Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Introduction of Unit-Dose Care in the 1,125 Bed Teaching Hospital: Practical Experience and Time Saving on Wards
Authors Herrmann S , Giesel-Gerstmeier J, Steiner T, Lendholt F, Fenske D
Received 29 November 2023
Accepted for publication 28 February 2024
Published 14 March 2024 Volume 2024:17 Pages 1137—1145
DOI https://doi.org/10.2147/JMDH.S450203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Saskia Herrmann,1,2 Jana Giesel-Gerstmeier,1 Thomas Steiner,3,4 Florian Lendholt,5 Dominic Fenske1
1Hospital Pharmacy, Helios Kliniken GmbH, Berlin, Berlin, Germany; 2Department of Pharmaceutical/Medicinal Chemistry, Institute of Pharmacy, Friedrich Schiller University Jena, Jena, Thuringia, Germany; 3Department of Urology, Helios Klinikum Erfurt, Erfurt, Thuringia, Germany; 4Medicine, HMU Health and Medical University Erfurt, Erfurt, Thuringia, Germany; 5Management, Helios Klinikum Erfurt, Erfurt, Thuringia, Germany
Correspondence: Dominic Fenske, Hospital Pharmacy, Helios Kliniken GmbH, Friedrichstraße 136, Berlin, Berlin, 10117, Germany, Tel +49 361 781 71100, Fax +49 361 781 71105, Email [email protected]
Purpose: The shortage of nursing staff as well as the slow progress in the German health care system’s digitalisation has gained much attention due to COVID-19. Patient-specific medication management using the unit-dose dispensing system (UDDS) has the potential for a lasting and positive influence on both digitalisation and the relief of nursing staff.
Methods: Nursing staff UDDS-acceptance was determined via a validated online survey. For the evaluation of stock keeping on the wards, the delivery quantities were determined for a comparative period before and after the introduction of the UDDS. The time required for on-ward medication-related processes on ward before and after the introduction of UDDS was recorded based on a survey form and the nursing relief in full-time equivalent (FTE) was calculated using the data obtained.
Results: We show that nurses appreciate the UDDS and confirm a significant reduction in drug stocks on the wards. The UDDS reduces the time needed to dispense medications from 4.52 ± 0.35 min to 1.67 ± 0.15 min/day/patient. In relation to the entire medication process, this corresponds to a reduction of 50% per day and per patient. Based on 40,000 patients/year and a supply of 1,125 beds with unit-dose blisters, 7.36 FTE nursing staff can be relieved per year. In contrast, 6.5 FTE in the hospital pharmacy are required for supplying the hospitals.
Conclusion: UDDS is well accepted by nurses, reduces stock levels on ward, and fulfils criteria as a nursing-relief measure.
Keywords: unit dose drug distribution systems, medication therapy management, nursing staff
Introduction
The patient-specific unit-dose dispensing system (UDDS) offers the possibility to dispense solid-oral-drugs such as capsules and tablets in hospital pharmacies. This system leads to fewer medication errors when dispensing oral drugs and improves patients’ safety.1–4 As part of the closed-loop medication management (CLMM), the UDDS has been receiving increased attention. Such a CLMM is characterised by computerised physician order entry (CPOE), prescription validation by clinical pharmacists (CP), as well as patient-orientated logistics, and electronic documentation of administration.5
Based on the CPOE, patient-orientated logistics can be processed by the UDDS in a hospital pharmacy, which also provides the basis for medication reconciliation by the CPs. For medication reconciliation, duplicate prescriptions, over/under dosing, as well as interactions and contraindications must be taken into account.6 The implemented clinical decision support system (CDSS) serves as a support for the CP. The interface between the electronic patient record and the CPOE makes it possible to link up not only allergies but also current and previous diagnoses as well as blood parameters. It is already known that patients with renal insufficiency have a high risk of experiencing adverse drug reactions.7 Patient transfers from outpatient to inpatient, and vice versa, especially harbours risks for drug-related problems.8,9 For improved patient safety, it is therefore essential to significantly reduce drug-related problems and prescription errors. Such errors may lead to longer hospital stays and readmissions, especially for multi-morbid patients.10 This results in additional costs for health care systems.11–13 Previous studies have already highlighted the importance of the role of a CP for this purpose.7,14–16 Including a CP in evaluating prescriptions significantly reduces prescription errors and drug-related problems, thus increasing patient safety.16
As a quality-assured centralised instrument, the UDDS relieves the nursing staff and reduces the stock on the wards.17,18 The administration and electronic documentation of drugs by the nursing staff on the ward completes the newly established medication cycle.19 By implementing the CLMM, errors in dispensing and administration of drugs are greatly decreased by scanning the UDDS blisters directly at the patient’s bedside.1 This concrete example of use shows that continuously digitalised and then automated processes can enhance safety and efficiency.
