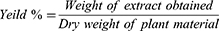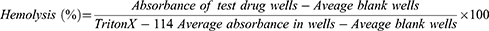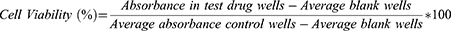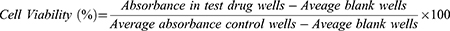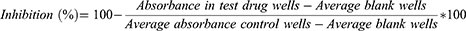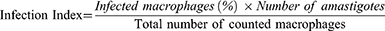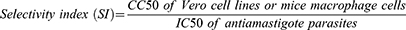Back to Journals » Journal of Experimental Pharmacology » Volume 15
In vitro Anti-Leishmanial Activities of Methanol Extract of Brucea antidysenterica J.F. Mill Seeds and Its Solvent Fractions
Authors Ketema T , Tadele M , Gebrie Z, Makonnen E , Hailu A, Abay SM
Received 1 December 2022
Accepted for publication 2 March 2023
Published 13 March 2023 Volume 2023:15 Pages 123—135
DOI https://doi.org/10.2147/JEP.S397352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tasisa Ketema,1 Markos Tadele,2 Zewdie Gebrie,1 Eyasu Makonnen,1,3 Asrat Hailu,3,4 Solomon M Abay1
1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 2Animal Health Research Program, Ethiopian Institute of Agricultural Research, Holetta, Ethiopia; 3Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT Africa), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 4Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Solomon M Abay, Department of Pharmacology and Clinical Pharmacy, Addis Ababa University, Zambia Street, P.O. Box 9086, Addis Ababa, Ethiopia, Tel +251 941 222169, Email [email protected]
Introduction: Leishmaniasis is one of the neglected tropical diseases, threatening lives of about 350 million people globally. Brucea antidysenterica seeds are used for the treatment of cutaneous leishmaniasis in the traditional medicine in Ethiopia.
Objective: This study aimed to evaluate Brucea antidysenterica seeds’ anti-leishmanial activity in vitro.
Methods: The crude (80% methanol) extract of Brucea antidysenterica seeds and its fractions were evaluated for their anti-leishmanial activities against promastigotes and intracellular amastigotes of Leishmania donovani and Leishmania aethiopica, and for their cytotoxic effects against mammalian cells. The quantitative estimations of total phenolic compounds (TPCs), flavonoids (TFCs) and alkaloids (TACs) were determined, spectrophotometrically. Median inhibitory concentration (IC50) and median cytotoxic concentration (CC50) of the extract and its solvent fractions were calculated using GraphPad Prism 9.1.0 computer software. Data was presented as mean ± standard error of the mean (SEM).
Results: The crude extract and its hexane, ethyl acetate and butanol fractions showed anti-leishmanial activities, with IC50 values of 4.14– 60.12 μg/mL against promastigotes, and 6.16– 40.12 μg/mL against amastigotes of both Leishmania species. They showed moderate cytotoxicity against Vero cell lines and peritoneal mice macrophages, with CC50 values of 100– 500 μg/mL, but > 1600 μg/mL against red blood cells. Selectivity indices ranged from 7.97 to 30.97. The crude extract, and its ethyl acetate and hexane fractions possessed 54.78– 127.72 mg of gallic acid equivalent TPC, 18.30– 79.21 mg of quercetin equivalent TFC, and 27.62– 97.22 mg of atropine equivalent TAC per gram of extracts.
Conclusion: The seeds of the plant possessed anti-leishmanial activities against L. aethiopica and L. donovani that might provide a scientific justification for its use in the treatment of leishmaniasis by traditional healers. Future works are recommended to isolate, purify and identify the possible secondary metabolites attributed to the anti-leishmanial activity.
Keywords: anti-leishmanial activity, Brucea antidysenterica seeds, Leishmania aethiopica, Leishmania donovani, promastigote, amastigote
Introduction
Over 28.96 million and 3.2 million people in Ethiopia inhabit areas with risk of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) infections, respectively.1,2 Ethiopia has the third largest number of VL cases per year in sub-Saharan Africa, next to South Sudan and Sudan.3 Therapeutic options for leishmaniasis are limited. It comprises amphotericin B,4 pentavalent antimonials,5 paromomycin,6 miltefosine7 and pentamidine8 – most of which have drawbacks in terms of variable efficacy and safety. Limited supply of the existing drugs in resource-limited settings, their high costs9 and the emergence of resistance10 are also other challenges in fighting leishmaniasis, which calls for looking for viable options.
Brucea antidysenterica J.F. Mill (genus: Brucea; family: Simaroubaceae) is one of the medicinal plants used in Ethiopian folk medicine for the treatment of leishmaniasis. It is an evergreen shrub 10–15 meter high, growing in the altitude range from 1400 to 2800 meters high.11 It is widely distributed in tropical African countries such as Nigeria, Ethiopia, Cameroon, Burundi, Sudan, Guinea, Congo, Angola, Zambia and Malawi.11 The plant got its name in honor of a Scottish traveler, James Bruce, who stayed in Ethiopia from 1769 to 1771.12
Ethnobotanical studies conducted in various parts of Ethiopia indicated that leaves, roots, bark, stems and seeds of B. antidysenterica J.F. Mill have been used for the treatment of numerous human medical disorders in folk medicine. For instance, the paste of leaf powder is applied topically to treat skin cancer,13 leprosy,14,15 eczema,16 scabies,16 and taken orally with water for the treatments of helminthiasis, anthrax, and malaria.17 The seeds of the plant are also used for the treatment of different diseases: the paste of seeds powder is used in wound healing and venereal diseases,18 and its powder is applied topically in the infected area for the treatment of cutaneous leishmaniasis.15 In addition to reports on the use of B. antidysenterica J.F. Mill in folk medicines, the in vitro and in vivo study indicated that the plant extract and its compound isolates exhibited activities against Mycobacterium tuberculosis,19 Entamoeba histolytica,20 Plasmodium berghei21 and against different strains of bacteria.22 The aim of the present study was to evaluate anti-leishmanial activity of 80% methanol extract of Brucea antidysenterica J. F. Mill seeds against L. donovani and L. aethiopica using in vitro models.
