Back to Journals » International Journal of Nanomedicine » Volume 11
Insights into the therapeutic potential of hypoxia-inducible factor-1α small interfering RNA in malignant melanoma delivered via folate-decorated cationic liposomes
Authors Chen Z , Zhang T, Wu B, Zhang X
Received 4 December 2015
Accepted for publication 14 January 2016
Published 11 March 2016 Volume 2016:11 Pages 991—1002
DOI https://doi.org/10.2147/IJN.S101872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Zhongjian Chen,1,* Tianpeng Zhang,2,* Baojian Wu,2 Xingwang Zhang2
1Department of Pharmaceutics, Shanghai Dermatology Hospital, 2Division of Pharmaceutics, College of Pharmacy, Jinan University, Gangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Malignant melanoma (MM) represents the most dangerous form of skin cancer, and its incidence is expected to rise in the coming time. However, therapy for MM is limited by low topical drug concentration and multidrug resistance. This article aimed to develop folate-decorated cationic liposomes (fc-LPs) for hypoxia-inducible factor-1α (HIF-1α) small interfering (siRNA) delivery, and to evaluate the potential of such siRNA/liposome complexes in MM therapy. HIF-1α siRNA-loaded fc-LPs (siRNA-fc-LPs) were prepared by a film hydration method followed by siRNA incubation. Folate decoration of liposomes was achieved by incorporation of folate/oleic acid-diacylated oligochitosans. The resulting siRNA-fc-LPs were 95.3 nm in size with a ζ potential of 2.41 mV. The liposomal vectors exhibited excellent loading capacity and protective effect toward siRNA. The in vitro cell transfection efficiency was almost parallel to the commercially available Lipofectamine™ 2000. Moreover, the anti-melanoma activity of HIF-1α siRNA was significantly enhanced through fc-LPs. Western blot analysis and apoptosis test demonstrated that siRNA-fc-LPs substantially reduced the production of HIF-1α-associated protein and induced the apoptosis of hypoxia-tolerant melanoma cells. Our designed liposomal vectors might be applicable as siRNA delivery vehicle to systemically or topically treat MM.
Keywords: malignant melanoma, HIF-1α siRNA, chitosan, cationic liposomes, gene therapy
Introduction
Malignant melanoma (MM) is a cancer that develops in pigment cells (ie, melanocytes). MM predominantly occurs in the skin and possesses a high blood metastasis and mortality. In addition, the incidence of MM is increasing year by year, which has been a prominent public health threat.1 Presently, basic means of MM treatment involve surgical resection at early stage, chemotherapy at later stage, and radiotherapy together with immunotherapy. However, these approaches are less effective in terms of advanced and metastatic MM. As an alternative, gene regimen provides a possibility for highly selective cancer treatment while avoiding adverse effects.2
Among various transcription factors, the hypoxia-inducible factor-1α (HIF-1α) that responds to hypoxic stress may be a potential therapeutic target because of its important role in glycolysis, erythropoiesis, and angiogenesis.3 In hypoxic conditions, HIF-1α is upregulated and forms a heterodimer with Arnt 1 to form the HIF-1α complex. The HIF-1α complex cognizes and binds to the hypoxia-responsive element of hypoxia-inducible genes, thereby activating transcription. This results in high expression of some mediators, such as glycolysis enzymes and vascular endothelial growth factor, which allow ATP synthesis in an oxygen-independent manner and the initiation of angiogenesis.4 Based on this, Folkman5 put forward the concept of tumor growth depending on angiogenesis, and since then, therapy against angiogenesis has received growing attention in tumor intervention.6 However, it has been found that anti-angiogenic therapy can just inhibit or kill hypoxia-sensitive tumor cells, and performs poorly in arresting the cloning of hypoxia-tolerant cells.7 Small interfering RNA (siRNA) are small pieces of double-stranded RNA, ~20–25 nucleotides in length, that can be used to interfere with the translation of proteins via binding to the messenger RNA (mRNA) at specific sequences of genes. Due to RNA interference, the synthesis of specific proteins based on the nucleotide sequences of their corresponding mRNA can be interrupted.8 The use of siRNA against HIF-1α to inhibit the angiogenesis of tumor by eradicating hypoxia-tolerant tumor cells is probably a promising approach for cancer prognosis.