Although the first UDDS was established in Germany in 1993, 30 years on, less than 10% of all hospital pharmacies use the system of patient-specific blistering routinely for their inpatients.20 In other words, only 6% of inpatient beds are currently supplied by UDDS.20 After consultation with the company Baxter B.V., the market leader in this sector in Europe (according to their own statement), there is a hesitancy not only in Germany but also on a European level. Despite the frequently proven increase in quality through improvement of drug safety, and the economic potential of a reduction in stock kept on the wards and the relief of nursing staff through an automated setting process, the question of full economic efficiency is not finally answered.1,18 The above-mentioned positive effects face high investment costs, reconstruction measures, the adaptation of new processes into daily structures of physicians, nurses, and CP, not to mention the increased need for pharmaceutical staff.1,21
In order to minimise the economic burden and counteract the lack of skilled nursing staff, it is possible in Germany to refinance nursing relief measures at 4%, according to § 5 of the Pflegebudgetverhandlung (care budget negotiation). Initial pilot projects show that UDDS can relieve the burden on nurses, which means that legislative refinancing is possible.17,18,22–24 Among other things, this is so far only an evaluation and subjective survey of a pilot ward, as well as preliminary results of a data collection.23,24 The results are therefore not reliable. There is a corresponding lack of representative economic studies not only for the purpose of refinancing UDDS, but also for showing the correlation between full-time equivalent (FTE) nurses and FTE pharmaceutical staff in international use.
In 2020, the CP started to roll out the CPOE with an integrated CDSS in the Helios Klinikum Erfurt (HK-EF) teaching hospital. The UDDS was introduced concurrently. This process took eleven months for 43 wards. HK-EF is part of a hospital trust and supplies three other hospitals. CPOE/CDSS and UDDS were also introduced in these hospitals in 2022 (Supplementary Figure 1). As a result, the hospital network has been completely rearranged to accommodate the new form of care. By supplying about 1,700 beds with UDDS, the pharmacy of HK-EF is one of the five largest UDSS pharmacies in Germany.20 The following data were collected in the setting of the teaching hospital, HK-EF.
For the first time, the study conditions allowed data to be collected in a single hospital setting. This included nurses’ opinions towards the introduction of UDDS and its impact on their daily nursing practice, as well as time spent on medication-related processes. The purpose of our data is to demonstrate how the UDDS affects the time allocation of nursing staff in medication-related processes. This should lead to an economic analysis, which will consider the impact on the refinancing of such a project at the national level, as well as the correlation between nurses on the ward and newly employed pharmaceutical staff worldwide.
Materials and Methods
Acceptance and Satisfaction of Nurses with UDDS
Four weeks after the introduction of UDDS on the wards at HK-EF, the acceptance and satisfaction of the nurses were determined anonymously by questionnaire via an online survey (LimeSurvey®, Supplementary Table 1). The evaluation is represented as relative frequency [%] using a bipolar Likert scale. The data collection took place between July and October 2021. The results were analysed in Microsoft Office Excel® 2016.
