g/kg/d), high (14g/kg/d) dosage groups, and perindopril positive control group (0.48mg/kg/d) for 12 weeks. Post-experiment, serum creatinine (SCr) and blood urea nitrogen (BUN) were analyzed using an automatic biochemical analyzer. Enzyme-linked immunosorbent assay (ELISA) measured 24-hour urinary albumin, neutrophil gelatinase-associated lipocalin (NGAL), TNF-α, IL-1β, VCAM-1, MCP-1, and HbA1c. Western blot assessed the protein expressions of p-PI3K, p-Akt, and p-NF-κB p65, while pathological kidney changes were observed.
Results: Compared to the blank group, the model group exhibited increased SCr, BUN, 24-hour urinary albumin, serum NGAL, TNF-α, IL-1β, VCAM-1, MCP-1, HbA1c, p-PI3K, and p-Akt, alongside increased p-NF-κB p65 expression, indicating significant kidney pathology. After treatment, the JWSJS group showed decreased SCr, BUN, 24-hour urinary microalbumin, NGAL, HbA1c, TNF-α, IL-1β, VCAM-1, MCP-1 levels, increased p-PI3K and p-Akt expression (P< 0.05), and reduced p-NF-κB p65 content (P< 0.05). Histopathological analysis revealed that JWSJS ameliorated renal tubular epithelial cell damage, glomerular capillary and basement membrane injuries, and facilitated the repair of damaged podocytes in diabetic nephropathy mice.
Conclusion: JWSJS demonstrated efficacy in reducing renal inflammation in diabetic nephropathy mice, with its mechanism likely associated with the inhibition of the PI3K/Akt/NF-κB signaling pathway.
Keywords: Jiawei Shengjiangsan, diabetic nephropathy, PI3K/Akt/NF-κB signaling pathway, db/db mice, inflammation
Introduction
Diabetic nephropathy (DN) stands out as a prominent microvascular complication of diabetes mellitus and represents the primary cause of end-stage renal disease. Recent years have witnessed a surge in the incidence of DN.1,2 This disease manifests progressively, with initial clinical stages marked by proteinuria and glomerular hyperfiltration. As the condition progresses, there is a gradual decline in the glomerular filtration rate.3 Studies have shown that diabetes-induced proteinuria primarily exhibits disruption of the fissure diaphragm’s integrity, loss of podocytes, and thickening of the glomerular basement membrane.4 Podocytes, being highly differentiated cells forming the glomerular filtration barrier alongside vascular endothelial cells and the basement membrane,5 play a crucial role. Impairment of this barrier has been linked to proteinuria, glomerular lesions, and renal function abnormalities.6,7 Due to the limited regenerative capacity of podocytes, their injury and loss contribute to the destruction of the glomerular filtration membrane, exacerbating the nephropathic process.8,9 Given the side effects associated with podocyte-protective drugs like angiotensin-converting enzyme inhibitors (ACEI) and endothelin receptor antagonists, such as hyperkalemia, exploring new and safe drugs for DN treatment is imperative.10 Therefore, naturally produced drugs like traditional Chinese medicine (TCM) have attracted significant interest in recent years. Jiawei Shengjiangsan, an empirical formula for DN treatment in TCM, has a historical track record, yet its impact on DN and the underlying mechanisms remain unclear, warranting further research.
The etiology of DN is complex and diverse, with inflammatory mechanisms being a major known pathogenic factor.11 It has been reported that the downstream signaling pathway of Phosphatidylinositol 3-kinase (PI3K) significantly influences cell growth, survival, and motility.12 The PI3K/Akt pathway activation under diabetic conditions affects renal tubular cell growth, epithelial-mesenchymal transition (EMT), and lipid metabolism.13 Hyperglycemia induces damage to glomerular and tubular cells, resulting in increased expression of inflammatory mediators that, in turn, induce renal injury through various mechanisms.14 Inflammatory mediators can contribute to extracellular matrix deposition and myofibroblast differentiation and proliferation through signaling pathways like NF-κB and JAK/STAT.15 Current evidence indicates that activation of the PI3K/Akt/NF-κB signaling pathway significantly increases downstream inflammatory cytokines like TNF-α, IL-1β, and IL-6,16 triggering renal inflammation and accelerating DN development. Therefore, intervening in the PI3K/Akt/NF-κB signaling pathway emerges as a promising strategy for DN prevention and treatment.