In melanoma patients, the hypoxia-inducible family of transcription factors is upregulated by key oncogenic drivers.9 It has also been investigated that HIF activity increases in MM cells under normoxic conditions.10 Considering the role of HIF-1α in the development of MM, it is hypothesized that the downregulation of HIF-1α by RNA interfering will be largely helpful to prevent the growth, progression, and metastasis of MM. However, the delivery of siRNA remains a challenge because of the lack of suitable vectors. Although viral vectors have been proved efficacious, the safety concerns compromise their further application. In recent years, nonviral vectors have been extensively attempted to deliver siRNA, such as polyamidoamine,11 poly(ethyleneimine),12 and poly(amino acid).13–15 Apart from poly(amino acid), the high toxicity of polymers limits their application in siRNA delivery. Alternative vectors or carriers, which are nontoxic, of low cost, and facilely fabricated, are highly desired to explore so as to efficiently deliver siRNA. Liu et al demonstrated that a transferrin-PEI-shRNA complex specific to HIF-1α could efficiently transfect the melanoma cells (A375) via transferrin receptor-mediated endocytosis and inhibit the tumor growth in the A375 xenograft model.16 In addition to polymeric nanoparticles, cationic liposomes in which positively charged materials are incorporated have been well investigated for DNA or siRNA delivery as biocompatible vectors.17 The materials that can be used to fabricate cationic liposomes for nucleotide therapeutics loading include cationic lipid or cholesterol,18 cationic peptides,19 and chitosan.20 Chitosan is a natural cationic polymer composed of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine. Poor water solubility and lack of amphiphilicity limit its biomedical uses. Oligochitosan is the degradation product of chitosan and able to dissolve in aqueous media due to the reduction of molecular mass. By linking with hydrophobic group, oligochitosan can be developed as a functional biomaterial. The introduction of oleic acid and folate confers oligochitosan amphiphilicity and targetability that can be used to decorate nanocarriers for drug delivery. However, cationic liposomes targeting tumor folate receptor as HIF-1α siRNA vector have not been fully investigated.
Herein, we developed folate-decorated cationic liposomes (fc-LPs) using oligochitosan derivatives to deliver HIF-1α siRNA with the aim of evaluating the suitability for MM treatment. Water-soluble oligochitosan was simultaneously amidated with oleic acid and folate to produce amphipathic oligochitosan derivatives. HIF-1α siRNA-loaded folate-cationic liposomes (siRNA-fc-LPs) were achieved by incorporation of the oligochitosan derivatives with folate ligand. The novel vectors of HIF-1α siRNA were characterized by particle size distribution, transmission electronic microscope (TEM), and electrophoresis assay. The performance of siRNA-fc-LPs in inhibiting the growth of hypoxia-tolerant cancer cells via downregulating the expression of HIF-1α was checked in human melanoma cells.
Materials and methods
Materials
Soybean lecithin (S100) was provided by Lipoid GmbH (Ludwigshafen, Germany). Folic acid, oleic acid, cholesterol, chitosan (molecular weight ~110,000–150,000), N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), and cobalt chloride were purchased from Sigma-Aldrich (St Louis, MO, USA). HIF-1α siRNA (human), control HIF-1α siRNA (fluorescein isothiocyanate [FITC] conjugate)-A, anti-HIF-1α antibody, goat anti-rabbit IgG-HRP, and GAPDH antibody were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Deionized water was prepared by a water purifying system (Woter, Chengdu, People’s Republic of China). All other chemicals were of analytical grade and used as received. The protocol of cell experiments was reviewed and approved by the Medical Ethics Committee of Shanghai Dermatology Hospital.
Synthesis of oligochitosan derivatives
Water-soluble oligochitosans were produced by depolymerization of chitosan with hydrogen peroxide.21 Briefly, 10 mL of 30% H2O2 was added into 490 mL of chitosan solution (1%, w/v, dissolved in 1% acetic acid), into which 0.5 g of phosphotungstic acid was supplemented. The hydrolysis reaction was initiated at 65°C in a thermostatic water bath. After 1 hour, the reaction was terminated by cooling down against ice water, and the reaction mixtures were collected by centrifugation. The supernatant fluid was then dialyzed against 0.05 M sodium bicarbonate solution to remove the inorganic acid used. Afterward, the dialyzed solution was filtrated with a Millipore membrane (0.22 μm) followed by lyophilization to obtain the final product. The molecular weight of oligochitosans was measured by static light scattering using Malvern Zetasizer (Nano ZS; Malvern Instruments, Malvern, UK).22
Oligochitosan derivatives diacylated by folate and oleic acid were synthesized via amidation of the carboxyl group of folate and oleic acid with the primary amino group of oligochitosan mediated by NHS and EDC.23 In detail, 1 g of oligochitosan was dissolved in 120 mL of deionized water in a 250 mL flask, and then, an aqueous solution of NHS and EDC was put in the flask. Under stirring, a dimethylsulfoxide solution containing folate and oleic acid was dropwise added into the system. After addition, the reaction was proceeded for 24 hours. Finally, the mixture solution was dialyzed against deionized water for three times, and then, the resulting product solution was lyophilized. To keep the amino groups of oligochitosan partially residual for siRNA complexation, the stoichiometric ratio of oligochitosan/folate/oleic acid for reaction was set to 3:1:5 (molar ratio) roughly. The concentrations of NHS and EDC in the reaction system were 5 and 2 mM, respectively. Furthermore, oligochitosan derivatives with no folate, as a control, were synthesized according to the same procedure. The products were characterized by 1H NMR at 400 MHz (Bruker AV-300; Bruker Corporation, Billerica, MA, USA) by dissolving them in a cosolvent of deuterated water and methanol (75/25, v/v).