Evaluation of Supply Volume on Ward
For the evaluation of the supply volume on ward, reports from the CGM AMOR A3 inventory management programme by AESCUDATA were analysed for HK-EF. For this purpose, the delivered quantity of three drugs was compared before UDDS (second quarter 2019) and after UDDS (second quarter 2022) implementation. So-called “fast movers” were selected for this purpose: metamizole 500 mg, metformin 1,000 mg, and metoprolol 47.5 mg.25 These drugs were available at all times of the evaluation in classic blister packs and in bulk for the UDDS. The data were tested for normal distribution using the Kolmogorov–Smirnov test. If a normal distribution was present (metformin, metoprolol), statistical analysis was performed using a two-tailed t-test. For non-normally distributed data (metamizole), the Wilcoxon test was used. The data were visualised in GraphPad PRISM 9.5.0 using a box-whisker plot (5–95 percentiles).
Temporal Evaluation of Medication-Related Processes without and with UDDS
For the quantitative survey of time spent on medication-related processes, a standardised survey form from the University Hospital Cologne was used as a template for Helios Kliniken GmbH (Supplementary Figure 2).24 The nurses completed it independently over 10–14 days, depending on weekends or holidays when the questionnaire was not collected. The term t0 corresponds to the classic medication process on the ward two weeks before the UDDS introduction. Four weeks after the UDDS introduction is defined as t1. Six months after the UDDS introduction corresponds to t2.
The medication process on the ward consists of six essential steps (Supplementary Figure 3). UDDS care variably influences the individual sub-processes variably. Based on the time recording on the wards, it should be determined whether a time saving can be achieved and which sub-processes are particularly influenced by changing the form of care.
The data were transferred to Microsoft Office Excel® 2016, and the median ± SEM was calculated as minutes in decimal numbers. For each row, the individual processes were corrected with the number of patients and summarised on a daily basis to obtain the time spent per patient per day. The Kolmogorov–Smirnov test was used to test for a normal distribution. Statistical analysis was performed using the Mann–Whitney U-test in GraphPad PRISM 9.5.0. The results were presented as median ± SEM in a bar chart.
Calculation of the Relieved Full-Time Equivalent Nurses
The results of the time recording (see “Temporal Evaluation of Medication-Related Processes without and with UDDS” and Table 1) serve as basis for the calculation of relieved FTE nurses. The time saved by UDDS was multiplied by cases/year and number of care days. This value was charged to the number of beds and hours of a FTE nurse. HK-EF accounting data for the years 2019 and 2022 were used for cases/year and care days.
 |
Table 1 Representation of the Time Spent for Sub-Processes in Minutes |
Results
Acceptance and Satisfaction of Nurses with UDDS
The introduction of UDDS care represents a complex changeover in the daily work of nurses. In order to identify challenges and counteract them, the nursing staff was asked about their satisfaction with the new form of care.
The response rate cannot be calculated because the survey was placed on the counter of the ward and was accessible multiple times. In total, 86 nurses participated (65% female, 35% male). Of the respondents, 87% stated that providing medicines is an essential part of their job description. Against this outcome, it should be emphasised that the clear majority (73%) do not fear a loss of competence due to the UDDS.
Delivery times by the pharmacy are reviewed positively and are well integrated into the daily routine of the ward (Figure 1; 61%). In contrast, only 23% of the nursing staff experienced the new form of supply as a form of time relief. In this context, 44% of the nurses consider the medication changes that occur during the day tolerable. To achieve this goal, the production times of the blisters were adapted to the ward rounds. The nursing staff confirms the assumption that the stock of solid oral drugs on the wards was reduced by the UDDS (Figure 1, 71% agreement).