Jiawei Shengjiangsan (JWSJS), known for its efficacy of ascending lucidity and descending turbidity, opening sweat pores, and activating channels, has shown promise in reducing proteinuria and improving renal function.17 Additionally, Song et al demonstrated that JWSJS reduced podocyte pyroptosis and attenuated renal injury in diabetic kidney disease (DKD) rats by down-regulating the TXNIP/NLRP3 signaling pathway.18 Moreover, mounting evidence suggests the involvement of the PI3K/Akt/NF-κB signaling pathway in renal disease.19–22 This study explored the intervention mechanism of JWSJS on renal injury in diabetic nephropathy, with emphasis on the PI3K/Akt/NF-κB signaling pathway and its associated inflammatory cytokines.
Materials and Methods
Animals
Thirty SPF-grade male db/db mice and six db/m mice (8 weeks of age) were procured from Hunan Skryginda Laboratory Co., Ltd. (Changsha, China), Animal License No. SCXK (Hunan) 2019-0004. These animals were maintained in accordance with the standards for experimental animals in the barrier environment of the Laboratory Animal Center of Henan University of Traditional Chinese Medicine, with Ethical Review Approval No.: DWLL202203012, and conducted according to the guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018.
Drugs, Reagents, and Instruments
JWSJS, consisting of Jiang Huang (Curcuma longa L.), Da Huang (Rheum officinale Baill), Jiang Can (Bombyx Batryticatus), Chan Tui (Cicadae Periostracum), Huang Qi (Astragalus mongholicus Bunge), and Hong Hua (Carthamus tinctorius L.) were obtained from the First Affiliated Hospital of Henan University of Traditional Chinese Medicine (Manufacturer: Sichuan New Green Pharmaceutical Science and Technology Development Co., Ltd.). Perindopril tert-butylamine tablets (Yashida), 4mg×30s, were also obtained from the same hospital. ELISA kits for neutrophil gelatinase-associated lipocalin (NGAL), interleukin 1β (IL-1β), tumor necrosis factor-alpha (TNF-α), vascular endothelial cell adhesion molecule 1 (VCAM-1), and monocyte chemotactic protein 1 (MCP-1) were procured from Jiangsu Enzyme Immuno. Antibodies for p-PI3K, p-Akt, p-NF-κB p65, and GAPDH were sourced from Servicebio. Other equipment utilized included a fully automatic biochemical analyzer (Shenzhen Radu Life Science and Technology), a vertical electrophoresis instrument (Servicebio), a panoramic section scanner (3DHISTECH), and a transmission electron microscope (Hitachi). (see Table 1)
 |
Table 1 Composition of JWSJS |
Grouping, Modeling, and Drug Administration
It is well-established that db/db mice, characterized by spontaneous gene mutations, exhibit elevated blood glucose levels at 4–8 weeks of age, develop urinary protein at 8 weeks, and display behavioral traits such as gluttony, daily thirst, and polyuria. Consequently, they represent a robust animal model for type 2 diabetic nephropathy.23,24 The db/m mice, serving as a wild-type blank control group, provided a comparative baseline for the db/db mice model.
Grouping and administration: After one week of acclimatization, thirty db/db mice were randomly assigned to the following groups: model group, high-dose group (JWSJS-H group) (14 g/kg/d), middle-dose group (JWSJS-M group) (7 g/kg/d), low-dose group (JWSJS-L group) (3.5 g/kg/d), and perindopril positive control group (0.48 mg/kg/d). The grouping was based on the body weight stratification method, with six mice in the model group and six mice in the db/m group forming the blank control group. Both normal and model groups received an equal volume of pure water through gavage once daily for 12 weeks. The experiments spanned 12 weeks, during which urine was collected in metabolic cages at weeks 0, 4, 8, and 12 to record the 24-hour urine volume and measure 24-hour urine albumin content using ELISA.
Observational Indicators and Methods
Biochemical Indicators Assay
After serum thawing, the Myriad automatic biochemistry instrument was used to assess biochemical indexes (serum creatinine [SCr] and blood urea nitrogen [BUN]) and evaluate renal injury levels.
Enzyme-Linked Immunosorbent Assay (ELISA)
Quantification of mice urinary 24-hour urine albumin,25 serum HbA1c,26 NGAL,27 TNF-α,28 IL-1β,29 VCAM-1,30 and MCP-131 in mice was conducted using the corresponding kits, strictly adhering to the manufacturer’s instructions.