Preparation of siRNA/liposome complexes
The siRNA/liposome complexes were prepared by film hydration method followed by incubation with HIF-1α siRNA. In brief, soybean lecithin S100 (425 mg), cholesterol (75 mg), and oligochitosan derivatives (50 mg) were dissolved in 2 mL of 75% ethanol (v/v) in a flask. Then, the solvents were removed by a rotary evaporator (Eppendorf, Hamburg, Germany) under vacuum at 45°C, in which a thin film was formed. After the complete removal of solvents, 20 mL of deionized water was introduced into the system to hydrate the lipid membrane. The hydration was performed at 200 rpm until the membrane completely peeled off from the flask wall and formed coarse liposome suspensions. To further homogenize the liposomes, a sonication procedure was carried out using an ultrasonic probe at 400 W for 5 minutes with 3-second interval. Finally, the resulting liposomes were incubated with HIF-1α siRNA for several minutes to obtain siRNA-fc-LPs with HIF-1α siRNA concentration of 1 μM. Additionally, HIF-1α siRNA-loaded conventional cationic liposomes (siRNA-cc-LPs) and blank fc-LPs (bk-fc-LPs) were prepared likewise.
Characterization of siRNA-fc-LPs
The particle size and ζ potential of siRNA-fc-LPs as well as bk-fc-LPs were determined by Malvern Zetasizer at 25°C based on dynamic light scattering and electrophoretic mobility principle, respectively. Briefly, liposomes were diluted appropriately with deionized water to appear translucent blue. After equilibrium for 120 seconds, the samples were subjected to laser diffraction or Doppler velocimetry for data collection. Z-average particle size and ζ potential were exported with the build-in software.
TEM was utilized to observe the morphology of bk-fc-LPs and siRNA-fc-LPs. The samples diluted 25 times were dropped on a carbon-coated copper grid and dried under vacuum. Then, the fixed nanoparticles were subjected to TEM inspection (Philips, Tecnai 10, NED) for the acquisition of micrographs at an acceleration voltage of 100 kV.
Electrophoresis assay for siRNA loading and protection
Agarose gel electrophoresis was performed to evaluate the siRNA encapsulation by liposomal vectors and their protective effect on siRNA degradation. The samples of naked siRNA and freshly prepared siRNA-fc-LPs in the absence or presence of RNase (0.1 mg/mL) were electrophoresed on agarose gel (1%) and visualized by ethidium bromide staining. In the case of stability test, the samples were incubated with RNase for 0.5 hours at 37°C prior to electrophoresis. To investigate the release of siRNA from vectors, siRNA-fc-LPs were treated with sodium heparin (10 mg/mL) for 2 hours at 37°C in an attempt to displace siRNA from the positively charged surface and then analyzed by electrophoresis.13
Hypoxic culture of human melanoma cells
Human melanoma cells (A375) were obtained from the American Type Culture Collection and cultured at 37°C in an atmosphere of 90% relative humidity and 5% CO2. The cells were maintained in T-75 flasks using Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotics. The culture medium was renewed every other day. The cells grew under normoxic condition for 48 hours to allow them to adhere. Afterward, the chemical anoxic agent CoCl2 was added to the cells to achieve a hypoxic induction with a concentration of 100 μM.24 The hypoxia-induced cells were subcultured in multi-well plates and subsequently used for various cell experiments.
In vitro transfection evaluation
To investigate the transfection efficacy of siRNA/liposome complexes, FITC-conjugated HIF-1α siRNA (FITC-siRNA) instead of HIF-1α siRNA were used to prepare fluorescence-labeled liposomal vectors as described earlier. The hypoxia-induced cells were seeded in six-well plates at a density of 1×105 cells/well and grew to 70%–80% confluence. After that, the culture media were removed and replaced with 500 μL of siRNA/liposome complexes (equal to 0.1 μM siRNA), which were diluted appropriately by serum-free media before transfection. After incubation for 1 hour, the incubation media were removed, and the cells were rinsed with phosphate-buffered saline (PBS; pH 7.4) for three times. As a control, the commercially available liposomal vectors Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) were adopted. All transfection experiments were performed in triplicate. The transfection efficacy, indicated as the mean fluorescence intensity (MFI), was assessed by flow cytometry. The MFI of A375 cells was recorded in the FL1 channel using a FACS Aria II flow cytometer (BD, San Jose, CA, USA).