Evaluation of Supply Volume on Ward
A clear improvement and immediately recognisable positive effect of introducing the UDDS is the decrease in the quantity of solid drugs delivered to the wards (Figure 1). The subjective assessment was examined through an economic analysis of ward consumption both before and after UDDS implementation. The second quarter of 2019 and 2022 were selected in order to be able to exclude the supply bottlenecks caused by COVID-19 and additional effects such as ward closures or staff shortages as much as possible.
A significant reduction in the supply volume of all three drugs could be seen on the wards in the second quarter of 2022; this can be attributed to the introduction of UDDS (Figure 2).
Temporal Evaluation of Medication-Related Processes without and with UDDS
Both the dispensing of oral medication by nurses and the control of the dispensed drugs in accordance with the principle of dual control are considered key processes in activities related to drugs (Figure 1).
Fifteen wards of the HK-EF teaching hospital participated in the survey; this corresponds to 35% of wards supplied with the UDDS blisters. As a result, we had 301 analysable days (Supplementary Table 2).
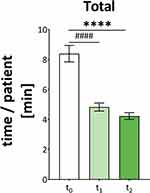
Before the implementation of the UDDS, there was a mean total time of 8.39 ± 0.55 min spent per patient per day for the entire medication process. At t1, four weeks after the introduction of the UDDS, the mean time per patient decreased significantly by 3.56 minutes to 4.83 ± 0.27 min (p < 0.05). After six months (t2), we suggest a further reduction in time due to the longer period of experience. The total time decreased by only another 0.61 min to 4.22 ± 0.23 min per patient per day (Figure 3). The sub-processes are shown in Table 1.
As expected, there was a significant time reduction for the entire medication process: the UDDS reduced the time spent by nurses on the ward by 4.17 min, which corresponds to a 50% decrease (p < 0.001).
Calculation of the Relieved Full-Time Equivalent Nurses
Using the determined time saving of 4.17 min/patient/day (Figure 3), we calculated how many FTE nurses can be relieved per year by UDDS care in HK-EF. Patient numbers from 2019 (50,000 cases) and 2022 (40,000 cases) were used for the calculation (Table 2). Because of the effects of COVID-19, the patient numbers in 2022 is lower than in 2019.26 The care days per case are 5.96 days, and 1,125 beds are supplied with the UDDS (Table 2).
 |
Table 2 Presentation of the Key Data Required for the Calculation of Relieved FTE Nurses for HK-EF Taking Gross and Net Working Time into Account |
Looking at the figures of the teaching hospital (Table 2), a relief of 7.36 FTE nurses can be achieved for 2022 and a relief of 9.20 FTE for 2019. In contrast, 8.5 FTE are employed in the pharmacy to supply the entire cluster. The calculated relief of FTE nurses corresponds to the gross figure without taking paid leave and sick leave into account. If leave days (30 days/year = 231 hours) and sick leave (25.7 days/year = 197.89 hours)27 are considered, the net working time is 1,573.11 hours/year. The number of relieved nurses therefore increases to 9.36 FTE (2022) and 11.70 FTE (2019).
Discussion
The purpose of this paper is to show that the introduction of the UDDS is accompanied by changes in the daily nursing routine but can bring advantages for the nurses, the patients, and from an economic point of view.
The survey on the acceptance of the nurses with regard to the introduction of UDDS (Figure 1) reveals that dispensing drugs is perceived as an essential part of their profession, which Frieß confirms.28 The 6-R rule is consistently followed by the nursing staff in the context of medication-related processes and plays a major role in their daily routine.29 This rule states that the right drug is administered to the right patient in the right dosage and at the right time in the right form, and that the drug administration is documented in the right way.29 Although UDDS interferes with the professional profile of the nursing staff, since drugs are blistered individually in the hospital pharmacy, the interviewees do not fear a loss of competence. This is based on the demarcation of responsibility, which clearly defines the UDDS as a support for the nursing staff, and additionally, as a quality-assuring and self-quality-assured measure. Therefore, the nursing staff sees UDDS as support rather than as competition, as they hold the increase in drug safety in high regard.