Kidney Pathology
The fixed renal tissues were embedded in paraffin blocks, sectioned on a microtome (thickness of 0.4 μm), and stained with hematoxylin and eosin (HE staining). The pathological changes in the mice kidneys were observed under an optical microscope. Electron microscope samples underwent double fixation with 2% glutaraldehyde starvation acid, followed by processing and photography under a 12,000× field of view by histopathologists. Glomerular basement membrane thickness was quantified using ImageJ software.
Western Blot
Kidney tissue was lysed using RIPA lysis buffer (containing phosphatase and protease inhibitors). The supernatant, obtained by centrifugation at 12,000 × g for 10 min at 4 °C, was subjected to Bicinchoninic Acid Assay (BCA) to determine protein concentration. Subsequently, polyacrylamide gel electrophoresis was performed, followed by membrane transfection and sealing with 5% skimmed milk. PI3K antibody, Akt antibody, NF-κB p65 antibody, p-PI3K antibody, p-Akt antibody, p-NF-κB p65 antibody, and the internal reference GAPDH were added separately and incubated overnight at 4°C. Akt antibody, p-NF-κB p65 antibody, and the internal reference GAPDH were also incubated at 4°C overnight. After TBST washing, horseradish peroxidase-labeled secondary antibody was added and incubated at room temperature for 2 hours, followed by ECL luminescence color development. The optical density of the target bands was analyzed using a gel image analyzer, and the results were expressed as p-PI3K/GAPDH, PI3K/GAPDH, p-Akt/GAPDH, Akt/GAPDH, p-NF-κB p65/GAPDH, NF-κB p65/GAPDH.
Statistical Analysis
Statistical analysis was conducted using SPSS 21.0 software, and data were presented as mean ± standard deviation (SD). Normality and variance chi-square tests were initially performed on all data. Between-group ANOVA was carried out using one-way ANOVA, and post hoc two-by-two comparisons were conducted using LSD two-by-two comparisons. Statistical significance was considered when P < 0.05.
Results
JWSJS Improves SCr and BUN in DN Mice
Compared to the normal group, the model group exhibited significantly elevated serum SCr and BUN levels in mice (P<0.05). Conversely, both the perindopril group and the JWSJS dosage group demonstrated a reduction in SCr and BUN levels compared to the model group (P<0.05). Notably, the JWSJS dosage group exhibited a significant decrease in SCr compared to the western medication group (P<0.05), with the medium-dose group showing a decrease in BUN levels (P<0.05). However, no significant difference was observed between the high and low-dose groups (P>0.05). These results demonstrate renal function injury in the model group, with JWSJS treatment showing the potential to mitigate renal injury (Figure 1 ).
JWSJS Reduced 24-Hour Urinary Protein Leakage in DN Mice
Compared to the normal group, the model group displayed a significant increase in 24-hour urine protein content in mice (P<0.05). The perindopril group and all dose groups of JWSJS exhibited a reduction in 24-hour urine protein content compared to the model group (P<0.05). Additionally, the JWSJS-M group showed a decrease in 24-hour urine protein content compared to the Western medicine group (P<0.05). However, there was no significant difference between the JWSJS-H and JWSJS-L groups (P>0.05). These findings indicate an elevation in urinary protein content in the model group, with JWSJS treatment reducing protein leakage (Figure 2 ).
JWSJS Decreased Blood Glucose and Levels of TNF-α, IL-1B, VCAM-1, MCP-1, and NGAL in DN Mice
Compared to the normal group, mice in the model group exhibited significantly elevated serum levels of TNF-α, IL-1β, VCAM-1, MCP-1, NGAL, and HbA1c (P<0.05). In contrast, the perindopril group and JWSJS dose groups demonstrated a decrease in these levels compared to the model group (P<0.05). In comparison to the Western medicine group, the medium-dose group of JWSJS exhibited a significant decrease in TNF-α, IL-1β, VCAM-1, MCP-1, NGAL, and HbA1c levels (P<0.05), with no significant difference between the high- and low-dose groups (P>0.05). These results indicate elevated blood glucose levels, renal inflammatory reactions, and renal function damage in the model group, with JWSJS treatment reducing blood glucose levels, alleviating renal inflammatory reactions, and protecting the kidneys (Figure 3 ).
