Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 15
Intramucosal Melanocytic Nevi – A Rare Cause for Gingival Enlargement. Report of a Case and Review of Literature
Authors Ramesh R , Sadasivan A
Received 10 March 2023
Accepted for publication 21 April 2023
Published 29 April 2023 Volume 2023:15 Pages 71—77
DOI https://doi.org/10.2147/CCIDE.S408425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Roshni Ramesh,1 Arun Sadasivan2
1Department of Periodontics, Government Dental College, Thiruvananthapuram, Kerala, India; 2Department of Periodontics, Sree Mookambika Institute of Dental Sciences, Kulasekharam, Tamil Nadu, India
Correspondence: Arun Sadasivan, Department of Periodontics, Sree Mookambika Institute of Dental Sciences, Kulasekharam, Tamil Nadu, India, Tel +91 9847246961, Email [email protected]
Background: Oral melanocytic nevi are infrequent oral lesions derived from nevus cells of oral mucosa which causes focal hyperpigmentation. The most common site of occurrence of oral nevi is the hard palate followed by buccal mucosa and gingiva. The mean age group affected are in their 3rd and 4th decade of life and there seems to be a predilection for females. Clinically, oral nevi are usually small, well-circumscribed macules but can also present as slightly raised papules. Histologically, nevi can be classified as Junctional, Compound or Intramucosal, with intramucosal being the more common type in the oral cavity.
Case Presentation: In this paper, we report a case of intramucosal nevus in a 25-year-old female patient. The lesion presented as a gingival enlargement in the mandibular anterior region involving the marginal and attached gingiva, which is an extremely rare presentation. The clinical findings, histologic features and surgical management are presented. The patient was followed up for one year and the one year follow up revealed a small area of focal hyperpigmentation at the site of the previous lesion which is being closely monitored.
Conclusion: Nevi located in the mucous membrane have been documented to pose a threat of malignant transformation. Hence, all pigmented lesions of the oral cavity should be cautiously diagnosed.
Keywords: focal hyperpigmentation, gingival enlargement, intramucosal, melanoma, nevi, oral
Introduction
Nevi are either congenital or acquired, benign, pigmented neoplasms of the skin or mucosa characterized by the presence of melanin-producing neuroectodermal-derived cells.1 Pigmented nevi in the oral cavity are distinctly uncommon and when present can occur anywhere in the oral cavity.2 King et al in a large-scale survey reported that oral pigmented nevi are a rare entity found only in 0.1% of the patients and coined a specific term, intramucosal nevus.3,4 The most common intraoral sites affected are the hard palate, buccal mucosa and gingiva. Pigmented nevi are seen in persons of all ages with the mean age group affected being the 3rd and 4th decade. Women seem to be more affected than men.
Clinically, a pigmented nevi is an asymptomatic, well-circumscribed, round, or oval, flat or slightly elevated spot or plaque and usually small in size. The colour varies from brown to blue or bluish grey to black. Histologically, nevi can be classified as intradermal/intramucosal, junctional or compound based on the pattern of proliferation of nevus cells. Even though nevi of the oral cavity are a rare finding, when they occur, the most common histological type is intramucosal.
Nevi of the mucous membrane have been identified as having the most potential for malignant transformation into melanoma although some studies refute this assertion. However, because of the malignant potential of nevi and due to the presence of pigmented macules in about one-third of patients with preliminary oral melanoma, it is advisable to diagnose all pigmented lesions promptly.5 The differential diagnosis of oral pigmentation disorders includes racial pigmentation, melanocytic macule, smoker’s melanosis, oral melanoacanthoma, in Addison’s disease and in certain syndromes like McCune-Albright syndrome, Laugier-Hunziker syndrome and Peutz Jegher syndrome. The role of racial pigmentation presenting on a gingival enlargement should also be carefully ruled out by clinical and histopathological evaluation.