Confocal laser scanning microscopy (CLSM) was used to visualize the internalization of siRNA vectors. The hypoxia-induced cells were grown on the circular slides for 24 hours and then incubated with siRNA/liposome complexes that were diluted in serum-free DMEM. The control group cells were treated with culture media alone. After transfection for 30 minutes, the cells were washed with PBS and observed with CLSM (Zeiss LSM510 Meta; Carl Zeiss AG, Oberkochen, Germany) at 488 nm excitation.
In vitro anti-melanoma activity
The anti-melanoma activity of siRNA/liposome complexes was evaluated in human MM cell line A375. Briefly, the hypoxia-induced cells were seeded in 96-well plates at a density of 5×105 cells/well. After growing for 24 hours, they were treated with bk-fc-LPs or three different concentrations of siRNA/liposome complexes (siRNA-cc-LPs and siRNA-fc-LPs) for another 24 and 48 hours, respectively. Cell viability was measured by MTT assay according to the literature reported previously.25
Western blot analysis for HIF-1α expression
The hypoxia-induced A375 cells were seeded in six-well plates at a density of 5×105 cells/well and cultured for 48 hours. Then, the cells were treated with 2 mL of preparations (bk-fc-LPs, siRNA-cc-LPs, and siRNA-fc-LPs), which were appropriately diluted by DMEM before use to reach an siRNA concentration of 1 μM. After incubation for 48 hours, whole-cell lysates were prepared by washing the cells with PBS and lysing them with radioimmunoprecipitation assay buffer for 30 minutes on ice. The lysates were centrifuged at 13,500 rpm for 10 minutes, and protein concentrations were determined by the bicinchoninic acid assay. Equal amounts of protein samples were loaded onto sodium dodecyl sulfate polyacrylamide electrophoresis gel. After electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes (Haoran Biotech, Shanghai, People’s Republic of China). The membrane was first blocked with 5% fat-free milk for 1 hour at room temperature and then incubated with primary antibodies against HIF-1α (1:1,000) overnight at 4°C. The signal of HIF-1α protein was detected by goat anti-rabbit IgG-HRP (1:5,000). Band intensities were measured by densitometry using the Quantity One software with GAPDH as a control.
Apoptosis detection
To evaluate the effect of liposomal vectors on cell apoptosis, the hypoxia-induced A375 cells were continuously cultured for 24 hours in the presence of siRNA-fc-LPs or the control preparations. The cells were rinsed with PBS for three times, trypsinized with 0.25% trypsin–ethylenediaminetetraacetic acid solution, and centrifuged for 5 minutes at 1,000 rpm. The collected cell pellets were resuspended in PBS ready for apoptosis. Apoptosis was measured by flow cytometry after staining with Annexin V-FITC and propidium iodide following the instruction of apoptosis detection kit (Keygen Biotech, Nanjing, People’s Republic of China). The cells treated with bk-fc-LPs and siRNA/Lipofectamine™ 2000 were designed as the negative and positive controls, respectively.
Results and discussion
Synthesis and identification of oligochitosan derivatives
Oligochitosans were produced through depolymerizing the macromolecular chitosans under the action of hydrogen peroxide. To revert the amino groups, the residual inorganic acids in the system were removed by dialysis against basic aqueous solution. The resultant oligochitosans possessed an average molecular weight of 1,150±116 based on static light scattering using the Debye plot, and they were completely soluble in water. The product yield was 73.1%, showing the convenience and feasibility of fabricating technique adopted.
The water-soluble oligochitosans were further used to synthesize functional folate/oleic acid-diacylated chitosan derivatives. Figure 1 depicts the synthetic route of such modifier that was used to prepare cationic liposomes. The repeated unit of obtained oligochitosan was ~7, counted based on the molecular weight. The number of overall amino groups was two times more than that of total carboxyl groups, which allowed a portion of amino groups of oligochitosan to be retained for siRNA complexation. The product structure was verified by 1H NMR (Figure 2). The 1H NMR spectrum of product clearly showed the signals of folate (δ 3.35, 6.62, and 7.67), oleic acid (δ 1.29, 2.38, and 5.33), and chitosan (δ 3.39, 4.02, and 5.03), indicating that folate and oleic acid have been successfully linked to oligochitosans. In addition, the system presented a translucent micellar suspension after the reaction solution was fully dialyzed by a large quantity of water. The particle size of the system was determined to be ~173 nm based on dynamic light scattering technique. This formation of micelles, generally self-assembled from amphipathic materials in aqueous media, further provided evidence of the successful synthesis of diacylated oligochitosan derivatives.