Materials and Methods
Leishmania Parasites Test Strains, Cell Lines and Laboratory Animals
Clinical isolates of L. aethiopica (CL-027/20) and L. donovani (VL-139/19) were obtained from Leishmaniasis Research and Diagnostic Laboratory (LRDL), Addis Ababa University. The strain of L. aethiopica (CL-027/20) was isolated from a 13-year-old male patient living in Guna Woreda, Arsi Zone, Oromia region, Ethiopia, while the strain of L. donovani (VL-139/19) was isolated from a 27-year-old male patient residing in Kolme Woreda, Konso Zone, Southern Nations, Nationalities, and People’s Region, Ethiopia. Red blood cells were collected from a 32-year-old health volunteer with no underlying chronic disease; Vero cell line (Vero ATCC CCL-81) was obtained from National Animal Health Diagnostic and Investigation Center, Sebeta, Ethiopia that was provided previously by the National Veterinary Institute (Bishoftu) previously; while Swiss Albino mice were obtained from Laboratory Animal House, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Science, Addis Ababa University, Ethiopia. Leishmania parasite test strains, cell lines and laboratory animals were used after getting ethical clearance from approved Ethics Review Board of the School of Pharmacy, as stated in the ethical clearance section of this paper.
Collection and Authentication of Plant Materials
The ripened seeds of Brucea antidysenterica J.F. Mill were collected from Dega Damot district 399 km away from capital city, Addis Ababa, in the month of January, 2020. The plant was identified and authenticated by Mr. Melaku Wondafrash, a botanist at the College of Natural and Computational Sciences, Addis Ababa University, Ethiopia. The voucher number TK-004 was given to B. antidysenterica J.F. Mill specimen and kept in Addis Ababa University National Herbarium for future reference.
Preparation of Plant Extract
The seeds of B. antidysenterica J.F. Mill were air-dried under shade for 3 weeks and grounded to coarse powder using mortar and pestle. Then, 400 gram of the powder was macerated with 1.2 liter of 80% methanol (1:3) in volumetric flask. The macerate was shaken occasionally to increase solvent penetration and solubility of bioactive agents. Post 72 h of maceration, the extract was filtered with muslin gauze, followed with Whatman No. 1 filter paper. By adding fresh solvents, the mark was re-macerated twice and filtered again to maximize the yield. The combined filtrates were concentrated using a rotary evaporator at 40°C, then frozen at −20°C overnight and dried using a lyophilizer.23 Finally, a gold-colored solid extract of B. antidysenterica J.F. Mill having sticky nature was obtained and its percentage yields were calculated from its dried mass using the following formula:
Preparation of Solvent Fractions
The dried 80% methanol extract was fractionated to n-hexane, ethyl acetate, n-butanol and aqueous fractions, respectively, based on increasing polarity of solvents according to a method described by Toma et al.24 The 80% methanol extract (16 gm) was transferred to a separatory funnel and dissolved in distilled water (150 mL). The aqueous solution of the extract was washed with 150 mL of n-hexane (3 times) followed by equal volumes of ethyl acetate (3 times) and n-butanol (4 times), respectively, until the extracting solvent became colorless. Fractions of organic solvents were concentrated using a rotary evaporator under reduced pressure at temperatures of 40°C and dried in an oven at temperatures of 30°C, while the aqueous fraction was dried using freeze dryer. The dried crude extract and fractions were weighed and stored in freezer until used for the procedure.
The yield of 80% methanol extract of B. antidysenterica J.F. Mill was 32.68 gm (8.17%). The yield of n-hexane, ethyl acetate, butanol and aqueous fractions of 80% methanol extract of B. antidysenterica were 1.38 gm, 3.12 gm, 4.37 gm and 5.82 gm, respectively.
Promastigote Cultures
The logarithmic stages of the clinical isolates of L. aethiopica and L. donovani, after being grown in Novy–MacNeal–Nicolle media, were seeded and grown in 25 mL tissue culture flasks containing M199 medium supplemented with 20% heat-inactivated fetal calf serum, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM L-glutamine and 100 IU/mL penicillin and 100 μg/mL streptomycin solution at 22°C for L. aethiopica and 26°C for L. donovani following the method described by Tariku et al25 with minor modification.
Vero Cell Line Culture
The Vero cells (the green African monkey kidney cell lines) were cultured in Roswell Park Memorial Institute (RPMI-1640) with L-glutamine and sodium bicarbonate medium supplemented with 10% heat inactivated newborn calf serum (HINBCS), 100U/mL penicillin, and 100μg/mL streptomycin in humidified 5% CO2 incubator at 37°C, as described by Ammerman and colleagues.26 The cells’ growth was monitored under inverted microscope and the media was changed every 3 days. The cells were sub-cultured when they reached >70% confluent monolayer.
Mice Peritoneal Macrophage Collection and Culture
The peritoneal macrophages were harvested from pathogen free, typically 6- to 8-week-old either sex of Swiss Albino mice according to the protocol previously described by Zhang et al,27 with minor modification. Each mouse was injected with 2 mL of 2% potato starch solution, intraperitoneally, to induce inflammatory response. Post 48 h of injection, each mouse was euthanized with chloroform, its abdomen was swabbed with 70% ethanol and the skin underlying the peritoneal cavity was opened. Then, 10 mL of sterile ice-cold phosphate buffered saline solution (PBS) supplemented with 3% HINBCS was injected into the peritoneal cavity of each animal, its abdomen was massaged for about 10–15 seconds and the macrophage cells were collected by drawing 6–8 mL of peritoneal exudates of PBS from each mouse. The peritoneal exudates were centrifuged at 450 × g (1500 rpm) for 10 min at 4 °C. The supernatant was discarded, and the resulting pellets were re-suspended in cold Minimum Essential Media (MEM) medium supplemented with 10% HINBCS, 25mM HEPES, 2mM L-glutamine and 100 U penicillin and 100 μg streptomycin/mL. The cells were counted using hemocytometer and adjusted to 3.5 × 106 cells/mL in complete MEM medium.