JWSJS Alleviated Renal Pathological Injury in DN Mice
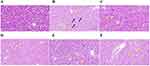
Results from HE staining revealed that mice in the blank group exhibited a uniform glomerular distribution with consistent cell numbers in both glomeruli and stroma. Tubular epithelial cells appeared rounded and plump, and there were no evident inflammatory changes or notable hyperplasia in the kidney interstitium. In the model group, renal tubular epithelial cells displayed vacuolar degeneration with tiny round vacuoles in the cytoplasm (black arrows), and glomerular capillaries showed slight bruising and dilation (yellow arrows). In comparison to the model group, the JWSJS-L and perindopril groups exhibited improved glomerular capillary stasis and dilation, with a more significant improvement observed in the JWSJS-H and JWSJS-M groups (yellow arrows) (Figure 4 ).
Electron microscopy results demonstrated that the glomerular basement membrane of mice in the blank group was intact, continuous, and uniformly thick. Podocytes were not swollen, and foot processes (FP) were well-structured and neatly arranged without noticeable fusion or widening. Conversely, the model group exhibited uneven thickness of the glomerular basement membrane, blurred local structure, swollen podocytes, shallow and dissolved intracellular stroma, proliferated and edematous endothelial cells, reduced FP abundance, localized swelling and fusion, and aggregation of red blood cells (RBC) in the vascular lumen. Treatment with JWSJS and perindopril led to varying degrees of improvement. For instance, in the JWSJS-H and JWSJS-L groups, although the thickness of the glomerular basement membrane was slightly uneven, podocyte swelling was significantly reduced, and FP arrangement remained neat with only a few instances of fusion and shortening. The JWSJS-M and perindopril groups exhibited more uniform glomerular basement membrane thickness, evenly distributed intracellular stroma in podocytes, and neatly arranged FP with uniform lengths, with only occasional fusion. The endothelial cells maintained a fair structure with homogeneous cytoplasm (Figure 5 ).
 |
Figure 5 Electron microscopic results of kidney in each group (×12000). Notes: (A) normal group; (B) model group; (C) JWSJS-H group; (D) JWSJS-M group; (E) JWSJS-L group; (F) perindopril group. |
The Effects of JWSJS on p-PI3K, p-Akt, and p-NF-κB p65 Proteins in Mice
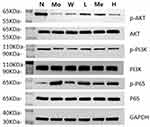
Compared to the normal group, mice in the model group displayed reduced protein expression content of p-PI3K and p-Akt, coupled with elevated protein expression content of p-NF-κB p65 (P<0.05). In contrast, the Chinese and Western medicine treatment groups exhibited increased protein expression content of p-PI3K and p-Akt, along with decreased protein expression content of p-NF-κB p65 in comparison to the model group (P<0.05). Compared to the Western medicine group, the JWSJS-L and JWSJS-M groups showed elevated p-PI3K and p-Akt protein expression content, while the low- and high-dose groups displayed decreased p-NF-κB p65 protein expression content (P<0.05). The differences between the remaining groups were not statistically significant (P>0.05) (Figures 6 and Figure 7).
Discussion
Shengjiangsan made its historical debut in the Ming Dynasty, documented in Zhang Heteng’s “The Complete Book of Summer Wounds”. Comprising Cicadae Periostracum, Curcuma longa L., Rheum officinale Baill, and Bombyx Batryticatus, this formulation holds specific therapeutic roles. Bombyx Batryticatus thermoregulatory properties and potential antidepressant effects.32 Flavonoids in Bombyx Batryticatus mainly include quercetin and kaempferol, among which kaempferol ameliorates renal injury and fibrosis by increasing the release of GLP-1 and insulin as well as inhibiting RhoA/Rho kinase in the mouse model of DN.33 Cicadae Periostracum clearing heat to release the exterior, and has anti-inflammatory, sedative analgesic antispasmodic, anti-convulsive, and anticoagulant effects.34 Cicadae Periostracum reduces proteinuria by inhibiting the overexpression of TLR4 in the renal tissues, inhibiting glomerular Mesangial cell proliferation, and attenuating mesangial matrix accumulation.35 Curcuma longa L. has the capacity to regulating qi and resolving stasis, and may possess antidepressant properties.36 Siwei Jianghuang Decoction Powder ameliorates renal injury in DN mice by regulating HIF-1α, VEGF, and TGF-β1 overexpression.37 Rheum officinale Baill constrains nature and remaining function and purging the lower and expelling stasis.38 Rheum officinale Baill can improve disorders of glucose-lipid metabolism