Back to Journals » Journal of Asthma and Allergy » Volume 17
Inhibitory Effect of Apolipoprotein A-I on Eosinophils in Allergic Rhinitis in vitro and in vivo
Authors Zeng Y, Li J, Wen Y, Xiao H, Yang C, Zeng Q, Liu W
Received 24 November 2023
Accepted for publication 8 February 2024
Published 13 February 2024 Volume 2024:17 Pages 89—96
DOI https://doi.org/10.2147/JAA.S449948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Yinhui Zeng,1,* Jinyuan Li,1,* Yueqiang Wen,2,* Haiqing Xiao,1 Chao Yang,1 Qingxiang Zeng,1 Wenlong Liu1
1Department of Otolaryngology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou, 510623, People’s Republic of China; 2Department of Nephrology, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, 510260, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenlong Liu, Department of Otolaryngology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou, 510623, People’s Republic of China, Email [email protected]
Purpose: Eosinophils have pivotal roles in the development of allergic rhinitis (AR) through the release of cytotoxic substances. Apolipoprotein A-I (Apo-AI) exhibits a strong inhibitory effect on eosinophil infiltration in allergic diseases. Nevertheless, the precise impact of Apolipoprotein A-I on eosinophils remains uncertain.
Methods: Our study recruited a total of 15 AR children and 15 controls. The correlation between Apo-AI expression and the counts of blood eosinophils was examined. Flow cytometry was employed to assess the role of Apo-AI in eosinophil apoptosis and adhesion. The Transwell system was performed to conduct the migration assay. An animal model using AR mice was established to test the effect of Apo-AI on eosinophils.
Results: Serum Apo-AI were negatively related to eosinophils counts and eosinophil chemotactic protein levels in AR. Apo-AI exerts a pro-apoptotic effect while also impeding the processes of adhesion, migration, and activation of eosinophils. The apoptosis triggered by Apo-AI was facilitated through the phosphoinositide 3-kinase (PI3K) pathway. The chemotaxis and activation of eosinophils, which are influenced by Apolipoprotein A-I, are regulated through the PI3K and MAPK signaling pathways. Apo-AI treated mice presented with decreased blood and nasal eosinophilic inflammation as well as down-regulated eosinophil related cytokines.
Conclusion: Our findings provide confirmation that Apo-AI exhibits inhibitory effects on the function of eosinophils in allergic rhinitis. This suggests that Apo-AI holds potential as a therapeutic target for future treatment strategies.
Keywords: apolipoprotein A-I, eosinophils, allergic rhinitis, apoptosis, adhesion, migration, activation
Introduction
Allergic rhinitis (AR) is a worldwide prevalent condition, with a prevalence rate exceeding 10% of the population.1 Eosinophils are known to have significant involvement in the pathophysiological mechanisms of AR through the secretion of T helper 2 (Th2) cytokines, which in turn produce type 2 inflammation.2 Besides, various toxic substance can be released by eosinophils, such as major basic proteins, etc. These proteins have the ability to promote tissue damage and amplify allergic inflammation.3 Eotaxins, secreted by epithelium after Th2 cytokines stimulation, recruit and activate eosinophils.4
Apolipoprotein A-I (Apo-AI), a prominent constituent of high-density lipoproteins, contributes to pathogenesis of atherosclerosis in coronary heart disease.5 Moreover, Apo-AI has anti-inflammatory effects by inhibiting dendritic cell maturation, suppress T cell activation, reducing cytokine production by macrophage.6–8 It is reported that Apo-AI protects the lung by decreasing eosinophil counts in the OVA-sensitized mice after 4F treatments.9 Genetic removal of Apo-AI can enhance pulmonary inflammation and airway hyperresponsiveness.10 However, the direct role of Apo-AI in eosinophilic inflammation was not fully understood. The primary aims of our research were to explore the effect of Apo-AI on the function of eosinophils using both in vitro and in vivo experimentation.
Methods and Methods
Patients
A total of 15 patients with persistent AR as defined by ARIA criteria and 15 healthy subjects were enrolled from our center.11 All AR patients fulfilled the following criteria: at least 2 years’ disease history, positive skin prick test or specific IgE values to at least one type of common aeroallergens. All healthy subjects have no nasal diseases and positive results to common aeroallergens. All participants included in the study did not have a history of asthma (excluded by spirometric measurements), recent respiratory system infection, or usage of anti-allergic medications, systemic or topical corticosteroids in one month before visits.