Hemolysis Assay
Hemolytic effect of 80% methanol extract of B. antidysenterica J.F. Mill and its solvent fractions were conducted using methods described by Abeje et al28 and Zohra et al.29 From a healthy volunteer person, about 4 mL of blood samples were collected using sterile syringe and transferred to a heparinized test tube. Two milliliter of the blood was added to 8 mL of PBS solution (PH 7.2) already transferred to 15 mL Eppendorf tube. The mixture was mixed well and centrifuged at 1000 × gm for 10 minutes. The supernatant was pipetted out using serological pipettes. The resulting pellet (1mL) was then transferred to 49 mL PBS solution (PH 7.2) to obtain 50 mL of 2% red blood cell suspension required for hemolytic tests.29 Two hundred microliter of the suspension was transferred to Eppendorf tubes containing 200 µL of serially diluted concentrations of extracts (1600–50 µg/mL) of standard drugs: amphotericin B (AMB) (40–1.25 µg/mL) and pentamidine (120–3.75 µg/mL) prepared in two fold dilutions with each test concentration in triplicates. PBS solution was used as a diluting medium for the preparation of serial dilutions. Triton X-114 (5 µL/mL) in PBS solution and 1% DMSO in PBS solution were used as positive and negative controls, respectively. The contents of Eppendorf tubes were mixed gently and incubated at 37°C in the incubator for 2 h, except for Triton X-114, which was incubated for 30 min. After incubation, the tubes were centrifuged at 1000 × g for 10 min. From each tube, 100 µL of supernatant was transferred to a separate well of a 96-well microplate. Finally, absorbance of the supernatant was measured at 540 nm using Victor Multilabel Reader.28 The hemolytic effect of each test substance was expressed as a percentage as per the following formula:29
Vero Cell Lines Cytotoxicity Assay
Vero cells at a density of 5 × 105 cells/mL were seeded in 96 microtiter plates. After incubation at 37°C in a 5% CO2 incubator for 24 h, 100μL of serially diluted concentration of extract (50–1600 μg/mL), standard drugs, AMB (1.25–40 µg/mL) and pentamidine (3.75–120 µg/mL) in complete RPMI-1640 medium were added into 96-well microtiter plates in triplicates. The microplate was then incubated at 37°C, 5% CO2 for 72 h. Post 68 h of incubation, 20 µL of 10% resazurin solution was added to each well and allowed to rest for 4 h. Finally, the viability of the cells was measured fluorometrically using multilabel plate reader at excitation and emission wavelengths of 544 nm and 590 nm, respectively, as described by Nigussie et al and Chan et al.30,31 Standard anti-leishmanial drugs and negative controls (medium alone and 1% DMSO) were used in this assay to provide reference values:
Mouse Macrophage Viability Assay
Cytotoxicity of each test substance against mouse peritoneal macrophage isolates was determined using methods described previously by Afrin et al,32 with minor modifications. Hundred microliter of mice peritoneal macrophages, suspended in a complete MEM medium (5 × 105 cells/mL), was seeded in 96-well microtiter plates. Serially diluted extracts (50–1600 μg/mL) and positive controls [AMB (1.25–40 μg/mL) and pentamidine (3.75–120 μg/mL)] and negative control (1% DMSO + Medium) in 100 µL of complete MEM medium were added to 96-well microtiter plates in triplicates and incubated in CO2 incubator for 72 h at 37°C, 5% CO2 and 95% humidity. After 68 h of incubation, 20µL of 10% resazurin solution (0.125 mg/mL) was added to each well and left for 4 h. Then, the fluorescence intensity of each well was measured using a Multilabel Reader at excitation and emission wavelengths of 544 nm and 590 nm, respectively.
The cytotoxic effects of test substances against mouse peritoneal macrophage isolates were expressed in terms of percentage of macrophage viability, which was determined using the following formula:
Anti-Promastigote Assay
The anti-promastigote activities of the extracts were ascertained using methods previously described by Tariku et al,25 with minor modifications. Serially diluted extract (400–6.25 μg/mL) contained in 100 μL of complete culture medium (M199 medium) was added to 96-well microtiter plates in triplicates. Then, 100 μL of logarithmic growth phase of parasite suspensions (3.00 × 106 promastigote cells/mL of L. aethiopica or L. donovani) were added to each well containing test substances. The contents of the microtiter plates were incubated for 72 h at 22°C (for L. aethiopica) and 26°C (for L. donovani). After 68 h of incubation, 20 μL of 10% fluorochrome resazurin solution (0.125 mg/mL, pH=7.2) was added to each well and allowed to rest for 4 h. Then, the fluorescence intensity was measured using a Multilabel Reader at an excitation wavelength of 544 nm and emission wavelength of 590 nm. Assays with standard anti-leishmanial drugs, AMB (10–0.156 μgm/mL), pentamidine (15–0.234 μg/mL) and medium with 1% DMSO were conducted as positive and negative controls, respectively.
The anti-promastigote activity of the extracts was expressed as a percentage of parasite growth inhibition, and it was determined using the following formula:33
Anti-Amastigote Assay
Intracellular anti-amastigote activity of the extracts and controls were assayed using methods previously described by Afrin et al32 and Utaile et al,34 with minor modifications. Mouse peritoneal macrophages suspended in a complete MEM medium (3 × 105 cells/mL, 200 µL) were seeded in 16-well chamber slides and incubated for 24 h at 37°C in a humidified 5% CO2 incubator. After 24 h, the chamber slides were washed with warm complete medium to remove non-adherent macrophages. The stationary stages of L. donovani and L. aethiopica promastigotes (parasite: macrophage: 10:1) were seeded in the chamber slides containing adherent macrophages and maintained at 31°C (L. aethiopica) or 37°C (L. donovani), 5% CO2 and 95% relative humidity for further 24 h. Then, non-internalized promastigotes were removed by extensive washing with warmed MEM medium and serially diluted concentrations of extracts (400–6.25 μg/mL) were added and incubated for a further 48 h in CO2 incubator. The culture medium was aspirated, and the slides were washed with PBS, fixated with methanol for 30 seconds and stained with Giemsa (10%) for 15 min. Finally, 50 macrophages per slide were counted and the number of amastigotes in each macrophage were enumerated. The assay was repeated with standard anti-leishmanial drugs: AMB (10–0.156 μgm/mL), pentamidine (15–0.234 μg/mL) and medium with 1% DMSO (negative standard). Serial concentrations of each test were prepared in two-fold dilutions with each test concentration in triplicates.