Preparation and characterization of siRNA-fc-LPs
In order to best preserve the activity of HIF-1α siRNA, siRNA-fc-LPs were fabricated through two steps, where blank liposomes were firstly prepared, and then followed by incubation with siRNA solution to achieve siRNA loading. The mean particle sizes of bk-fc-LPs and siRNA-fc-LPs were 104.1 and 95.3 nm, respectively, with polydispersity <0.3 (Figure 3A). The particle size of siRNA-fc-LPs was a little smaller than that of blank liposomes, which was possibly attributed to the siRNA condensation toward the liposomes. The ζ potential of liposomes containing no oligochitosan derivatives was determined to be −1.62 mV. However, bk-fc-LPs possessed a ζ potential of 42.6 mV, showing a positively charged surface. It was suggestive that incorporation of oligochitosan derivatives into the liposomes changed the interfacial potential of liposomes. After coupling with HIF-1α siRNA, the ζ potential of liposomes decreased apparently, as reflected by the ζ potential of siRNA-fc-LPs that was 2.41 mV. The differences in interfacial potential of liposomes demonstrated the complexation of siRNA with cationic liposomes to be successful, to a certain extent. Generally, nanoparticles with absolute ζ potential >30 mV are considered stable. However, for shell–core-type nanoparticle, the hydrophilic shell or corona also plays an important role in particle stability, for instance, charged less or uncharged PEGylated nanoparticles. In our case, the oligochitosan derivative and soluble siRNA formed a hydrophilic shell outside the liposomes, which allowed siRNA/liposome complexes to be stable.
The resulting bk-fc-LPs and siRNA-fc-LPs were nearly spherical, as revealed by TEM (Figure 3B). The complexing of siRNA somehow affected the morphology of liposomes, where the roundness of liposomes appeared to reduce. This was likely related to the siRNA squeezing that interrupted the membrane flexibility of liposomes.
Figure 4 shows the electrophoresis phenomena of naked HIF-1α siRNA and its liposome complexes with different treatments. It could be seen that HIF-1α siRNA has been effectively loaded onto the cationic liposomes. There was no evident electrophoresis shift for siRNA-fc-LPs (lane 3) observable, and the staining signals were basically retained in the upper position. The negatively charged sodium heparin also failed to release siRNA from siRNA/liposome complexes, suggesting a firm binding occurrence between HIF-1α and cationic liposomes. After treatment with RNase, a distinct electrophoresis outcome was presented compared with the untreated HIF-1α siRNA, apart from siRNA-fc-LPs. The cleaved fragments of HIF-1α siRNA were faintly visualized from the electrophoresis band (lane 5). Nevertheless, HIF-1α siRNA, as loaded onto the liposomal vectors, was less affected by the treatment of RNase as revealed by the electrophoresis of siRNA-fc-LPs (lane 6). These results demonstrate that the engineered vectors are provided with excellent capacities in siRNA loading and protection from degradation before uptake by the target cells.
In vitro transfection efficacy
The transfection efficiency of HIF-1α siRNA mediated by liposomal vectors was assessed by the count of positively transfected cells and the value of MFI. Figure 5 exhibits the histograms of cell transfection and the MFI of cells following treatment with various siRNA/liposome complexes. The transfection efficiency of siRNA was significantly enhanced through liposomal vectors relative to the control (Figure 5A). FITC-siRNA-loaded conventional cationic liposomes (FITC-siRNA-cc-LPs) increased the positive cell count close to an order of magnitude. However, more conspicuous transfection was produced by FITC-siRNA-loaded fc-LPs (FITC-siRNA-fc-LPs) and FITC-siRNA/Lipofectamine™ 2000, where the rates of positive events of two vectors were up to 87.3% and 85.4%, respectively. Figure 5B presents a quantitative comparison of the MFI of cells treated with FITC-siRNA-cc-LPs, FITC-siRNA-fc-LPs, and FITC-siRNA/Lipofectamine™ 2000. Apparently, the MFI of FITC-siRNA-fc-LPs group was significantly higher than that of FITC-siRNA-cc-LPs group (paired t-test, P<0.01). It was noted that there was no significant difference between fc-LPs and the positive control Lipofectamine™ 2000 in terms of MFI, showing an equivalent transfection efficiency.