In this paper, we report a rare case of intramucosal melanocytic nevi presenting as a gingival enlargement in relation to gingiva of mandibular anterior region. This case report has been prepared in accordance with the CARE (Comparison to self and others, adaptability, resourcefulness and emotional well-being) criteria.6
Case Report
A 25-year-old woman reported to our dental clinic with the chief complaint of a swelling in the gums in her lower anterior region of two years duration. The patient reported no significant medical history. She had undergone fixed orthodontic treatment fifteen years back. She noticed a small growth in her gums two years back which slowly increased to the present size. The growth was asymptomatic. Intraoral examination revealed the presence of a localized pigmented gingival enlargement [GE] extending from lower left central incisor [31] to lower left canine [33] region, measuring 13 × 8 mm in size. It involved the labial, marginal as well as attached gingiva (Figure 1). The enlargement was brownish black in colour, indurated, rough textured with a sessile base and firm in consistency. The GE also extended partially over the crown surface. Plaque and calculus deposits were minimal, with no bleeding on probing seen. Deep pockets (6–8 mm) were recorded on the labial aspect of the affected teeth. Radiographically, both the orthopantomograph (OPG) and Cone beam computed tomography (CBCT) images showed only mild bone loss in the labial plate of lower anterior teeth (Figure 2).
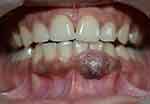 |
Figure 1 Pre-operative photograph of gingival enlargement. |
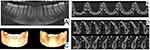 |
Figure 2 OPG and CBCT images showing minimal bone loss in the labial plate beneath the gingival overgrowth (A–D). |
The patient was informed about the treatment options available and the necessity for a biopsy. Before starting the treatment, a written informed consent was obtained. The patient provided written consent to publish the case report. A provisional diagnosis of pyogenic granuloma was made. The differential diagnosis included racial pigmentation on a gingival enlargement, fibroma, peripheral giant cell granuloma, melanotic macule and malignant melanoma.
Case Management
The initial treatment included full mouth scaling and root planing. At one-week post scaling, an excisional biopsy was done, and a collagen sponge dressing (FIX PLUG from Synerheal Pharmaceuticals, Chennai) was used to cover the excision site (Figure 3A and B). The use of a collagen dressing helped in the rapid healing of excision site and did not create a mucogingival defect at the excision site as seen in the one month post operative photograph (Figure 3C). The tissue specimen was sent for histopathological examination.
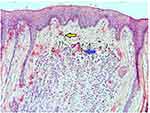
The hematoxylin and eosin (H&E 100x) stained section showed a stratified squamous surface epithelium in association with a fibrous connective tissue (CT). The CT showed cohesive islands of polygonal to oval epithelioid cells with focal melanin pigment production. The nests of nevus cells were separated by a hyalinised band beneath the epithelium. The nevus cells did not show any significant cellular atypia or dysplasia. At the periphery adjacent to the epithelium, cells were large and round without dendritic processes (Type A) with an intense accumulation of melanin. The cells in the deeper layers were uniform with a round to polygonal shape (Type B) and showed decreased melanin deposition. The associated CT was fibrous with dense bundles of collagen fibres. The lesional cells were seen close to the excision borders. The histopathological features were suggestive of intramucosal nevi (Figure 4).
Clinical Outcomes
The patient remained uneventful for a year following the surgical excision. Follow-up of the patient at one year revealed a small area of focal hyperpigmentation at the site of the previous lesion which is being closely followed up (Figure 5).
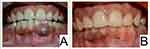 |
Figure 5 (A) Preoperative and (B) one-year post-operative photograph, showing a focal area of hyper-pigmentation. |
Discussion and Literature Review
Melanocytic nevi are said to be benign neoplasms arising from genetically altered melanocytes that undergo clonal expansion but soon cease dividing, explaining clinical regression.7 Oral melanocytic nevus is also thought to be a benign tissue malformation or tumour due to the excessive proliferation of nevus cells. These cells are neuroectodermal derived and produce melanin. During early stages of intrauterine life, there is a migration of the precursors of melanocytes, ie melanoblasts from the neural crest to the epidermis. These later differentiate into dendritic cells.