The anti-amastigote activity of the extracts per well was expressed as infection index, and it was calculated using the following formula:35
Selectivity Index
The selectivity index (SI) of each test substance was determined from its CC50 against mammalian cells (Vero cell line and mice macrophage) and their corresponding IC50 against Leishmania parasites (IC50 of amastigotes). The SI of the extracts and standards in killing parasites as opposed to mammalian cells was estimated using the following formula:31
Quantitative Determination of Total Phenolic Compounds, Flavonoids and Alkaloids
The total phenol, flavonoid and alkaloid contents of 80% methanol extract of the seeds of B. antidysenterica J.F. Mill and its ethyl acetate and hexane fractions were determined using UV-spectrophotometry as per the following protocols.
Determination of Total Phenolic Compounds Content
The total phenolic contents of 80% methanol extract of the plant and its fractions were determined using Folin–Ciocalteu’s method, as described by Nigatu et al.36 Serial concentrations of gallic acid (100–6.25 µL/mL) as a standard solution were prepared in methanol. From each aliquot of this solution, 1 mL was transferred into a separate 10mL tube. Five milliliters of methanol, 0.5 mL of Folin–Ciocalteu’s reagent and, 5 minutes later, 1.5 mL of 20% Na2CO3 were added into each Eppendorf tube. Each tube content was mixed gently, its final volume adjusted to 10 mL with methanol and incubated at room temperature. After 90 min incubation, the absorbance of each solution was measured at a wavelength of 760 nm using UV-Vis spectrophotometer. The solutions of the crude extract and its fractions at concentration of 100 µg/mL were prepared in methanol. One mL of each solution of 80% methanol extract, ethyl acetate fraction, hexane fraction and methanol (blank) were transferred to separate Eppendorf tubes. The total phenol estimates were expressed as mg of gallic acid equivalent per g of dry extract/fractions (mg of GAE/g).
Determination of Total Flavonoids Content
The total flavonoids content of the 80% methanol extract and its fractions were determined using a complex formed by aluminum chloride as per methods previously described by Nigatu et al.36 Serial concentrations of standard, quercetin solution, (1–0.065 mg/mL) were prepared in methanol to establish calibration curve. One mL of each aliquot of standard solution, followed by 0.3 mL of 5% NaNO2, was transferred to separate 15 mL tubes and left for 5 min. Another 0.3 mL of 10% AlCl3 was added to the contents, mixed with the solution and left to stand for 5 min once again. Then, 2 mL of 1M NaOH solution was added, and the final concentration of the complex was adjusted to 10 mL with distilled water. Each complex was incubated for 30 min at room temperature. Finally, the absorbance of the complex was measured at a wavelength of 510 nm using UV-Vis spectrophotometer. The crude extract and its fractions were also dissolved in methanol to obtain 1 mg/mL. Then, 1 mL of each solution of 80% methanol extract, its fractions and methanol (blank) were transferred into separate Eppendorf tubes and the same procedure was followed as standard solution. Serial concentrations of solution were prepared in two-fold dilutions, and all tests were performed in triplicate. The total flavonoid estimates were expressed as mg of quercetin equivalent per g of dry extract/fractions (mg of QE/g).
Determinations of Total Alkaloid
Total alkaloid content of the 80% methanol extract and its fractions were determined, using the method previously described by Ajanal et al.37 Two mL solutions of each 80% methanol extract and its fractions (1 mg/mL) in methanol were mixed with 2 mL of 2N HCl solution in separate 50 mL tubes and filtered. One mL of the filtrate was transferred to a separatory funnel and washed with 5 mL of chloroform 2 times. The chloroform extract was discarded, and the pH of the rest of the solution was adjusted to neutral with 0.1 M NaOH solution. To the neutralized solution, 5 mL of bromocresol green solution and 5 mL of buffer solution (PH, 4.7) were added and shaken. The complex was extracted with 4 mL of chloroform, and repeated once with 4 mL of chloroform upon vigorous shaking. The extract was collected in separate 50 mL tubes and its final volume was adjusted to 10 mL with chloroform. The absorbance of the chloroform extract was measured at a wavelength of 470 nm using UV‑spectrophotometer.
The average absorbance of blank solution was subtracted from standard and crude extract/fraction solutions and the total alkaloid content was determined using standard curve of Atropine. The total alkaloidal estimates were expressed as mg of atropine equivalent per g of dry extract/fractions (mg of AE/g).
Statistical Analysis
Anti-promastigote activity (IC50) and anti-amastigote activity (IC50) were calculated from sigmoidal dose–response curves of percentage inhibition and infection index, respectively. The cytotoxic effect (CC50) of the extract against mammalian cells was calculated from sigmoidal dose–response curves of percentage viability of mammalian cells. Data was presented as mean ± standard error of the mean (SEM). The total content of each secondary metabolite in the crude extract and its fractions was calculated using a calibration curve of absorbance of standards. GraphPad Prism 9.1.0 computer software (GraphPad Software, Inc., CA, USA) was used to estimate IC50 and CC50. Selectivity index (SI) was also obtained from the ratio between CC50 to IC50.