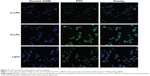
The internalization level of siRNA following the in vitro transfection was evaluated using CLSM (Zeiss LSM510 Meta; Carl Zeiss AG). As shown in Figure 6, the cell nuclei were clearly stained blue by Hoechst 33258, while the cells were transfected to different extent as they were treated with different transfection media. The fluorescence intensity of FITC-siRNA-cc-LPs group was weakest among three liposomal vectors containing FITC-siRNA. The group of FITC-siRNA-fc-LPs displayed a more intense green fluorescence than that of FITC-siRNA-cc-LPs, showing that fc-LPs possessed stronger transfection ability. Compared with Lipofectamine™ 2000, the fluorescence intensity of FITC-siRNA-fc-LPs group was almost parallel, and even more intensive. As known, Lipofectamine™ 2000 contain lipid subunits that can form liposomes in an aqueous environment, composed of cationic phospholipids (dioleoylpropyl trimethyl ammonium chloride/dioleoylphosphatidylethanolamine =1/1), whose transfection efficacy for gene therapeutics has been well proved.26,27 The transfection ability of fc-LPs was not inferior to that of Lipofectamine™ 2000 at all. Besides the low toxicity and cost, the high transfection efficacy rendered fc-LPs rather promising as nonviral vectors for HIF-1α siRNA delivery. They can effectively internalize siRNA by the folate receptor-mediated transport,28 since the overexpression of folate receptor on MM cells has been confirmed.29,30
In vitro anti-melanoma activity
The antitumor activity of siRNA/liposome complexes was characterized by their cytotoxicity that was performed on human MM cells (A375) based on MTT assay. Figure 7 exhibits the cytotoxic profiles of siRNA/liposome complexes on A375 cells with different siRNA concentrations as well as the negative control of bk-fc-LPs. To investigate the effect of handling time on the cell viability, the cells were respectively treated for 24 and 48 hours. Overall, the cell viability decreased as a function of siRNA concentration and treatment time. The cells treated with bk-fc-LPs maintained a survival rate of ~90%, even though subjected to a high carrier concentration for 48 hours, demonstrating a low cytotoxicity of liposomal vector itself. A small quantity of dead cells were probably drawn by the natural apoptosis. Relative to bk-fc-LPs, siRNA-cc-LPs produced a moderate cell killing effect, resulting in 25.4% and 32.1% cell death at 24 and 48 hours, respectively. This could be attributed to the induction of HIF-1α siRNA that was uptaken by cells via siRNA-cc-LPs. Although no folate ligand was involved, the conventional cationic liposomes improved the anti-melanoma activity of HIF-1α siRNA, manifesting the chitosan-modified liposomes effective for siRNA delivery. In comparison with siRNA-cc-LPs, siRNA-fc-LPs induced considerable death of A375 cells, especially at 0.2 μM siRNA level. After treatment for 48 hours, the cell viability was merely preserved to 44.8%. The results show that siRNA-fc-LPs are more effective than siRNA-cc-LPs in causing death of hypoxia-induced MM cells.
The anoxic cells are greatly dependent on the upregulation of HIF-1α for growth and differentiation.31 HIF-1α serves as a pivotal mediator of hypoxia that mediates the metabolic switch from oxidative phosphorylation to anaerobic glycolysis. Owing to HIF-1α siRNA interference, the first step of protein synthesis of HIF-1α was cut off, leading to the decline in production of downstream effectors, such as glycolysis enzymes and various protein kinases, for proliferation.32 As a result, the cells under anoxic condition went toward the prompt death. RNA interference is an attractive tool to surmount diseases that are difficultly treated through regular remedy, though the delivery of siRNA is always challenging because of easy siRNA degradation and low delivery efficacy. Liu et al developed micellar nanoparticles to deliver siRNA with an activity of HIF-1α silencing, and they found that the hypoxic tumor growth could be effectively inhibited in a prostate cancer model.33 Folate-grafted polymeric nanoparticles have also been explored to systemically deliver HIF-1α siRNA for targeting to ovarian cancer. Enhanced in vitro/in vivo stability and tumor targeting of siRNA were achieved by such vectors.34 In the present study, the anti-melanoma activity of HIF-1α siRNA/liposomal complexes was first confirmed, especially the high activity of folate-decorated liposomal vectors.