Three theories have been put forward regarding the development of oral nevi:
- “Abtropfung” theory of nevogenesis was proposed Paul Gerson Unna who was a German physician in 1893. It is the most widely accepted theory which postulates that during the development of the melanocytic tumour, there is a migration of nevus cells from the epidermis to dermis and which then starts to proliferate.
- “Hochsteigerung” theory was put forward by Dr Stewart F Cramer, a pathologist in the year 1984. This theory is essentially the reverse of the Abtropfung theory. It states that the neural crest-derived melanocytes tend to migrate upward from the dermis to the epidermis.
- “Dual origin” theory refers to the possibility of dual origin of nevus cells. The nevus cells seen in the epithelial basal layer and juxta epithelial region of the submucosa are thought to have a melanocytic origin. Whereas the nevus cells present in the submucosa or deep in the dermis are thought to have an origin from nerve cells, specifically Schwann cells.8
There is a homeostatic regulatory and safeguard mechanism that restrains the proliferation of genetically altered nevus cells which explains the phenomenon of nevocyte proliferation. The regulatory mechanisms responsible for the “oncogene induced senescence” include telomerase associated regulation, tumour suppressor gene activity and DNA (deoxyribonucleic acid) damage responses. These maintain the benign nature of melanocytic nevi.9,10
Buchner and Hansen in a review of multiple cases of oral nevi reported that the frequency of occurrence of clinical variants are 55% intramucosal type followed by common blue nevus (32%), compound nevi (6%), junctional nevi (5%) and combined nevi (2%). They also reviewed the incidence percentage of nevus at various intra oral sites11 (Table 1). By far, the commonest type of oral nevi is the intra mucosal type and represents 63% to 70% of all oral nevi.12 The most common location of oral nevi as reported in the literature is the hard palate (42%). The second most common site is the buccal mucosa (17%), and 8% of all types of oral nevi are found on gingiva. Among intramucosal nevi, approximately 17% are seen on gingiva.12 Clinically, the lesions usually present as well-circumscribed, round, or oval, flat, or slightly elevated spots or plaques. In the present case, the intramucosal nevi presented as a gingival enlargement of the mandibular left anterior region, involving the marginal and attached gingiva, which is a rare finding.
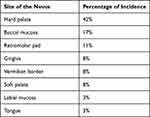 |
Table 1 Distribution of Incidence Percentage of Oral nevus11 |
Nevi are most often found in females in the third and fourth decades of life. In our case, the patient was a 25-year-old female. Nevi are typically small measuring between 0.1 and 0.6 cm in diameter and the lesions are usually asymptomatic.2 Literature suggests that about 75% of nevi are smaller than 0.6 cm and those larger than 1.3 cm were present in only 5% of the cases.13 In the present case, the lesion was comparatively large, about 1.3×0.8 cm in diameter, which is a relatively uncommon finding, and presented on the mandibular left anterior gingiva as a gingival enlargement, involving the marginal and attached gingiva. The lesion was asymptomatic. Rithika et al reported a similar lesion in the maxillary anterior gingival mucosa and with an unusual history of pain.14
Oral nevi are usually smooth and rarely have rough or papillated surface. In our case, the swelling was well circumscribed, firm in consistency but with a rough surface and there was a whitish plaque in the centre of the swelling. Macroscopically, nevi are thought to proliferate in two configurations, Unna’s and Miescher’s nevi. An exophytic outlook with papillary or round pattern of nevus cells are seen in Unna’s nevi. Miescher’s nevi have an endophytic outlook with diffuse insinuation of nevus cells into the subepithelial region.5 Based on their histological features, nevi can be classified as Junctional, Compound or Intramucosal. Intramucosal nevi is the most common type in the oral cavity. In junctional nevi, the nevus cells are found at the epithelial mesenchymal junction; in compound nevi, the lesional cells exist in the junctional and underlying connective tissue area; and in intramucosal nevi, lesional cells are located solely within the connective tissue.15 Junctional activity does not exist in intramucosal nevi, and the nevus cells are grouped in the lamina propria and submucosa; there is a conjunctive band separating these cells from the surface epithelium.