Kleinjung showed in 2015 that stockpiling on wards could be reduced. He confirms, on the one hand, the perception of the nursing staff (Figure 1) and, on the other hand, the results of the significant reduction of solid oral drugs on the wards (Figure 2). The decrease in quantity of medicines leads to reduced discards due to expiry and lower storage costs. Regarding medicines with supplementary remuneration (ZE, Zusatzentgelt) and medicines that are classified as reimbursable, in addition to new examination and treatment methods (NUB, neue Untersuchungs- und Behandlungsmethoden) in Germany, this is an advantage. Patient-specific billing is necessary for these drugs due to their high price. Because of the patient-specific blistering, the billing automatically takes place on a patient-specific basis, and the drug package remains centrally in the pharmacy.
From the nursing point of view, the UDDS is not a time relief (Figure 1). This stands in contrast to the results of the time recording, which already shows a direct positive effect after four weeks. With the new introduction of UDDS on the wards, the medication-related process steps are impaired, and a new routine must be established. The step of dispensing oral drugs is immediately significantly reduced, whereas the control time for medication does not change after four weeks of being supplied with the UDDS blisters. By developing new routines, confidence has increased and the control process has decreased. The discrepancy between the results can be explained, inter alia, by the fact that trust in the new form of care must first be developed.30 On a pilot ward in the Convent Hospital Linz, a time saving of 62% is shown for the processes of dispensing and controlling dispensed drugs.23 These two essential steps were reduced by 56% in our study. The difference in outcomes from Linz results from interviewing the pilot ward, so no comparability is possible over a longer period.
The results published so far by the University Hospital of Cologne include the steps of ordering, sorting, dispensing oral drugs, checking those drugs, and other processes related to medicines.24 Following the survey from the University Hospital Cologne, only clearly assignable processes were recorded in the cluster; other processes related to medicines were not considered. Taking into account only these process steps from the results of the University Hospital Cologne, a time reduction of approx. 46%, or 3.87 min per patient per day, is shown there.24 Correspondingly, the results are comparable. It should be noted that the data in the cluster were collected on the same wards before and after the introduction of the UDDS, so this was the only relevant variable at the time. In contrast, the data from the University Hospital of Cologne was generated in cooperation with various hospitals and wards. The preliminary results do not show the required personnel demand in the hospital pharmacy for the supply of the UDDS. Nevertheless, it should be noted that the recorded times at the HK-EF teaching hospital might be biased due to interruptions during the process.
With reference to the results for the hospital trust, this yields a saving of 11 FTE nurses (70,000 patients/year, 1,700 beds, 5.49 care days and a net working time of 1,573.11 hours/year). The outcomes demonstrate that the relief of nursing staff through UDDS supply is a scalable effect: the more patients are supplied, the more nursing staff are relieved in the medication-related process. This effectively leaves more time for patient care. Nevertheless, the UDDS also requires staff in the pharmacy. On the one hand, CP for medication reconciliation, and on the other hand, pharmaceutical technical assistants (PTA) for producing the ready-to-use forms. This results in a personnel expenditure of 6.5 FTE for the coverage of the HK-EF and 8.5 FTE for the care of the hospital trust (3.5 CP, 5 PTA). With a focus on German regulations, a care-relieving measure can be clearly shown. From an international point of view, it is obvious that in our hospital trust, a relief of at least 11 FTE nurses can be achieved. Nevertheless, there are costs for the procurement of materials (eg, unit-dose machines, storage space, and consumables) and conversion of the premises. Therefore, further studies are necessary for the economic assessment of the purchase of unit-dose machines, consumables, technical support, and other details.
It should be noted that in our study, the time recording was based on personal assessments by the nursing staff rather than being carried out using a stopwatch. The processes of dispensing and controlling were also affected by digitalisation. Before the introduction of UDDS, the steps were clearly defined, but this was not standardised following the introduction of the UDDS, especially in the beginning. Some wards included not only the medication dispensed by the nurses under the control step but also the control of all unit-dose blister packs. The pharmacy staff had already checked these blisters for completeness and errors, resulting in duplicated work in this case. Additionally, in some cases, the documentation in the CPOE was also considered part of the setting process.