and effectively treat DN by up-regulating the glucagon-like peptide-1 (GLP-1) receptor and decreasing the levels of TGF-β1 and α-SMA.39,40 The addition of Astragalus mongholicus Bunge and Carthamus tinctorius L. to the formula targets the disease mechanism of qi deficiency and blood stasis. Astragalus mongholicus Bunge, being the most abundant component, replenishing qi to rise yang and abating fever. Carthamus tinctorius L., with its pungent and warm nature, activates blood circulation, removes blood stasis, and clears the channels. The overall composition, predominantly featuring pungent herbs, capitalizes on their dispersing, developing, running away, and propagating nature. This imparts the formula with the efficacy of ascending clarity, descending turbidity, dredging collaterals, and opening sweat pores. Research has identified the therapeutic potential of Astragalus mongholicus Bunge components, Astragali polysaccharides,41 and Astragaloside,42,43 as well as Carthamus tinctorius L.’s key component, Hydroxysaffron yellowness A monomer (HSYA).44,45 These components can reportedly ameliorate inflammation-associated renal lesions by inhibiting inflammatory responses. Furthermore, JWSJS exhibited positive outcomes in addressing myocardial and lung injuries in septicemic rats through the down-regulation of P38-MAPK phosphorylation.46,47 However, the specific mechanism of JWSJS’s action on DN remains unexplored.
The early clinical manifestations of DN often lack specific symptoms, necessitating the use of laboratory tests for early detection of renal lesions. Among the various indicators for assessing renal injury, serum creatinine, blood urea nitrogen, and urinary microalbumin48,49 exhibit high sensitivity, while neutrophil gelatinase-associated lipocalin (NGAL) serves as an early marker for chronic renal injury.50 Additionally, elevated levels of glycated hemoglobin (HbA1c) play an indispensable role in DN diagnosis.51 Results from the current experimental study indicate that JWSJS effectively reduces serum SCr, BUN, NGAL, and HbA1c levels, diminishes 24-hour urinary protein excretion, and alleviates kidney injury in mice.
The pathogenesis of DN is intricate, with chronic inflammatory responses emerging as a major, yet unavoidable, contributing mechanism.52 The PI3K/AKT/NF-κB signaling pathway is closely intertwined with the development of inflammatory diseases.53–55 Activation of the PI3K/Akt signaling pathway in DN has been reported to initiate a cascade of immune-inflammatory responses, leading to damage and degeneration of the glomerular basement membrane.56 This process involves the mediation of swelling, fusion, and loss of podocytes, ultimately impairing glomerular filtration and causing leakage of urinary proteins.57,58 NF-κB, a crucial inflammatory transcription factor downstream of the PI3K/Akt pathway, is normally bound to its inhibitory protein IκB in the cytoplasm during the resting state, keeping the PI3K/Akt/NF-κB signaling pathway inactive. However, under the high glucose conditions of diabetes, this pathway is easily activated.59 Consequently, rapid activation of NF-κB proteins occurs, leading to the release of substantial inflammatory factors, including TNF-α, IL-1, and IL-6, initiating an inflammatory cascade known as an “inflammatory storm”.60 This storm can result in severe and rapid damage to the kidneys. The findings of our study demonstrate that JWSJS can increase the expression of p-PI3K and p-Akt proteins while downregulating the expression of p-NF-κB p65 protein. This dual action serves to inhibit the activation of the PI3K/Akt/NF-κB signaling pathway. Consequently, JWSJS effectively reduces the expression of inflammatory factors such as TNF-α, IL-1β, VCAM-1, and MCP-1. This reduction in inflammatory response contributes to the attenuation of renal injury, protection of the kidney, improvement in renal tubular epithelial cell vacuolar degeneration, amelioration of glomerular capillary stasis and dilatation, repair of glomerular vascular endothelial cells, mitigation of glomerular basement membrane damage, repair of podocyte injuries, and enhancement of the fusion and degeneration of foot processes.
In summary, JWSJS emerges as a promising therapeutic agent capable of mitigating renal injury, reducing renal inflammatory responses, and ameliorating pathological injuries in mice with diabetic nephropathy. This therapeutic effect is likely achieved through the inhibition of the PI3K/Akt/NF-κB signaling pathway. Nonetheless, further research is imperative to unveil the comprehensive molecular mechanisms underlying JWSJS’s efficacy in diabetic nephropathy.