Ethical Clearance
All procedures conducted in this research work were reviewed and approved by Ethics Review Board of the School of Pharmacy, College of Health Sciences, Addis Ababa University, with a letter number ERB/SOP/177/13/2020 dated September 2020. This Ethics Review Board reviews basic research involving experimental animals and clinical research involving patients and human volunteers. Informed consent was obtained from the patients to use the parasite isolates and from the volunteer human to use the collected red blood cells for research purposes. Experimental animal rearing and handling were in compliance with the European Directive 2010/63/UE.
Results
Anti-Leishmanial Activity
The 80% methanol extract of B. antidysenterica J.F. Mill seeds and its solvent fractions showed activities against clinical isolates of promastigotes and intracellular amastigotes of L. aethiopica and L. donovani with varying IC50. The ethyl acetate fraction revealed the highest anti-leishmanial activity, with 4.14 ± 0.62 ≤ IC50 ≤ 6.85 ± 1.46 µg/mL against promastigotes and amastigotes of test strains (Table 1 and Table 2).
 |
Table 1 The IC50 of Test Substances Against Promastigotes of Leishmania aethiopica |
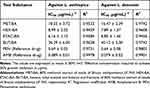 |
Table 2 The IC50 of Test Substances Against Intracellular Amastigotes of Leishmania aethiopica and Leishmania donovani |
The aqueous fraction (189.3 ± 8.70 ≤ IC50 ≤ 208.9 ± 20.2 µg/mL) and the butanol fraction (36.29 ± 6.00 ≤ IC50 ≤ 40.12 ± 5.30 µg/mL) exhibited the lowest activities against the promastigotes and amastigotes of leishmania species, respectively (Table 1 and Table 2).
Cytotoxicity Effects
The cytotoxic effects (CC50) of 80% methanol extract of the plant and three other solvent fractions against human red blood cells, Vero cell lines and peritoneal mice macrophages were also presented. All solvent fractions and crude extract showed CC50 > 1600 µg/mL against red blood cells. The crude extract and its hexane, ethyl acetate and butanol fractions revealed 8.28 ± 0.01, 7.76 ± 0.23, 7.63 ± 0.47 and 4.57 ± 0.42% of hemolysis at 1600 µg/mL, respectively. On the other hand, the hexane fraction revealed the highest cytotoxic effect, with CC50 values of 126.75 ± 6.55 and 134.35±12.95 µg/mL against Vero cell lines and macrophages, respectively (Table 3). The finding also exhibited that the 80% methanol extract of the plant and its solvent fractions (7.97 ≤ SI ≤ 30.97) showed selective toxicities toward leishmania parasites (Table 4).
 |
Table 3 CC50 of Test Substance Against Vero Cell Lines and Peritoneal Mice Macrophage Isolates |
 |
Table 4 Selectivity Indices of Test Substances Between Parasite and Animal Cell Lines |
Total Contents of Phenolic Compounds, Flavonoids and Alkaloids
The TPC and TFC of 80% methanol extract of seeds of B. antidysenterica and its ethyl acetate and hexane fractions are presented in Table 5. The TPC of the 80% methanol extract and its fractions were obtained from the equation of the calibration curve of gallic acid: Y = 0.006352*X + 0.08988, R2 = 0.9778. On the other hand, their TFCs were calculated from the standard curve of quercetin: Y = 0.6187*X – 0.01021, R2 = 0.9952. Additionally, the total alkaloid contents were extrapolated from the calibration curve of atropine: Y = 0.001312*X + 0.001750, R2 = 0.9934.
 |
Table 5 Total Phenolic, Flavonoid and Alkaloid Content of 80% Methanol Extract of Seeds of Brucea antidysenterica J.F. Mill and Its Fractions |
Discussion
The highest and the lowest anti-promastigote activities were recorded by ethyl acetate and aqueous fractions, respectively. The IC50 of the crude extract and its solvent fractions against promastigotes of L. donovani were ranging from 27 to 1170, and 5 to 217 times those of amphotericin B and pentamidine isethionate, respectively. Similarly, their IC50 against promastigotes of L. aethiopica were ranging from 38 to 1170 and 8 to 238 times the IC50 of amphotericin B and pentamidine isethionate, respectively, suggesting that the anti-promastigote activities of the crude extract and its solvent fractions were much lower than the activities shown by reference drugs (Table 1).
The level of in vitro activities of the reference drugs, crude extract and its solvent fractions were appreciated based on the following criteria: IC50 ≤ 5 μg/mL pronounced activity; 5 < IC50 ≤ 20 μg/mL good activity; 20 < IC50 ≤ 30 μg/mL moderate activity; 30 < IC50 ≤ 64 μg/mL low activity; and IC50> 64 μg/mL inactive.38 Accordingly, the crude extract, n-hexane fraction and n-butanol fraction possessed moderate, good and low anti-promastigote activity, respectively, against both L. aethiopica and L. donovani isolates. The ethyl acetate fraction demonstrated good anti-leishmanial activity against L. aethiopica and pronounced activity against L. donovani, while aqueous fraction was found to be inactive against both L. donovani and L. aethiopica promastigotes (Table 1).
The in vitro promastigote parasites culture in cell free media is simple and reproducible,40 even though the ecology, metabolism, morphology, composition of the surface glycocalyx of the promastigote differ from those of amastigote.40,41 Hence, the results obtained from anti-promastigote screening may not be correlated with ex vivo intracellular amastigotes or in vivo animal models. In the current study, the potential anti-amastigote activities, clinically relevant stages of Leishmania, of the crude extract and hexane, ethyl acetate and butanol fractions were evaluated as confirmatory tests of their anti-promastigote activities.
Eighty percent methanol extract, hexane and ethyl acetate fractions were found to have good activity against the intracellular amastigotes of both L. donovani and L. aethiopica, whereas butanol fraction was found to have low anti-amastigotes activity against both species of Leishmania (Table 2). Like anti-promastigotes assay, ethyl acetate fractions exhibited the highest activity against intracellular amastigotes (Table 2).
