Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Is Serum Uric Acid Level Associated with Disease Activity in Rheumatoid Arthritis Patients
Authors Alkhudir D, Al-Herz A , Saleh K, Alawadhi A , Al-Kandari W , Hasan E, Mokaddem K, Ghanem A, Bartella YA, Hussain M, AlHadhood N, Ali Y , Nahar E, Alenizi A, Aldei A , Abutiban F, Hayat S, Behbehani H, Baron F, Alhajeri H, Alkadi A , Alsaber A
Received 18 May 2023
Accepted for publication 1 November 2023
Published 14 November 2023 Volume 2023:15 Pages 223—230
DOI https://doi.org/10.2147/OARRR.S418814
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Dalal Alkhudir,1 Adeeba Al-Herz,1 Khulood Saleh,2 Adel Alawadhi,1,3 Waleed Al-Kandari,2 Eman Hasan,1 Khaled Mokaddem,1 Aqeel Ghanem,4 Youssef Attia Bartella,1 Mohammed Hussain,1 Naser AlHadhood,2 Yaser Ali,4 Ebrahim Nahar,4 Ahmad Alenizi,5 Ali Aldei,1 Fatemah Abutiban,5 Sawsan Hayat,4 Hussain Behbehani,2 Fatemah Baron,5 Hebah Alhajeri,4 Amjad Alkadi,6 Ahmed Alsaber7
1Department of Rheumatology, Amiri Hospital, Kuwait city, Kuwait; 2Department of Rheumatology, Farwania Hospital, Farwaniya, Kuwait; 3Department of Rheumatology, Faculty of Medicine, Kuwait University, Jabriya, Kuwait; 4Department of Rheumatology, Mubarak Al-Kabeer Hospital, Jabriya, Kuwait; 5Department of Rheumatology, Jahra Hospital, Jahra, Kuwait; 6Department of Rheumatology, Sabah Hospital, Sabah, Kuwait; 7College of Business and Economics, The American University of Kuwait, Salmiya, Kuwait
Correspondence: Dalal Alkhudir, Amiri Hospital, Ministry of Health, Kuwait, Email [email protected]
Background: An association between serum uric acid (UA) and disease activity in rheumatoid arthritis (RA) patients has not been well studied. We describe RA patients with high and normal UA and study its association with RA activity.
Methods: Adult RA patients from the Kuwait Registry for Rheumatic Diseases (KRRD) were studied from February 2012 through March 2022. Patients with documented UA levels were included. UA of > 357 μmol/L (6mg/dL) was considered high. Statistical comparison and correlation were made using multivariate logistic regression.
Results: Overall, 1054 patients with documented UA. A total of 158 patients (15%) had high UA level with a mean of 409± 44.4μmol/L. The mean age for the high UA group and low UA group were 59.3 ± 10.7 years and 54.5 ± 12.4 years, respectively (p< 0.001). 49.4% were female in high UA group, and 62.2% were female in low UA group, respectively (p< 0.05). Logistic analysis showed an inverse relation between DAS28 and UA, as lower DAS28 score was associated with higher UA level (p=0.032) OR 1.39. There was a direct relation with HAQ, creatinine and UA. A higher HAQ is associated with a higher UA level (p=0.019) OR 0.78. High creatinine level is also associated with high UA level (p< 0.001) OR 0.24. The use of antirheumatic drugs was similar among patients with high and normal UA.
Conclusion: RA patients with a higher UA had a lower disease activity despite using similar antirheumatic drugs. The reasons behind this association need to be further studied.
Keywords: rheumatoid arthritis, uric acid, disease activity, DAS28, registry, KRRD, Kuwait, Middle East
Introduction
Rheumatoid arthritis (RA) is a chronic and destructive disease of the joints. Disease activity in patients with RA represents the ultimate measure that guides various therapeutic interventions, other goals including quality of life represented by HAQ and radiographic erosions. Many studies have investigated the role of other factors in disease activity. Long-standing RA and comorbidities were associated with higher disease activity.1 In another study, smoking was found to be associated with worse disease activity.2 Disease activity was found to be worse in females compared to males.3 Reduction of Rheumatoid Factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA) were closely linked to a reduction of disease activity.4
Uric acid is an insoluble compound that is a product of nitrogen product metabolism, which is usually excreted in the urine. High levels of uric acid in the blood can cause uric acid crystals to form and precipitate in the joints and can lead to gouty arthritis. In 1983, Turner et al have reported that high uric acid may modulate RA autoimmunity in an animal model.5 The association between serum uric acid (UA) and disease activity in rheumatoid arthritis (RA) patients has not been well studied, despite many attempts to explain the association in the literature. We describe RA patients with high and normal UA and study its association with RA activity.