Effect of siRNA-fc-LPs on HIF-1α expression
To appraise the potency of HIF-1α siRNA alone and its liposome complexes in blocking HIF-1α expression, the protein level of HIF-1α in hypoxia-induced A375 cells was analyzed by Western blot after siRNA interference. As shown in Figure 8, in contrast with the culture medium or bk-fc-LPs, siRNA-cc-LPs or siRNA-fc-LPs significantly downregulated the expression of HIF-1α protein that was normalized to the GAPDH control. However, a more evident difference in HIF-1α expression was observed between siRNA-cc-LPs and siRNA-fc-LPs. The protein level of HIF-1α in siRNA-fc-LPs group was far lower than that of siRNA-cc-LPs group. It was noted that the HIF-1α-associated protein was almost undetectable in siRNA-fc-LPs-treated cells, simultaneously accompanying reduced GAPDH protein. As known, GAPDH is an enzyme that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy.35 When the cells are in hypoxic condition, oxidative stress, or glucose metabolic disturbance, the expression and activity of GAPDH will decline. The decline of GAPDH as a loading control further proved the high activity of siRNA-fc-LPs against MM. In addition, there was a corroborative evidence that the amount of living cells significantly decreased in the group treated with siRNA-fc-LPs. These results indicate that siRNA-fc-LPs can more effectively inhibit the expression of HIF-1α of MM cells, and hence the growth of tumor.
Apoptosis assay
The effects of siRNA/liposome complexes on apoptosis of the hypoxia-induced A375 cells are shown in Figure 9. Less apoptosis took place in the control group (blank liposomes) as observed from the quadrants of 2 and 4, which signify the later and early apoptotic cells, respectively. This provides evidence that the devised liposomal vector itself is of low toxicity depending on the use of highly biocompatible materials, such as chitosan and lecithin. However, considerable apoptotic cells (Q2) were observable in the groups of siRNA/liposome complexes, especially in the group of siRNA-fc-LPs as a result of the induction of HIF-1α siRNA. It was indicative that fc-LPs have effectively mediated HIF-1α siRNA into the cytoplasm, thereby causing the apoptosis of hypoxia-induced cells. The apoptosis rate of siRNA-fc-LPs group (37.8%) was somewhat higher than that of Lipofectamine™ 2000 (33.6%). In the case of our study, it may need longer time to induce an in-depth apoptosis. Nevertheless, these results have demonstrated that fc-LPs have an excellent intracellular siRNA delivery efficacy as well as low toxicity.
Conclusion
In this study, fc-LPs were developed, and their potential as economic nonviral vectors of HIF-1α siRNA for MM treatment was preliminarily evaluated. The designed liposomal vectors exhibited excellent siRNA loading and protection from degradation properties. In comparison with siRNA alone or conventional liposomal vectors, enhanced anti-melanoma activity of HIF-1α siRNA was achieved through fc-LPs. The potentialization of antitumor activity can be ascribable to the enhanced transfection of siRNA mediated by fc-LPs. Western blot assay and apoptosis test lent a strong support that liposomal vectors targeting to folate receptor were more competent in siRNA delivery than conventional ones. Our findings provided new insights into the use of HIF-1α siRNA, by delivering it with nontoxic cationic liposomes, to achieve the therapy enhancement of MM via RNA intervention.
Acknowledgment
This work was supported by the Research Program of Shanghai Municipal Health and Family Planning Commission (201440025).
Disclosure
The authors report no conflicts of interest in this work.
References
Gordon R. Skin cancer: an overview of epidemiology and risk factors. Semin Oncol Nurs. 2013;29(3):160–169. | ||
Viola JR, Rafael DF, Wagner E, Besch R, Ogris M. Gene therapy for advanced melanoma: selective targeting and therapeutic nucleic acids. J Drug Deliv. 2013;2013:897348. | ||
Formenti F, Constantin-Teodosiu D, Emmanuel Y, et al. Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2010;107(28):12722–12727. | ||
Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. | ||
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. | ||
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. | ||
Nakazawa Y, Kawano S, Matsui J, et al. Multitargeting strategy using lenvatinib and golvatinib: maximizing anti-angiogenesis activity in a preclinical cancer model. Cancer Sci. 2015;106(2):201–207. | ||
Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev. 2015;29(87):108–119. | ||