The toxicity level of bioactive agents was classified previously based on the following criteria: <10μg/mL very strong cytotoxicity, 10–100 μg/mL strong cytotoxicity, 100–500 μg/mL moderate cytotoxicity.42 Accordingly, the crude (80% methanol) extract and its fractions are non-toxic to RBC. On the other hand, the crude extract and its solvent fractions exhibited a moderate cytotoxic effect against both Vero cells and mice macrophages (Table 3). However, extrapolating how the in vitro cytotoxicity test results relate to the in vivo results is problematic as pharmacokinetics of a bioactive compound in the tissue is not considered in the environment of in vitro study.39
A selectivity index (SI) value >1 is considered to be selective against the Leishmania parasites and a value <1 is considered as selective against mammalian host cells.42 In view of that, all test groups were selective against amastigotes of both strains of Leishmania. But the crude extract and its fractions showed less selectivity than amphotericin B. Conversely, they showed comparable selectivity against Leishmania spps as pentamidine isethionate except ethyl acetate fraction, which is more selective against the parasites (Table 4).
Preliminary phytochemical screening conducted previously by Tessema et al indicated the presence of alkaloids, tannins, flavonoids, triterpenoids, phenols, steroids and glycosides in 80% methanol extract of seeds of B. antidysenterica J.F. Mill.23 Phenolic compounds include simple phenols, cinnamic acid derivatives, coumarins, benzoic acid derivatives, tannins, flavonoids, lignins and lignans, among others.43 Phenolic compounds obtained from various plants having inhibitory activities against protozoan parasites including Leishmania parasites were reported. Cinnamic acid derivatives (eg o-coumaric acid and p-coumaric acid), flavonol derivatives (morin and rutin), hydroxybenzoic acid derivatives (gallic acid, gentisic acid, vanillic acid) and ellagic acid were reported for their in vitro activities against L. amazonensis. Moreover, gentisic acid and p-coumaric acid were also reported to have in vivo activity against L. amazonensis in BALB/c mouse model.44 Treatment of L. donovani promastigotes and intracellular amastigotes with rosmarinic acid also led to alteration of membrane integrity of mitochondrial and other cells as a result of its iron chelation capability.45 The phenolic compounds contained in 80% methanol extract and its ethyl acetate and hexane fractions might be attributable to their anti-leishmanial activities (Table 5).
Furthermore, flavonoids which include chalcones, flavones, flavonols, and isoflavones are also largely known for their wide spectrum of activities against leishmaniasis.46 Quercetin, another flavonoid compound, was also found to chelate iron and inhibit topoisomerase II, the enzymes used in the replication of parasites within the phagolysosomes of macrophage.47 In the current study, TFC of 80% methanol extract and its ethyl acetate and hexane fractions might be responsible for their ant-leishmanial activities (Table 5).
Canthine alkaloids (canthin-6-one and 5-methoxycanthin-6-one) isolated from stem bark of Zanthoxylum chiloperone49 also previously isolated from B. antidysenterica50 demonstrated in vitro anti-leishmanial activity against L. braziliensis, L. amazonensis, and L. donovani. Canthin-6-one also displayed an interesting leishmanicidal activity against L. amazonensis-infected BALB/c mice when administered intralesionally, even though it did not show any activity against the parasites when administered orally.49 It might be due to inactivation of the compound in gastrointestinal tract of the mice as a result of digestive enzymes or gastric acid contents. Another alkaloid isolate, β-Carboline-1-propionic acid (β-CPA), obtained from stem bark of Quassia amara L. (Simaroubaceae)51 and previously isolated from B. antidysenterica,52 displayed potent anti-leishmanial activity of L. amazonensis and L. infantum against promastigotes and intracellular amastigotes, respectively.51 The alkaloids contained in 80% methanol extract and its ethyl acetate and hexane fractions might be responsible for their anti-leishmanial activities (Table 5).
The crude extracts and compound isolates obtained from other members of genus Brucea also revealed activities against Leishmania and Trypanosoma. Aqueous, and 80% methanol extracts of seeds and leaves of Brucea sumatrana and their fractions revealed anti-leishmanial activities against L. infantum.38,53 The plant was also found to be active against two Trypanosoma (T. cruzi and T. brucei brucei), the parasites related to Leishmania species.38,53
This finding indicated that the high concentrations of phenolic compounds and flavonoids were detected in 80% methanol extract while high concentration of alkaloids was detected in its ethyl acetate fraction, respectively. Conversely, the lowest concentrations of TPC, TAC and TFC were found in hexane fractions (Table 5). This suggested that the anti-leishmanial activities of 80% methanol extract of the seeds of B. antidysenterica and its ethyl acetate fraction were attributed to phenols and alkaloids, respectively, or it might be the combined effects of phenols, flavonoids, alkaloids and other secondary metabolites contained in the plant.
Conclusion
Ethyl acetate and hexane fractions were found to exhibit high anti-leishmanial activities against L. donovani and L. aethiopica isolates. The report validates use of B. antidysenterica J.F. Mill seeds in the treatment of leishmaniasis by traditional healers. Isolation and identification of specific compounds from the active fractions are required to find hits and lead compounds that can be an input in anti-leishmanial product development.
Abbreviations
AMB, Amphotericin B; AE/g, Atropine equivalent per gram of extract/fraction; ATCC, American Type Culture Collection; CC50, Cytotoxic Concentrations that kill 50% of the cells; CL, Cutaneous leishmaniasis; DMSO, Dimethylsulfoxide; GAE/g, Gallic acid equivalent per gram of extract/fraction; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HINBCS, Heat Inactivated New Born Calf Serum; GAE/g, Gallic acid equivalent per gram of extract/fraction; IC50, Effective Concentration that inhibits 50% of the cells; LRDL, Leishmaniasis Research and Diagnostic Laboratory; M-199, Medium −199 with Earle’s salts; MEM, Minimum Essential Media; QE/g, Quercetin equivalent per gram of extract/fraction; RPMI, Roswell Park Memorial Institute; SI, Selectivity Index; TAC, Total alkaloidal content; TFC, Total flavonoid content; TPC, Total phenolic content; VL, Visceral leishmaniasis; WHO, World Health Organization.