Different types of nevus cells are often appreciated during the development of the lesion: (1) Epithelioid or Type A nevus cells, which are superficial cells and appear as large epithelioid cells with ample cytoplasm; (2) Lymphocyte like or Type B which are round to polygonal with less cytoplasm and present in intermediate portion of the lesion and (3) Spindle shaped or Type C which are small and round with spindle-shaped nuclei and found in the deeper portion of the lesion. In the present case, Type A and B cells could be appreciated on histopathological examination.
The chances of malignant transformation of melanocytic nevi are reported to be rare. For the transformation of benign nevi to malignant melanoma, the somatic oncogenic mutations seen in nevi are by themselves not sufficient. It is reported that additional cumulative genetic alterations are required. The primary oncogenic events in the progression would involve the signaling molecules within the mitogen-activated kinase (MAP-kinase) pathways and phosphatidyl-inositol-3-phosphate kinase (PI3-kinase pathways).9,10 Research has been done to comparatively analyze genetic abnormalities seen in the tumour lesions and characterize during tumour progression the progressive accumulation of these abnormalities. Benign intramucosal nevi exhibit point mutations or kinase fusions in BRAF, NRAS, HRAS, CDKN2A and GNAQ or GNA11. These may represent early acquired events during tumour progression. In a landmark study by Shain et al, some important conclusions were reached. At the level of precursor lesions such as nevi, the initial mutagenic events triggering the neoplastic transformation are represented by several mutations which lead to the activation of the MAPK pathway (such as BRAF V600E), while at an intermediate stage of tumour progression, NRAS and additional driver mutations are seen. In these tumour lesions, TERT promoter mutations are very frequently observed (seen in 77% of cases). Only in advanced melanoma lesions were biallelic inactivation of CDKN2A and PTEN and TP53 mutations seen.16
The ideal treatment of intramucosal nevi is excisional biopsy with a safety margin of 2 mm and a histopathological exam to rule out malignant transformation. In this case, also we followed the same standard operating procedures reported in the current literature for diagnosis and treatment.
Nevi, though uncommon, can occur in the oral cavity and sometimes have unusual presentations. Intramucosal nevi presenting as gingival enlargement as in the present case is a rare finding. It is extremely important to differentiate these lesions from other pigmented lesions including oral melanomas which have a high mortality rate.1
Conclusion
Pigmented lesions of the oral cavity often have similar presentations which pose a diagnostic dilemma to the dental surgeon. These lesions are often noticed in routine dental examinations. Since nevi in the mucous membrane have shown to display a threat for malignant transformation into melanoma, it is advisable to cautiously diagnose all pigmented lesions of the oral cavity. Knowledge regarding diagnosis and treatment of oral pigmented lesions can help dental professionals in timely and accurate management of these lesions.
Abbreviations
GE, Gingival enlargement; OPG, Orthopantomograph; CBCT, Cone beam computed tomography; H&E, hematoxylin and eosin; CT, Connective tissue; DNA, Deoxyribonucleic acid; MAPK, Mitogen activated protein kinase; PI3, Phosphatidyl inositol 3-phosphate; BRAF, proto-oncogene B-raf; NRAS, Neuroblastoma RAS viral oncogene homolog; HRAS, Harvey rat sarcoma viral oncogene homolog; CDKN2A, Cyclin-dependent kinase inhibitor 2A; GNAQ, Guanine nucleotide-binding protein G(q) subunit alpha (Gaq); GNA11, G protein subunit alpha 11; TERT, Telomerase reverse transcriptase; PTEN, Phosphatase and TENsin homolog deleted on chromosome 10; TP 53, Tumor protein 53.