Conclusion
JWSJS demonstrated efficacy in reducing renal inflammation in diabetic nephropathy mice, with its mechanism likely associated with the inhibition of the PI3K/Akt/NF-κB signaling pathway.
Abbreviations
DN, Diabetic nephropathy; ACEI, Angiotensin-converting enzyme inhibitors; TCM, Traditional Chinese medicine; P13K, Phosphatidylinositol 3-kinase; TNF-α, Tumor necrosis factor-alpha; IL-1β, Interleukin-1β; IL-6, Interleukin-6; NGAL, neutrophil gelatinase-associated lipocalin; VCAM-1, vascular endothelial cell adhesion molecule-1; MCP-1, monocyte chemotactic protein 1; SCr, serum creatinine; BUN, blood urea nitrogen; ELISA, Enzyme-Linked Immunosorbent Assay; BCA, Bicinchoninic Acid Assay; FP, foot processes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Special Foundation for Scientific Research on Traditional Chinese Medicine in Henan Province (No.20-21ZY1036).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society, et al. National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61(1):12–50. Chinese. doi:10.3760/cma.j.cn112138-20211027-00751
2. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15. doi:10.1111/dom.14007
3. Mauer SM, Steffes MW, Ellis EN, et al. Structural-functional relationships in diabetic nephropathy. J Clin Investig. 1984;74(4):1143–1155. doi:10.1172/JCI111523
4. Li JJ, Kwak SJ, Jung DS, et al. Podocyte biology in diabetic nephropathy. Kidney Int. 2007;106:S36–S42. doi:10.1038/sj.ki.5002384
5. Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77(7):571–580. doi:10.1038/ki.2009.424
6. Liu M, Liang K, Zhen J, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8(1):413. doi:10.1038/s41467-017-00498-4
7. Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi:10.1172/JCI119163
8. Dai H, Liu Q, Liu B. Research Progress on Mechanism of Podocyte Depletion in Diabetic Nephropathy. J Diabetes Res. 2017;2017:2615286. doi:10.1155/2017/2615286
9. Nishad R, Mukhi D, Tahaseen SV, et al. Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. J Biol Chem. 2019;294(44):16109–16122. doi:10.1074/jbc.RA119.008966
10. AKh L. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–381. doi:10.2147/IJNRD.S40172
11. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–152. doi:10.1042/CS20120198
12. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi:10.1126/science.296.5573.1655
13. Xue M, Cheng Y, Han F, et al. Triptolide attenuates renal tubular epithelial-mesenchymal transition Via the MiR-188-5p-mediated PI3K/AKT Pathway in Diabetic Kidney Disease. Int J Biol Sci. 2018;14(11):1545–1557. doi:10.7150/ijbs.24032
14. Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017;312(4):F716–F731. doi:10.1152/ajprenal.00314.2016
15. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi:10.1038/nrdp.2015.18
16. Brosius FC, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2503–2512. doi:10.1681/ASN.2009070721
17. Zhang S, Yang C, Wang P. Modified Shengjiangsan Powder Has Effect on Protein Expression of Nephrin and Podocin in Podocytes of Diabetic Rats. New J Trad Chin Med. 2019;51:71–74 doi:10.13457/j.cnki.jncm.2019.03.022
18. Song R, Zhang X, Gao F, et al. Effects of Jiawei Shengjiangsan on TXNIP/NLRP3 pathway and podocyte pyroptosis in rats with diabetic kidney disease. Pharmacol Clin Chin Mater Med. 2022;38:2–9 doi:10.13412/j.cnki.zyyl.20220628.001
19. Chen F, Sun Z, Zhu X, Ma Y. Astilbin inhibits high glucose-induced autophagy and apoptosis through the PI3K/Akt pathway in human proximal tubular epithelial cells. Biomed Pharmacother. 2018;106:1175–1181. doi:10.1016/j.biopha.2018.07.072