Method
Subjects
Subjects were recruited from Kuwait Registry for Rheumatic Diseases (KRRD). This is a national registry for adult patients with RA in Kuwait, who satisfied the American College of Rheumatology (ACR) criteria for RA.1 Departments of Rheumatology in five major government hospitals in Kuwait (Amiri, Farwania, Mubarak Al-Kabeer, Jahra, and Sabah Hospitals) are participating in this registry. We have enrolled all patients from the beginning of the registry in February 2013 through April 2022 who had uric acid (UA) documented.
Data and Method
In this registry, the data collected included patient’s demographic data, laboratory data including level of uric acid, ESR, ANA, ACPA and RF. To assess RA disease activity, we have used the following measures: 1) patient reporting of morning stiffness, 2) examination of the joints for swelling and tenderness, 3) blood markers of inflammation including ESR & CRP, 4) Health Assessment Questionnaire (HAQ) and 5) visual analogue scale for pain (VAS) and Disease Activity Score (DAS-28) (Table 1).
 |
Table 1 Comparison of Disease Activity of the Two Groups |
We used hospital visits where uric acid was documented. Patients were divided into two groups, high uric acid group (UA ≥ 357 µmol/L, 6mg/dL), and low uric acid group (UA < 357 µmol/L, 6mg/dL).
The KRRD, from which this study originated, was approved by the Ethics Committees of the Faculty of Medicine, Kuwait University, and the Ministry of Health, Kuwait. Written informed consent was obtained from patients enrolled in the registry.
Statistical Analysis
The relevant data were extracted, and statistical analyses were done using the Statistical Package for the Social Sciences (SPSS), version 28.0. Descriptive data analysis was used to evaluate means, SD, percentages and frequencies of distributions for the following variables: gender, age, disease duration, ESR, and serological makers including RF, ANA, and ACPA. An independent two-sample t test was used to assess the significance between means. A χ2 test was used to assess the statistical significance of associations between categorical variables. Multivariate logistic regression was used to identify factors affecting DAS 28 with calculation of the odds ratio (OR).
Results
In our KRRD registry, there were 1860 patients from February 2013 to April 2022. These patients had a total of 12,548 visits. There were 1054 (57%) who had had UA documented and these patients had 6320 visits. A total of 158 (15%) had high UA level. The mean uric acid level for the high group UA was 409± 44.4 µmol/L compared to the mean of 247.9± 57.4 µmol/L for the low uric acid group (p<0.001). The two groups were compared using different variables including gender, age, smoking, duration of RA, serology and treatment (Table 2). Age was similar in both groups. The majority of females had lower UA level by 62.2%, as compared to high UA group who had 49.4% females, respectively (p= 0.002). Mean age was similar in both UA groups (p<0.001). There was a significant difference noted between anti-CCP serology and UA levels, CCP positivity was associated with lower UA level and vice versa (p= 0.036). In terms of comorbidities, we looked through diabetes mellitus (DM), hypertension and coronary artery disease (CAD). Hypertension was noticed to be more in patients with low UA level compared to patients with higher UA level (p<0.001). No significant difference is noted in DM and CAD in terms of UA level. Duration of RA was similar in both groups.
 |
Table 2 Demographic and Serological Characteristics of the Two Groups |
As noticed in the logistic regression (Table 3) with all the variables combined, higher DAS score was associated with lower UA level (p= 0.032) OR 1.39, 95% CI. The same thing was noticed with steroids treatment, as patients who are on steroid treatment are associated with lower UA level (p= 0.048) OR 1.95.
 |
Table 3 Logistic Regression |
On the other hand, there was a direct relation with HAQ, creatinine and UA. A higher HAQ is associated with a higher UA level (p=0.019) OR 0.78, 95% CI. High creatinine level is also associated with high UA level (p<0.001) OR 0.24.
We compared creatinine level in both genders with UA level. UA level has a direct proportion with creatinine level in subgroups such as male and female (p<0.001) (Figure 1). Similarly, creatinine was proportional to UA regardless of DAS28 score (DAS28<3.2 p<0.001, DAS28≥3.2 p<0.002) (Figure 2).
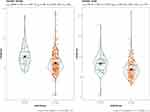 |
Figure 1 Plot chart correlation between UA and creatinine levels in both genders. |
 |
Figure 2 Plot chart correlation between UA and creatinine levels in high and low DAS28 scores. |
However, after adjusting all the variables in multivariable logistic regression (Table 3 and Figure 3), it was found that a low UA level was associated with a high DAS28 score (p=0.032) CI 95%.
 |
Figure 3 Forest plot of multivariable logistic regression analysis. |
Discussion
In this study, we tried to find out the correlation between serum UA level and RA disease activity. Since the DAS28 score is the most commonly used parameter to monitor disease activity in our practice, we compared it to uric acid level in our patient’s database. As noted with the results, there is an inverse relation between uric acid level and DAS28 score, after adjusting for variables like age, gender, duration of RA, creatinine level and current treatment.