Hanna SC, Krishnan B, Bailey ST, et al. HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J Clin Invest. 2013;123(5):2078–2093. | ||
Kuphal S, Winklmeier A, Warnecke C, Bosserhoff AK. Constitutive HIF-1 activity in malignant melanoma. Eur J Cancer. 2010;46(6):1159–1169. | ||
Boe SL, Jorgensen JA, Longva AS, Lavelle T, Saeboe-Larssen S, Hovig E. Light-controlled modulation of gene expression using polyamidoamine formulations. Nucleic Acid Ther. 2013;23(2):160–165. | ||
Islam MA, Park TE, Singh B, et al. Major degradable polycations as carriers for DNA and siRNA. J Control Release. 2014;193:74–89. | ||
Liu Y, He X, Kuang Y, et al. A bacteria deriving peptide modified dendrigraft poly-l-lysines (DGL) self-assembling nanoplatform for targeted gene delivery. Mol Pharm. 2014;11(10):3330–3341. | ||
Asayama S, Kumagai T, Kawakami H. Synthesis and characterization of methylated poly(L-histidine) to control the stability of its siRNA polyion complexes for RNAi. Bioconjug Chem. 2012;23(7):1437–1442. | ||
Plianwong S, Opanasopit P, Ngawhirunpat T, Rojanarata T. Chitosan combined with poly-L-arginine as efficient, safe, and serum-insensitive vehicle with RNase protection ability for siRNA delivery. Biomed Res Int. 2013;2013:574136. | ||
Liu Y, Tao J, Li Y, et al. Targeting hypoxia-inducible factor-1alpha with Tf-PEI-shRNA complex via transferrin receptor-mediated endocytosis inhibits melanoma growth. Mol Ther. 2009;17(2):269–277. | ||
Safinya CR, Ewert KK, Majzoub RN, Leal C. Cationic liposome-nucleic acid complexes for gene delivery and gene silencing. New J Chem. 2014;38(11):5164–5172. | ||
Hattori Y, Nakamura A, Arai S, Kawano K, Maitani Y, Yonemochi E. siRNA delivery to lung-metastasized tumor by systemic injection with cationic liposomes. J Liposome Res. 2014;25(4):279–286. | ||
Pirollo KF, Zon G, Rait A, et al. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17(1):117–124. | ||
Wang B, Zhang S, Cui S, et al. Chitosan enhanced gene delivery of cationic liposome via non-covalent conjugation. Biotechnol Lett. 2012;34(1):19–28. | ||
Xia Z, Wu S, Chen J. Preparation of water soluble chitosan by hydrolysis using hydrogen peroxide. Int J Biol Macromol. 2013;59:242–245. | ||
Puskas I, Szemjonov A, Fenyvesi E, Malanga M, Szente L. Aspects of determining the molecular weight of cyclodextrin polymers and oligomers by static light scattering. Carbohydr Polym. 2013;94(1): 124–128. | ||
Zhang J, Chen XG, Huang L, Han JT, Zhang XF. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: biocompatibility, protein adsorption and cellular uptake. J Mater Sci Mater Med. 2012;23(7):1775–1783. | ||
Zhong X, Lin R, Li Z, Mao J, Chen L. Effects of Salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells. Biol Pharm Bull. 2014;37(7):1199–1206. | ||
Zhang X, Zhang T, Ye Y, et al. Phospholipid-stabilized mesoporous carbon nanospheres as versatile carriers for systemic delivery of amphiphobic SNX-2112 (a Hsp90 inhibitor) with enhanced antitumor effect. Eur J Pharm Biopharm. 2015;94:30–41. | ||
Han NR, Lee H, Baek S, Yun JI, Park KH, Lee ST. Delivery of episomal vectors into primary cells by means of commercial transfection reagents. Biochem Biophys Res Commun. 2015;461(2):348–353. | ||
Martin TM, Plautz SA, Pannier AK. Temporal endogenous gene expression profiles in response to lipid-mediated transfection. J Gene Med. 2015;17(1–2):14–32. | ||
Li H, Miteva M, Kirkbride KC, et al. Dual MMP7-proximity-activated and folate receptor-targeted nanoparticles for siRNA delivery. Biomacromolecules. 2015;16(1):192–201. | ||
Sanchez-del-Campo L, Montenegro MF, Cabezas-Herrera J, Rodriguez-Lopez JN. The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell Melanoma Res. 2009;22(5):588–600. | ||
Motoyama K, Onodera R, Tanaka N, et al. Evaluation of antitumor effects of folate-conjugated methyl-beta-cyclodextrin in melanoma. Biol Pharm Bull. 2015;38(3):374–379. | ||
Nutsch K, Hsieh C. When T cells run out of breath: the HIF-1α story. Cell. 2011;146(5):673–674. | ||
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. | ||
Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm. 2012;9(10):2863–2874. | ||
Li TS, Yawata T, Honke K. Efficient siRNA delivery and tumor accumulation mediated by ionically cross-linked folic acid-poly(ethylene glycol)-chitosan oligosaccharide lactate nanoparticles: for the potential targeted ovarian cancer gene therapy. Eur J Pharm Sci. 2014;52:48–61. | ||
Zala D, Hinckelmann MV, Yu H, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152(3):479–491. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.