Data Sharing Statement
Data supporting the results reported in the manuscript will be available upon request.
Acknowledgments
We are grateful to Dr. Daniel Gizaw and Dr. Dereje Shegu from National Animal Health Diagnosis and Investigation Center, Sebeta, Ethiopia for providing Vero cell lines. We also thank Mr. Dawit Araya and Mr. Mulugeta Gichile, the staff of Leishmania Research and Diagnostic Laboratory (LRDL) of Addis Ababa University, for all their support and assistance throughout the study period. This paper is based on a thesis by Mr. Tasisa Ketema. It has been published on the institutional website: http://etd.aau.edu.et/handle/123456789/.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa) and Addis Ababa University through its thematic research grant supported this research. The funders have no involvement in the study design or submission of the paper for publication.
Disclosure
The authors declare no competing interests in this work.
References
1. Seid A, Gadisa E, Tsegaw T, et al. Risk map for cutaneous leishmaniasis in Ethiopia based on environmental factors as revealed by geographical information systems and statistics. Geospat Health. 2014;8(2):377–387. doi:10.4081/gh.2014.27
2. Gebremichael D. Zoonotic impact and epidemiological changes of leishmaniasis in Ethiopia. Open Vet J. 2018;8(4):432–440. doi:10.4314/ovj.v8i4.13
3. Hailu A, Gramiccia M, Kager PA. Visceral leishmaniasis in Aba-Roba, south-western Ethiopia: prevalence and incidence of active and subclinical infections. Ann Trop Med Parasitol. 2009;103(8):659–670. doi:10.1179/000349809X12554106963555
4. Sundar S, Chakravarty J. Liposomal Amphotericin B and Leishmaniasis: dose and Response. J Glob Infect Dis. 2010;2. doi:10.4103/0974-777X.62886
5. An I, Harman M, Esen M, Çelik H. The effect of pentavalent antimonial compounds used in the treatment of cutaneous leishmaniasis on hemogram and biochemical parameters. Cutan Ocul Toxicol. 2019;38(3):294–297. doi:10.1080/15569527.2019.1610887
6. Id S, Miguel J, Id P, et al. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl Trop Dis. 2019;1–12.
7. Sunyoto T, Potet J, Boelaert M. Why miltefosine — a life-saving drug for leishmaniasis — is unavailable to people who need it the most. BMJ Global Health. 2018:1–10. DOI:10.1136/bmjgh-2018-000709
8. Priscilla E, Gadelha N, Augusto J, et al. Patients with cutaneous leishmaniasis caused by L. guyanensis: a pilot study *. Anais brasileiros de dermatologia. 2015;90(6):807–813. doi:10.1590/abd1806-4841.20153956
9. Hendrickx S, Guerin PJ, Caljon G, Croft SL, Maes L. Evaluating drug resistance in visceral leishmaniasis: the challenges. Parasitology. 2018;145(4):453–463. doi:10.1017/S0031182016002031
10. Nagle AS, Khare S, Kumar AB, et al. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Cheml Rev. 2014;114(22):11305–11347.
11. Tariku Nefo D. Isolation and structural elucidation of an alkaloid constituent from the berries of brucea antidysenterica. Chem Process Eng Res. 2019. 59:13–14. doi:10.7176/CPER
12. Jansen PCM. Spices, Condiments and Medicinal Plants in Ethiopia, Their Taxonomy and Agricultural Significance. Wageningen University and Research; 1981.
13. Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in Eleven Districts, Ethiopia. Evid Based Complement Alternat Med. 2020;2020:1–23. doi:10.1155/2020/7683450
14. Van Wyk BE. A review of African medicinal and aromatic plants. Med Arom Plants World Afr. 2017;3:19–60. doi:10.1007/978-94-024-1120-1
15. Wubetu M, Abula T, Dejenu G, Freetly HC. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern. BMC Res Notes. 2017;10(1):1–13. doi:10.1186/s13104-017-2482-3
16. Avigdor E, Wohlmuth H, Asfaw Z, Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10. doi:10.1186/1746-4269-10-38
17. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Survey of medicinal plants used to treat malaria by sidama people of Boricha District, Sidama Zone, South Region of Ethiopia. Evid Based Complement Alternat Med. 2016;2016. doi:10.1155/2016/9690164
18. Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethnopharmacol. 2006. doi:10.1016/j.jep.2006.11.019
19. Shakila R, Narihiko F, Masayoshi O, Kiyoshi T, Kuo-Hsiung L. Anti-tuberculosis activity of quassinoids. Chem Pharm Bull. 1997;45(9):1527–1529. doi:10.1248/cpb.45.1527
20. Gillin FD, Reiner DS, Suffness M. Bruceantin, a Potent Amoebicide from a Plant, Brucea antidysenterica. Antimicrob Agents Chemoth. 1982;22(2):342–345. doi:10.1128/AAC.22.2.342
21. Kefe A, Giday M, Mamo H, Erko B. Antimalarial properties of crude extracts of seeds of Brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Complement Altern Med. 2016;16:1–8. doi:10.1186/s12906-016-1098-9
22. Zewdie KA, Bhoumik D, Wondafrash DZ. Evaluation of in-vivo antidiarrhoeal and in- vitro antibacterial activities of the root extract of Brucea antidysenterica J. F. Mill (Simaroubaceae). BMC Complement Med Ther. 2020;7:1–11.
23. Tessema Z, Makonnen E, Debella A, Molla Y. Evaluation of in vivo wound healing and anti-inflammatory activity of crude extract of the fruits of Brucea antidysentrica in mice. Biochem Pharmacol. 2018. doi:10.1016/j.wndm.2018.05.005
24. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015:1–8. DOI:10.1186/s12906-015-0779-0
25. Activity A, Profile T, van der A DL, Verschuren WMM, Hendriks HFJ, Beulens JWJ. NPC natural product communications. Am J Clin Nutr. 2010;91(6):1777–1783. doi:10.3945/ajcn.2010.29170
26. Manuscript A. NIH public access; 2009: 1–10. doi:10.1002/9780471729259.mca04es11.Growth.
27. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008:1–14. DOI:10.1002/0471142735.im1401s83
28. Abeje F, Bisrat D, Hailu A, Asres K. Phytochemistry and antileishmanial activity of the leaf latex of aloe calidophila reynolds. Phytother Res. 2014;1805:1801–1805.