Eosinophil Culture
Peripheral blood (20 mL, 1×108/mL) was collected into sterile vacutainers. Subsequently, 50 mL of PBS was added to the whole blood and carefully mixed. After the blood was spread out on Ficoll-Paque (GE Healthcare, Finland), it was centrifuged for 30 minutes at room temperature at 400 × g force. After removing the supernatant, the bottom layer, which contained the erythrocyte and granulocyte fraction of the cells, was collected. The next step was lysis of erythrocytes at a low pH. Half of the amount of sterile water was added to the tubes containing cells, and they were gently mixed for a maximum of 10 seconds. Afterward, the mixture was supplemented with PBS, and it was centrifuged for 10 minutes at 300 × g.
Eosinophils were isolated from peripheral blood by magnetic eosinophil isolation Kit (Miltenyi Biotec, USA). Following the lysis of erythrocytes, eosinophils (1×106/mL) were resuspended in RPMI 1640 media. Giemsa and trypan blue staining were used to confirm the purity and viability of eosinophils (98–100%).
Functional Assay of Eosinophils
Eosinophils were subjected to incubation with fluorescein isothiocyanate-labeled annexin V and propidium iodide for the purpose of staining (Sigma-Aldrich, USA). The stained eosinophils were then analyzed using flow cytometry (BD, USA). The presence of apoptotic cells was verified by determining the ratio of total cells that presented a positive fluorescence signal for FITC and a negative signal for PI.
For adhesion assay, eosinophils were stained by FITC-conjugated cluster of differentiation (CD18), intercellular cell adhesion molecule (ICAM-1), ICAM-3 or IgG1 isotype (eBioscience, USA). The staining process was carried out for 30 minutes at 4°C in dark environment. The confirmation of staining was performed using flow cytometry.
For migration assay, a concentration of eosinophils at 105/mL was introduced into the top chamber of the Transwell system. Apo-AI (R&D systems, 1 and 100 μg/mL) or eotaxin (positive control, 10 ng/mL) was subsequently added to the bottom chamber. After a duration of 30 minutes, the cells that had migrated into the bottom chamber were quantified by counting them in 20 fields using optical microscopy. The ECP release from eosinophils were examined by ELISA kits (Cusabio, China). The MAPK inhibitor (LY294002, 100 μg/mL) and PI3K inhibitor (SB203580, 100 μg/mL) were purchased from R&D systems.
Animal Models
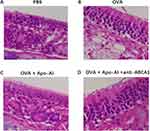
Four-week-old male C57BL6/J mice of wild-type phenotype were acquired and provided with a regular diet. The mice were sensitized by 20 μg ovalbumin (OVA) (Sigma-Aldrich) and 2 mg Al(OH)3 in 0.2mL PBS through intraperitoneal injection on day 1, 8 and 14. From day 14 to 18, the mice were subjected to a daily administration of OVA (40mg/mL) or PBS (control group) through intranasal instillation. After the last provocation, the mice were anaesthetized and sacrificed. The nasal symptoms were recorded for 15-minutes. The 100 μg of recombinant mouse Apo-AI or anti-ABCA1 in 40 mL of PBS was given intranasally 2 hours before each OVA challenge in some groups. Nasal tissues slice (4- to 5-μm) were stained by haematoxylin–eosin (HE) to evaluated the general morphology.
Animals were fed in the SPF animal room. All animal studies were approved by the Animal Ethics Committee of Guangzhou Women and Children’s Medical Center and conducted according to the guidelines of the Animal Care and Use local Committee.
Rt-Pcr
To isolate total RNA, nasal turbinate tissues were chopped into 1-mm pieces. The mirVana kit (Ambion) was used for total RNA isolation. The cDNA Reverse Transcription kit (Applied Biosystems, USA) was applied to reverse transcribe the RNA after it had been treated with 10 U of DNase I per 20 μg of RNA. The reactions were heated to 95°C for 10 minutes. Thereafter, there were 40 cycles of denaturation (10 s at 95°C) and annealing extension (60 s at 60°C). The specific mRNA relative expression was normalized to the expression of housekeeping gene. The primers were listed as follows: IL-5, sense, 5-TGCTTCTGCATTTGAGTTTG-3, 5-CAGCCTATTCATGGGACTTTG-3;CCL11, sense, 5-CCTAGGGCCTTGGTTTTCTT-3, antisense, 5-GAAGGGATTCCTGATTGCTG-3; CCL24, sense,5-ACATCATCCCTACGGGCTCT-3, antisense 5-TGTACCTCTGGACAGCCACA-3; GAPDH, sense, 5-GCTGCCCAGAACATCATCC-3, antisense 5-GTCAGATCCACGACGGACAC-3.
Ethics Approval and Consent to Participate
Ethical approval has been obtained from Ethics Committee of Guangzhou Women and Children’s Medical Center, in accordance with the Helsinki Declaration protocol (Research study number NO. [2020] 26901) to ensure that the research is conducted ethically and in compliance with internationally recognized standards. Informed consent was obtained from children’s legal guardians.