20. Zhang Y, Wang B, Guo F, et al. Involvement of the TGFβ1-ILK-Akt signaling pathway in the effects of hesperidin in type 2 diabetic nephropathy. Biomed Pharmacother. 2018;105:766–772. doi:10.1016/j.biopha.2018.06.036
21. Hong J, Wang X, Zhang N, Fu H, Li W. D-ribose induces nephropathy through RAGE-dependent NF-κB inflammation. Arch Pharm Res. 2018;41(8):838–847. doi:10.1007/s12272-018-1061-z
22. Li X, Wang M, Hong H, et al. Sophocarpine attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Immunol Res. 2018;66(4):521–527. doi:10.1007/s12026-018-9012-9
23. Breyer MD, Böttinger E, Brosius FC, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi:10.1681/ASN.2004080648
24. Ziyadeh FN, Hoffman BB, Han DC, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000;97(14):8015–8020. doi:10.1073/pnas.120055097
25. Liu R, Zhu H, Yang JH, et al. [Can urine albumin/creatinine ratio replace 24 hours urinary albumin?]. Zhonghua Nei Ke Za Zhi. 2019;58(5):377–381. Chinese. doi:10.3760/cma.j.issn.0578-1426.2019.05.009
26. Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33(6):393–400. doi:10.3343/alm.2013.33.6.393
27. Gharishvandi F, Kazerouni F, Ghanei E, et al. Comparative assessment of neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C as early biomarkers for early detection of renal failure in patients with hypertension. Iran Biomed J. 2015;19(2):76–81. doi:10.6091/ibj.1380.2015
28. Martínez-Aguilar VM, Carrillo-ávila BA, Sauri-Esquivel EA, et al. Quantification of TNF-α in patients with periodontitis and type 2 diabetes. Biomed Res Int. 2019;2019:7984891. doi:10.1155/2019/7984891
29. Chang PY, Wu TL, Tsao KC, et al. Microplate ELISAs for soluble VCAM-1 and ICAM-1. Ann Clin Lab Sci. 2005;35(3):312–317.
30. Kostopoulou E, Kalavrizioti D, Davoulou P, et al. Monocyte Chemoattractant Protein-1 (MCP-1), activin-a and clusterin in children and adolescents with obesity or type-1 diabetes mellitus. Diagnostics. 2024;14(4):450. doi:10.3390/diagnostics14040450
31. Sun J, Wei S, Zhang Y, Li J, Andreucci E. Protective effects of astragalus polysaccharide on sepsis-induced acute kidney injury. Anal Cell Pathol. 2021;2021:7178253. doi:10.1155/2021/7178253
32. Jiang Q, Wang L-N, Liu Y, et al. Research progress on processing historical evolution, chemical constituents, and pharmacological action of Bombyx Batryticatus. China J Chin Mater Med. 2023;48(12):3269–3280 doi:10.19540/j.cnki.cjcmm.20230113.201
33. Sharma D, Kumar Tekade R, Kalia K. Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: an in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine. 2020;76:153235. doi:10.1016/j.phymed.2020.153235
34. Zhao Z, Zhou G, Wang Y, et al. Study on chemical constituents and pharmacological effects of periostracum cicada. Jilin J Chin Med. 2017;37(05):491–493 doi:10.13463/j.cnki.jlzyy.2017.05.018
35. Yu J, Du Y, Wang H. Effect of periostracum cicadae and bombyx batryticatus on toll - like receptor 4 expression in renal tissue of mesangial proliferative glomerulonephritis rats. Chin Arch Trad Chin Med. 2015;33:7–9+1 doi:10.13193/j.issn.1673-7717.2015.01.001.
36. Meini C, Wei G, Qin H et al Progress in the pharmacology, clinical application and mechanism of curcumin., et al. J Yan’an Univ. 2021;19(03):96–99. doi:10.19893/j.cnki.ydyxb.2020-0166
37. Lai X, Tong D, Ai X, et al. Amelioration of diabetic nephropathy in db/db mice treated with tibetan medicine formula Siwei Jianghuang Decoction Powder extract. Sci Rep. 2018;8(1):16707. doi:10.1038/s41598-018-35148-2
38. Wu C, Zhou Y, Shangguan L, et al. Progress of research on the pharmacological effects and mechanisms of Emodin. J China Pharm Univ. 2023;54(05):634–643.