29. Zohra M, Fawzia A. Hemolytic activity of different herbal extracts used in Algeria. Int J Pharm Sci Res. 2014;5(08):495–500.
30. Chan SM, Khoo KS, Sit NW. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: implications for ethnopharmacological studies. Trop J Pharm Res. 2015;14:1991–1998.
31. Leishmania A, Nigussie D, Tasew G, et al. In-vitro investigation of fractionated extracts of Albizia gummifera seed. J Clin Cell Immunol. 2015;6(6). doi:10.4172/2155-9899.1000373
32. Afrin F, Chouhan G, Islamuddin M, Want MY, Ozbak HA, Hemeg HA. Cinnamomum cassia exhibits antileishmanial activity against Leishmania donovani infection in vitro and in vivo. PLoS Negl Trop Dis. 2019;13(5):1–28.
33. Tadele M, Abay SM, Makonnen E, Hailu A. Leishmania donovani growth inhibitors from pathogen box compounds of medicine for malaria venture. Drug Design Dev Ther. 2020;14:1307–1317. doi:10.2147/DDDT.S244903
34. Utaile M, Kassahun A, Abebe T, Hailu A. Experimental parasitology susceptibility of clinical isolates of Leishmania aethiopica to miltefosine, paromomycin, amphotericin B and sodium stibogluconate using amastigote-macrophage in vitro model. Exp Parasitol. 2013;134(1):68–75. doi:10.1016/j.exppara.2013.01.022
35. Berry SL, Hameed H, Thomason A, et al. Development of NanoLuc-PEST expressing Leishmania mexicana as a new drug discovery tool for axenic- and intramacrophage-based assays. PLoS Negl Trop Dis. 2018;12(7):1–20.
36. Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exper Pharmacol. 2021;Volume 13:15–22. doi:10.2147/JEP.S285079
37. Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV - Spectrophotometer. Anc Sci Life. 2018;31(4):2–5. doi:10.4103/0257-7941.107361
38. Ehata MT, Phuati AM, Lumpu SN, et al. In vitro antiprotozoal and cytotoxic activity of the aqueous extract, the 80 % methanol extract and its fractions from the seeds of brucea sumatrana Roxb. (Simaroubaceae) growing in democratic Republic of Congo. Chin Med. 2012;2012:65–71. doi:10.4236/cm.2012.31011
39. De Muylder G, Ang KKH, Chen S, Arkin MR, Engel JC, James H. A screen against leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Neglect Trop Dis. 2011;5(7). doi:10.1371/journal.pntd.0001253
40. Gupta S. Visceral leishmaniasis: experimental models for drug discovery. Indian J Med Res. 2011;133:27–39.
41. Aulner N, Danckaert A, Rouault-hardoin E, et al. High content analysis of primary macrophages hosting proliferating Leishmania amastigotes: application to anti-leishmanial drug discovery. To cite this version: HAL Id: pasteur-01433415 High Content Analysis of Primary Macrophages Hosting Proliferatin. Methods Mol Biol. 2017;1535:173–195. doi:10.1371/journal.pntd.0002154
42. Indrayanto G, Putra GS, Suhud F. Validation of in-vitro Bioassay Methods: Application in Herbal Drug Research.
43. Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18(2):2328–2375. doi:10.3390/molecules18022328
44. Monzote L, Córdova WHP, García M, Piñón A, Setzer WN. In-vitro and in-vivo activities of phenolic compounds against cutaneous in-vitro and in-vivo activities of phenolic compounds against cutaneous Leishmaniasis. Rec Nat Prod. 2016;10(3):269.
45. Antwi CA, Mmalebna C, Id A, et al. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLOS Negl Trop Dis. 2019;13(2):1–22.
46. Silva-silva JV, Moragas-tellis CJ. Antileishmanial activity of flavones- rich fraction from Arrabidaea chica Verlot (Bignoniaceae). Front Pharmacol. 2021;12:(July):1–14. doi:10.3389/fphar.2021.703985
47. De Arias AR, Pandolfi E, Celeste M, Rolón M. Selected natural and synthetic phenolic compounds with antileishmanial activity: a five-year review. Curr Bioact Compd. 2012. doi:10.2174/1573407211208040002
48. Liu J, Jin H, Zhang W, Yan S, Shen Y. Chemical constituents of plants from the Genus Brucea. Chem Biodivers. 2009;6:57–70.
49. Ferreira ME, Arias De AR, De Ortiz ST, Inchausti A, Nakayama H. Leishmanicidal acti v ity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone v ar. angustifolium. J Ethnopharmacol. 2002;80:7–10.
50. Ju-ichi M, Lee KH, Martin MS, Kempczinski RF, Gordon RD. Antitumor agents, 79. cytotoxic antileukemic alkaloids from. J Vasc Surg. 1986;4(3):428–434.
51. Gabriel RS, Garcia AR, Alviano CS, et al. β -Carboline-1-propionic acid alkaloid: antileishmanial and cytotoxic effects. Turkish J Pharma Sci. 2019;29:755–762.
52. Makong YS, Mouth G, Liliane J, et al. Cytotoxic stilbenes and canthinone alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules. 2019 24(23):1–10.
53. Ehata MT, Lumpu SN, Munduku CK, Kabangu OK, Cos P, Maes L. Study of antiparasitic and cytotoxicity of the aqueous, the 80 % methanol extract and its fractions, and the acute toxicity of the aqueous extract of Brucea sumatrana (Simaroubaceae) leaves collected in mai-ndombe, democratic Republic of Congo. Chin Med. 2016;50(7):93–109. doi:10.4236/cm.2016.73011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.