In terms of drug safety alone, UDDS care is considered a quality-assuring and self-quality-assured measure through automation and process optimisation.2,3,20 In addition, patient-specific blistering is a further step towards CLMM. The information to close the loop is available, but the providers still have to find appropriate technical solutions to ensure both the blisters and the patient’s wristband can be scanned, and so that patient safety is also increased in accordance with the 6-R rule.
Conclusion
Drug and patient safety, as well as the opportunity to refinance UDDS and the shortage of nursing staff, represent incentives for an introduction across Germany for this form of care. Furthermore, internationally, the UDDS will be a key resource in hospitals to increase patient care and safety. However, additional analysis of the cost-effectiveness ratio would be useful. Improving the closure of the CLMM, particularly with regard to medication scanning and patient wristband verification, would also improve patient safety not only for solid medicines but also for cytostatics. Our study shows that, when used efficiently, the UDDS can influence several medication-related processes on the ward in a resource-efficient way.
Data Sharing Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Ethical Approval
The research conducted is a survey of clinic staff, with a focus on ensuring the quality of new processes. Participants were informed of the voluntary and anonymous nature of their participation through a declaration of consent, including information on data processing. Authorisation for this study was obtained from the Works Council with appropriate data protection measures in place. As this study does not involve diagnostic or therapeutic procedures on patients, no ethical approval is required.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hänninen K, Ahtiainen HK, Suvikas-Peltonen EM, Tötterman AM. Automated unit dose dispensing systems producing individually packaged and labelled drugs for inpatients: a systematic review. Eur J Hosp Pharm. 2021;33(4):1–8.
2. Fanning L, Jones N, Manias E. Impact of automated dispensing cabinets on medication selection and preparation error rates in an emergency department: a prospective and direct observational before-and-after study. J Eval Clin Pract. 2016;22(2):156–163. doi:10.1111/jep.12445
3. Malik M, Eisend S, Kunze T. Einführung der Unit-Dose-Versorgung: einfluss auf die Arzneimitteltherapiesicherheit im Universitätsklinikum Schleswig-Holstein Kiel. Krankenhauspharmazie. 2019;40:421–430.
4. Baehr M, Van der linde A, König R, et al. Kopplung von elektronischer Verordnung und patientenorientierter Logistik: signifikante Verbesserung der Arzneimitteltherapiesicherheit. Krankenhauspharmazie. 2014;35(04):110–117.
5. Berger V, Sommer C, Boje P, et al. The impact of pharmacists’ interventions within the closed loop medication management process on medication safety: an analysis in a German university hospital. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.1030406
6. Eisend S, Lemmer L, Melzer S, et al. Leitlinie: anforderungen an eine Unit-Dose-Versorgung in der Krankenhausapotheke; 2024.