These findings can be attributed to the fact that patients in remission are on DMARDs and with no or minimal dose of steroids (prednisolone), in contrast to patients with active disease (high DMARDs) who would have been on higher doses of steroids. Prednisolone can have an effect on serum uric acid by increasing uric acid clearance.6 Another explanation is that patients with active disease (high DAS28) would have an active inflammatory cascade, including cytokines that result in the activation of macrophages, neutrophils, and dendritic cells. Neutrophils subsequently release numerous proteases that can adversely affect the lubricating properties of synovial fluid and the integrity of the cartilage by digesting native collagen in cartilage resulting in significant cartilage damage.
Uric acid is the end product of purine metabolism. However, 90% of the resulting nucleotide metabolites adenine, guanine, and hypoxanthine are reused in that they are reformed to AMP, IMP, and GMP by adenine phosphoribosyl transferase (APRT) and hypoxanthine-guanine phosphoribosyl transferase (HGPRT), respectively. Only the reminder is converted to xanthine and further to uric acid by xanthine oxidase. The physiological properties or uric acid molecules are that they have low solubility in synovial fluid, and this solubility can be reduced with cold temperature. With active RA, and with all the inflammatory changes in the synovium, the properties of the synovial fluid changes and well the temperature might explain more solubility of uric acid molecules.
A study by Garg et al showed no significant difference in uric acid level in RA patients compared to control.7 They attributed the findings to the increased utilization of uric acid in trapping the free radicals produced in rheumatoid arthritis. Another study by Lee et al compared UA in patients on methotrexate to nonuser patients and did find that serum UA decreases with methotrexate therapy,8 but the exact mechanism behind these findings was not fully understood.
In contrast, a study from Egypt by Nada et al concluded that higher UA levels in active RA patients, and might have been an inflammatory marker for the severity of joint inflammation. At the same time, they have also suggested that higher doses of steroids could be considered a cause of hyperuricemia.9
Further studies are needed to evaluate the correlation between UA level and disease activity in RA patients.
The limitations of our study are that the results showed association not causation, and the data were extracted from a retrospective database.
Ethical Considerations
This study complies with the Declaration of Helsinki.
Disclosure
This is an investigator study from Kuwait Registry for Rheumatic Diseases (KRRD). The KRRD registry is supported by an unrestricted grant from Pfizer. The authors report no other conflicts of interest in this work.
References
1. Jung MS, Kwok KS, Ju JH, Lee SW, Song JJ, Yoon CH. Risk factors associated with inadequate control of disease activity in elderly patients with rheumatoid arthritis: results from a nationwide Korean College of Rheumatology BIOlogics (KOBIO) registry. PLoS One. 2018;13(10):e0205651.
2. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology. 2016;55(11):1969–1977. doi:10.1093/rheumatology/kew285
3. Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11:1–2.
4. Böhler C, Radner H, Smolen JS, Aletaha D. Serological changes in the course of traditional and biological disease modifying therapy of rheumatoid arthritis. Ann Rheum Dis. 2013;72(2):241–244. doi:10.1136/annrheumdis-2012-202297
5. Turner RA, Pisko EJ, Agudelo CA, Counts GB, Foster SL, Treadway WJ. Uric acid effects on in vitro models of rheumatoid inflammatory and autoimmune processes. Ann Rheum Dis. 1983;42(3):338–342. doi:10.1136/ard.42.3.338
6. Liu C, Zhen Y, Zhao Q, Zhai JL, Liu K, Zhang JX. Prednisone lowers serum uric acid levels in patients with decompensated heart failure by increasing renal uric acid clearance. Can J Physiol Pharmacol. 2016;94(7):797–800. doi:10.1139/cjpp-2015-0490
7. Garg C, Mishra MK, More NV A study for the level of adenosine Deaminase Crp and uric acid in rheumatoid arthritis patients. 2019; 16–19.
8. Lee JJ, Bykerk VP, Dresser GK, et al. Reduction in serum uric acid may be related to methotrexate efficacy in early rheumatoid arthritis: data from the Canadian Early Arthritis Cohort (CATCH). Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:S38092. doi:10.4137/CMAMD.S38092
9. Nada D, Gaber R, Mahmoud AS, Elkhouly R, Alashkar D. Hyperuricemia among Egyptian rheumatoid arthritis patients. Is it an association or an inflammatory marker? A cross-sectional observational study. Open Access Rheumatol. 2021;5:305–314. doi:10.2147/OARRR.S331488
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