Statistical Analysis
The data were analyzed by GraphPad Prism 8.0 software. Two-way ANOVA and Bonferroni’s test were done to compare difference among multiple groups. A Spearman rank correlation analysis was done to evaluate the associations among different factors. A significance level of less than 0.05 was defined as statistically significant.
Results
Serum Apo-AI Levels and Its Relation with Blood Eosinophils in AR Subjects
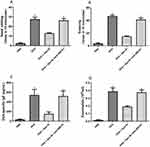
Table 1 displays the demographic information of the subjects and controls in the study. Serum Apo-AI protein level was significantly lower in AR subjects when compared to controls (Figure 1A, 123.3±8.27 vs 155±9.7 mg/mL, P<0.001). The levels of the Apo-AI protein were negatively correlated with the number of eosinophils (r=−0.53, P<0.001) and ECP levels (r=−0.64, P<0.001) in AR subjects (Figure 1B and C).
 |
Table 1 Demographic Characteristic of Allergic Rhinitis Children and Controls |
The Regulation of Eosinophils by Apo-AI
The apoptosis of eosinophils was promoted significantly by Apo-AI as shown in Figure 2A. Anti-ABCA1 and MAPK inhibitor (LY294002) alleviated the apoptosis of eosinophils mediated by Apo-AI, suggesting the key role of Apo-AI receptors and MAPK pathway in the apoptosis of eosinophils (Figure 2A). Apo-AI inhibited the expression of CD-18, ICAM-1 and ICAM-3, while anti-ABCA1 alleviated these effects (Figure 2B–D). Moreover, Apo-AI inhibited the chemotaxis and activation of eosinophils in a dose-dependent manner, while anti-ABCA1 and MAPK and PI3K inhibitor alleviated these effects (Figure 2E and F).
The Role of Apo-AI in Allergic Mice Model
The numbers of nasal rubbing and sneezing, the OVA-specific IgE, the number of blood eosinophils in Apo-AI treated AR mice were significantly lower compared to AR mice, while anti-ABCA1 alleviated these effects (Figure 3A–D). HE staining demonstrated a statistically significant reduction of eosinophils and lymphocytes and less epithelial hyperplasia in AR mice treated with Apo-AI compared to AR mice, while anti-ABCA1 reversed these effects (Figure 4). Our results also showed that the mRNA expression of IL-5, CCL11 and CCL24 in nasal turbinate tissue decreased significantly in AR mice treated with Apo-AI compared to AR mice, while anti-ABCA1 reversed these effects (Figure 5).
Discussion
Apo-AI, the major constituent of HDL, possesses widely recognized antiatherogenic characteristics that are facilitated through the process of cholesterol efflux from cells.12 Recently, the anti-inflammatory properties have been discovered by various groups. Tani et al found a noteworthy inverse relationship between Apo-AI levels with neutrophil, monocyte, eosinophil and total leukocyte counts.13 Barochia’s study reported a negative correlation between eosinophil and Apo-AI in nonasthmatics.14 Consistently, our data also presented negative correlation between Apo-AI and eosinophils number and activation marker (ECP).
To prove the direct role of Apo-AI on eosinophil, we treated eosinophil with Apo-AI of different concentration. The MAPK pathway was involved in eosinophils apoptosis, while PI3K and MAPK was involved in eosinophils chemotaxis and activation. Consistently, previous studies also reported that PI3K-Akt is correlated with the survival of eosinophils and MAPK is involved in chemotaxis and degranulation in eosinophils.15
In mice model, Apo-AI can inhibit the progression of asthma by suppressing eosinophil infiltration of lung via a reduction of IL-5.16 Apo-AI mimetic peptides can lower airway hyperresponsiveness (AHR), allergic inflammation, and mucous cell metaplasia in asthma models.17 The Apo-AI levels were also correlated with ECP levels during the early response. The numbers of BALF eosinophils, lymphocytes, and neutrophils were reduced significantly by Apo-AI mimetic peptide in mice model.18
Our data also showed Apo-AI decreased both blood and nasal eosinophils inflammation and activation in mice model. Despite the direct regulation of Apo-AI on eosinophils, our study also found that Apo-AI treated mice presented with down-regulated IL-5, CCL11 and CCL24. IL-5 is a well-known cytokine, which can stimulate eosinophil proliferation, differentiation, and activation. CCL11 (eotaxin-1) and CCL24 (eotaxin-2) are key chemotactic factors for eosinophils. Although the detailed mechanism was not clear, the downregulation of the above cytokines may inhibit eosinophils indirectly.