Consent for Publication
The authors certify that they have obtained written informed consent from the patient before starting the treatment. In the consent form, the patient was informed about the disease condition and the treatment options available. The patient provided written consent to publish the case report. The patient understood that due efforts will be made to conceal her identity and that her name will not be published. Institutional approval was not required to publish the case details.
Funding
The study was conducted without external funding.
Disclosure
The authors declare that they have no competing interests.
References
1. Popa C, Stelea C, Popa R, Popescu E. Leziuni pigmentare endogene orale ĩ periorale [Oral and perioral endogenous pigmented lesions]. Rev Med Chir Soc Med Nat Iasi. 2008;112(4):1054–1060. Romanian. PMID: 20209786.
2. Freitas DA, Bonan PR, Sousa AA, Pereira MM, Oliveira SM, Jones KM. Intramucosal nevus in the oral cavity. J Contemp Dent Pract. 2015;16(1):74–76. PMID: 25876954. doi:10.5005/jp-journals-10024-1638
3. King OH, Blankenship JP, King WA, Coleman SA. The frequency of pigmented nevi in the oral cavity. Report of five cases. Oral Surg Oral Med Oral Pathol. 1967;23(1):82–90. PMID: 5224929. doi:10.1016/0030-4220(67)90489-6
4. Kumar RV, Kranthi K, Seshan H. Pigmented intramucosal nevus of gingiva: a case report. Int J Contemp Dent. 2010;1:14–19.
5. Dutta D, Kamath VV, Rajkumar K. Oral melanocytic nevi: report of two cases with immunohistochemical elaboration of their probable origin and maturation. Indian J Dermatopathol Diagn Dermatol. 2015;2:29–33. doi:10.4103/2349-6029.173413
6. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013:bcr2013201554. PMID: 24155002; PMCID: PMC3822203. doi:10.1136/bcr-2013-201554
7. Rogers T, Marino ML, Raciti P, et al. Biologically distinct subsets of nevi. G Ital Dermatol Venereol. 2016;151(4):365–384. PMID: 27119653; PMCID: PMC5445663.
8. Krengel S. Nevogenesis--new thoughts regarding a classical problem. Am J Dermatopathol. 2005;27(5):456–465. PMID: 16148419. doi:10.1097/01.dad.0000175532.27368.3f
9. Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. PMID: 24460190; PMCID: PMC4831647. doi:10.1146/annurev-pathol-012513-104658
10. Damsky WE, Bosenberg M. Melanocytic nevi and melanoma: unraveling a complex relationship. Oncogene. 2017;36(42):5771–5792. PMID: 28604751; PMCID: PMC5930388. doi:10.1038/onc.2017.189
11. Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part I: a clinicopathologic study of 36 new cases. Oral Surg Oral Med Oral Pathol. 1987;63(5):566–572. PMID: 3473378. doi:10.1016/0030-4220(87)90229-5
12. Buchner A, Leider AS, Merrell PW, Carpenter WM. Melanocytic nevi of the oral mucosa: a clinicopathologic study of 130 cases from northern California. J Oral Pathol Med. 1990;19(5):197–201. PMID: 2359037. doi:10.1111/j.1600-0714.1990.tb00825.x
13. Agrawal J. Intramucosal melanotic nevi - A case report of an unusual gingival enlargement. J Indian Soc Periodontol. 2013;17(2):239–241. PMID: 23869134; PMCID: PMC3713759. doi:10.4103/0972-124X.113087
14. Bashamalla R, Rao GV, Ramulu S, Sravya T. Pigmented intramucosal nevus of gingiva with a special insight on its pathophysiology: report of a rare entity. J NTR Univ Health Sci. 2017;6:181–184. doi:10.4103/2277-8632.215529
15. Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139(3):282–288. PMID: 12622618. doi:10.1001/archderm.139.3.282
16. Shain AH, Yeh I, Kovalyshyn I, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373(20):1926–1936. PMID: 26559571. doi:10.1056/NEJMoa1502583
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.