7. Corsonello A. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med. 2005;165(7):790–795. doi:10.1001/archinte.165.7.790
8. Blassmann U, Morath B, Fischer A, Knoth H, Hoppe-Tichy T. Arzneimitteltherapiesicherheit im Krankenhaus: einbindung von Stationsapothekern zur Reduktion von arzneimittelbezogenen Problemen im stationären Medikationsprozess. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. Med saf hosp. 2018;61(9):1103–1110. doi:10.1007/s00103-018-2788-x
9. Ensing HT, Stuijt CCM, van den Bemt BJF, et al. Identifying the Optimal Role for Pharmacists in Care Transitions: a Systematic Review. J Manag Care Spec Pharm. 2015;21(8):614–636. doi:10.18553/jmcp.2015.21.8.614
10. Davies EC, Green CF, Mottram DR, Rowe PH, Pirmohamed M. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol. 2010;70(5):749–755. doi:10.1111/j.1365-2125.2010.03751.x
11. Walsh EK, Hansen CR, Sahm LJ, Kearney PM, Doherty E, Bradley CP. Economic impact of medication error: a systematic review. Pharmacoepidemiol Drug Saf. 2017;26(5):481–497. doi:10.1002/pds.4188
12. Darcis E, Germeys J, Stragier M, Cortoos P. The impact of medication reconciliation and review in patients using oral chemotherapy. J Oncol Pharm Pract. 2023;29(2):270–275. doi:10.1177/10781552211066959
13. Dean BS, Allan EL, Barber ND, Barker K. Documentation of pharmacists’ interventions in an emergency department and associated cost. Am J Health Syst Pharm. 1995;52(22):2543–2549. doi:10.1093/ajhp/52.22.2543
14. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi:10.1128/CMR.18.4.638-656.2005
15. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. doi:10.1016/S0002-9343(97)89519-8
16. Franklin BD, O’Grady K, Donyai P, Jacklin A, Barber N. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before-and-after study. Qual Saf Health Care. 2007;16(4):279–284. doi:10.1136/qshc.2006.019497
17. Ghaffarzadeh J, Maher A, Alimohammadzadeh K, Hosseini S, Bahadori M. The role of “dose-unit” drug distribution system in the professionalism of pharmacists and job satisfaction of nurses: a case study. J Health Admin. 2022;25(1):92–102. doi:10.52547/jha.25.1.92
18. Kleinjung H. Ökonomische Vorteile einer Unit-dose-Versorgung. das Krankenhausheft. 2015;2024(3):234–239.
19. Bundesverband Deutscher Krankenhausapotheker e.V. Ausschuss Elektronische Verordung; Available from: https://www.adka.de/adka/ausschuesse/elektronische-verordnung/.
20. Eisend S, Geckler T, Lemmer L, et al. Unit-Dose-Versorgung in deutschen Krankenhäusern; 2022. Available from: https://www.adka.de/adka/ausschuesse/unit-dose/.
21. Baehr M, Melzer S, eds.. Closed Loop Medication Management: Arzneimitteltherapiesicherheit Im Krankenhaus. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2018.
22. Ahtiainen HK, Kallio MM, Airaksinen M, Holmström A-R. Safety, time and cost evaluation of automated and semi-automated drug distribution systems in hospitals: a systematic review. Eur J Hosp Pharm. 2020;27(5):253–262. doi:10.1136/ejhpharm-2018-001791
23. Steindl-Schönhuber T, Gittler G. Umstellung der Arzneimittelversorgung auf Unit-Dose: eine Antwort auf COVID-19 mit entscheidenden Vorteilen im Krankenhaus. Krankenhauspharmazie. 2022;43(11):440–446.
24. Düpmeier S, Lenssen R, Sommer B, Richardt A, Möller D, Liekweg A Unit-Dose als pflegeentlastende Maßnahme - eine bundesweite Erhebung; 2022.
25. Dudenredaktion. Schnelldreher; Available from: https://www.duden.de/rechtschreibung/.
26. Klauber J, Wasem J, Beivers A, Mostert C. Krankenhaus-Report 2021. Berlin, Heidelberg: Springer Berlin Heidelberg; 2021.
27. Knieps F, Pfaff H, eds.. Pflegefall Pflege? Berlin: MWV Medizinisch Wissenschaftliche Verlagsgesellschaft; 2022.
28. Frieß J. Medikamente sicher verabreichen. Heilberufe. 2015;67(2):42–43. doi:10.1007/s00058-015-1305-y
29. Dietmaier O, Schmidt S, Laux G. Pflegewissen Psychopharmaka. Berlin, Heidelberg: Springer Berlin Heidelberg; 2019.
30. Barghorn K. Einstellungen und Verhalten von Mitarbeitern in betrieblichen Veränderungsprozessen. Osnarbrück; 2010.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.