39. Zeng J-Y, Wang Y, Miao M, et al. The effects of rhubarb for the treatment of diabetic nephropathy in animals: a systematic review and meta-analysis. Front Pharmacol. 2021;12:602816. doi:10.3389/fphar.2021.602816
40. Liu J, Sun Y, Zheng H, et al. Emodin attenuated the kidney damage of high-fat-diet mice via the upregulation of glucagon-like peptide-1 receptor. Biomed Res Int. 2021;2021:6662704. doi:10.1155/2021/6662704
41. Zhang WJ, Frei B. Astragaloside IV inhibits NF- κ B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314. doi:10.1155/2015/274314
42. Zhao P, Wang Y, Zeng S, et al. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol Immunotoxicol. 2015;37(5):428–433. doi:10.3109/08923973.2015.1080266
43. Lee M, Zhao H, Liu X, et al. Protective effect of hydroxysafflor yellow a on nephropathy by attenuating oxidative stress and inhibiting apoptosis in induced type 2 diabetes in rat. Oxid Med Cell Longev. 2020;2020:7805393. doi:10.1155/2020/7805393
44. Li Y, Zheng D, Shen D, et al. Protective effects of two safflower derived compounds, kaempferol and hydroxysafflor Yellow A, on hyperglycaemic stress-induced podocyte apoptosis via modulating of macrophage M1/M2 polarization. J Immunol Res. 2020;2020:2462039. doi:10.1155/2020/2462039
45. Ibrahim M, Parveen B, Zahiruddin S, et al. Analysis of polyphenols in Aegle marmelos leaf and ameliorative efficacy against diabetic mice through restoration of antioxidant and anti-inflammatory status. J Food Biochem. 2022;46(4):e13852. doi:10.1111/jfbc.13852
46. Yan S, Jiang Y, Yu T, et al. Shengjiang San alleviated sepsis-induced lung injury through its bidirectional regulatory effect. Chin Med. 2023;18(1):39. doi:10.1186/s13020-023-00744-6
47. Qian Y, Qian F, Zhang W, et al. Shengjiang Powder ameliorates myocardial injury in septic rats by downregulating the phosphorylation of P38-MAPK. J Biosci. 2019;44(2):40. doi:10.1007/s12038-019-9857-7
48. Khan NU, Lin J, Liu X, et al. Insights into predicting diabetic nephropathy using urinary biomarkers. Biochim Biophys Acta Proteins Proteom. 2020;1868(10):140475. doi:10.1016/j.bbapap.2020.140475
49. Kishore L, Kaur N, Singh R. Distinct biomarkers for early diagnosis of diabetic nephropathy. Curr Diabetes Rev. 2017;13(6):598–605. doi:10.2174/1573399812666161207123007
50. Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res. 2018;2018:2852398. doi:10.1155/2018/2852398
51. Liang S, Li Q, Zhu HY, et al. Clinical factors associated with the diagnosis and progression of diabetic nephropathy. Cell Biochem Biophys. 2014;70(1):9–15. doi:10.1007/s12013-014-9892-9
52. Matoba K, Takeda Y, Nagai Y, et al. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int J Mol Sci. 2019;20(14):3393. doi:10.3390/ijms20143393
53. Lv S, Wang W, Wang H, et al. PPARγ activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer. 2019;19(1):204. doi:10.1186/s12885-019-5426-6
54. Fu Q, Huang Y, Ge C, et al. SHIP1 inhibits cell growth, migration, and invasion in non-small cell lung cancer through the PI3K/AKT pathway. Oncol Rep. 2019;41(4):2337–2350. doi:10.3892/or.2019.6990
55. Zhang Z, Yao L, Yang J, et al. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol Med Rep. 2018;18(4):3547–3554. doi:10.3892/mmr.2018.9375
56. Huang G, Lv J, Li T, et al. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38(4):1179–1189. doi:10.3892/ijmm.2016.2713
57. Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi:10.1038/nrneph.2011.51
58. Sucosky P, Balachandran K, Elhammali A, et al. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-β1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29(2):254–260. doi:10.1161/ATVBAHA.108.176347
59. Zhang Z, Li K. Curcumin attenuates high glucose-induced inflammatory injury through the reactive oxygen species-phosphoinositide 3-kinase/protein kinase B-nuclear factor-κB signaling pathway in rat thoracic aorta endothelial cells. J Diabetes Investig. 2018;9(4):731–740. doi:10.1111/jdi.12767
60. O’Sullivan AW, Wang JH, Redmond HP. NF-kappaB and p38 MAPK inhibition improve survival in endotoxin shock and in a cecal ligation and puncture model of sepsis in combination with antibiotic therapy. J Surg Res. 2009;152(1):46–53. doi:10.1016/j.jss.2008.04.030
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.