Our findings provide confirmation that Apo-AI exhibits inhibitory effects on the function of eosinophils in allergic rhinitis. This suggests that Apo-AI holds potential as a therapeutic target for future treatment strategies.
Acknowledgments
Yinhui Zeng, Jinyuan Li and Yueqiang Wen are co-first authors for this study. This study was supported by grants from the National Natural Science Grant of China (No.81970861, No.82271142), the Guangdong Province Natural Science Grant (No. 2021A1515010940), and the Science and Technology Program of Guangzhou (No. 202201020600, No.202201011844), Scientific Research Capacity Improvement Project of Guangzhou Medical University (02-410-2302151XM).
Disclosure
The authors declare that they have no relevant conflicts of interest in this work.
References
1. Ma T, Chen Y, Pang Y, et al. Prevalence and risk factors of allergic rhinitis and asthma in the southern edge of the plateau grassland region of northern China: a cross-sectional study. World Allergy Organ J. 2021;14(7):100537. doi:10.1016/j.waojou.2021.100537
2. Lee WWL, Puan KJ, Lee B, et al. Eosinophilic allergic rhinitis is strongly associated with the CD45RB(lo) subset of CD161(+) Th2 cells that secretes IL-2, IL-3, IL-4, IL-5, IL-9, and IL-13. Allergy. 2023;78(10):2794–2798. doi:10.1111/all.15846
3. Rasheed Z, Zedan K, Saif GB, et al. Markers of atopic dermatitis, allergic rhinitis and bronchial asthma in pediatric patients: correlation with filaggrin, eosinophil major basic protein and immunoglobulin E. Clin Mol Allergy. 2018;16(1). doi:10.1186/s12948-018-0102-y
4. Wang WW, Zhu K, Yu HW, Pan YL. Interleukin-17A potentiates interleukin-13-induced eotaxin-3 production by human nasal epithelial cells from patients with allergic rhinitis. Int Forum Allergy Rhinol. 2019;9(11):1327–1333. doi:10.1002/alr.22382
5. Mehta A, Shapiro MD. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol. 2022;19(3):168–179. doi:10.1038/s41569-021-00613-5
6. Kim KD, Lim HY, Lee HG, et al. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005;338(2):1126–1136. doi:10.1016/j.bbrc.2005.10.065
7. Huang H, Li Z, Huang J, et al. Apolipoprotein A1 modulates teff/treg balance through scavenger receptor class B type I-dependent mechanisms in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2022;63(8):23. doi:10.1167/iovs.63.8.23
8. Chen RX, Jiang W-J, Liu S-C, et al. Apolipoprotein A-1 protected hepatic ischaemia-reperfusion injury through suppressing macrophage pyroptosis via TLR4-NF-κB pathway. Liver Int. 2023;43(1):234–248. doi:10.1111/liv.15448
9. Nandedkar SD, Weihrauch D, Xu H, et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011;52(3):499–508. doi:10.1194/jlr.M012724
10. Wang W, Xu H, Shi Y, et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res. 2010;51(9):2560–2570. doi:10.1194/jlr.M004549
11. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
12. Rye KA. High density lipoprotein structure, function, and metabolism: a new thematic series. J Lipid Res. 2013;54(8):2031–2033. doi:10.1194/jlr.E041350
13. Tani S, Nagao K, Hirayama A. Association of systemic inflammation with the serum apolipoprotein A-1 level: a cross-sectional pilot study. J Cardiol. 2016;68(2):168–177. doi:10.1016/j.jjcc.2015.08.016
14. Barochia AV, Gordon EM, Kaler M, et al. High density lipoproteins and type 2 inflammatory biomarkers are negatively correlated in atopic asthmatics. J Lipid Res. 2017;58(8):1713–1721. doi:10.1194/jlr.P077776
15. Wong CK, Ng SS, Lun SW, Cao J, Lam CW. Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: implications for mast cell-eosinophil interaction in allergic inflammation. Immunology. 2009;126(4):579–587. doi:10.1111/j.1365-2567.2008.02916.x
16. Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging roles of apolipoprotein E and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am J Respir Cell Mol Biol. 2016;55(2):159–169. doi:10.1165/rcmb.2016-0060TR
17. Yao X, Vitek MP, Remaley AT, Levine SJ. Apolipoprotein mimetic peptides: a new approach for the treatment of asthma. Front Pharmacol. 2012;3:37. doi:10.3389/fphar.2012.00037
18. Yao X, Gordon EM, Barochia AV, Remaley AT, Levine SJ. The a’s have it: developing apolipoprotein A-I mimetic peptides into a novel treatment for asthma. Chest. 2016;150(2):283–288. doi:10.1016/j.chest.2016.05.035
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.