Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 16
Impact of Spondyloarthritis on Pregnancy Outcome: A Descriptive Analysis from a Specialized Center in Qatar
Authors Al Emadi S, Hadwan NN , Saleh R, Satti E, Singh R
Received 11 November 2023
Accepted for publication 18 January 2024
Published 22 January 2024 Volume 2024:16 Pages 21—29
DOI https://doi.org/10.2147/OARRR.S449343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Samar Al Emadi,1 Nawal Nouman Hadwan,1 Rawan Saleh,1 Eman Satti,1 Rajvir Singh2
1Department of Rheumatology, Hamad Medical Corporation, Doha, Qatar; 2Medical Research Center, Statistics, Hamad Medical Corporation, Doha, Qatar
Correspondence: Samar Al Emadi, Department of Rheumatology, Hamad General Hospital, PO Box 3050, Doha, Qatar, Tel +974 55550087, Email [email protected]
Introduction: Spondyloarthritis (SpA) most commonly presents at childbearing age; thus, pregnancy is of concern. However, data on pregnancy outcomes in these patients are limited.
Purpose: This study aimed to retrospectively describe pregnancy outcomes in patients with SpA from the Middle East.
Patients and Methods: We reviewed the electronic health records of all pregnant women attending a specialized pregnancy and rheumatic disease clinic between 2016 and 2022. All pregnant patients diagnosed with axial spondyloarthritis (axSpA) and peripheral SpA were included. Data on adverse maternal and fetal outcomes were collected.
Results: Fifty-seven eligible pregnancies were identified from hospital records: 10 pregnancies ended in early miscarriage. Forty-seven pregnancies resulted in live singleton births, 25 in patients with peripheral SpA and 22 with axSpA. Human leukocyte antigen B27 was positive in 7 (15%) patients and only in women with axSpA. Twenty-nine (64%) patients received treatment throughout pregnancy. Consistent biologic disease-modifying antirheumatic drug (bDMARD) use was high, in eight (32%) patients with peripheral SpA and in nine (41%) with axSpA. A conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) was used as treatment in 11 (50%) patients with peripheral SpA and two (8%) with axSpA. Twenty-two (53%) neonates were delivered by cesarean section, 19 (40%) by normal vaginal delivery and three (6%) by assisted delivery. Additionally, 44 (94%) deliveries were at term, and 42 (91%) neonates had a normal birth weight. Exploration of a subgroup showed no difference in reported outcomes between patients treated with bDMARD and those treated with csDMARD.
Conclusion: This descriptive study reports a high rate of favorable pregnancy outcomes in patients with SpA. There was no evidence to suggest a difference in pregnancy outcomes between women with axSpA and those with peripheral SpA. This study was one of the first reports from the Middle East. Further studies with larger sample size are warranted.
Keywords: axial spondyloarthritis, pregnancy, pregnancy outcomes, neonatal health, maternal health
Introduction
Spondyloarthritis (SpA) characterizes a group of chronic progressive inflammatory disorders that includes axial spondyloarthritis (axSpA), peripheral SpA, psoriatic arthritis, reactive arthritis, and enteropathic spondylitis associated with inflammatory bowel disease, with axSpA as the most common, affecting the sacroiliac joint and spine and often associated with reduced activity and quality of life.1
The prevalence of axSpA shows regional differences owing to genetic background. Recent estimates of axSpA prevalence per 10,000 population are 23.8 in Europe, 16.7 in Asia, 31.9 in North America, 10.2 in Latin America, and 7.4 in Africa.2 A strong association was observed between axSpA and the human leukocyte antigen B27 (HLA-B27) genotype, which forms part of the Assessment of Spondyloarthritis International Society (ASAS) criteria for axSpA diagnosis.3 However, HLA-B27 prevalence was further reported to show regional differences, with lower prevalence in Middle Eastern than in Western European populations, for both healthy and axSpA patient groups.4 A recent systematic review has shown prevalence estimates for healthy populations to be between 0.3 and 6.8%, and in axSpA patients, estimates range from 26.2 to 91%.4
AxSpA most commonly presents in the second or third decade of life; hence, childbearing years and pregnancy are of particular concern. While often historically reported to be more common in men than in women, the increasing awareness of axSpA in women suggests that the prevalence could be similar to that in men.5
Chronic inflammatory disorders have long been shown to be associated with adverse outcomes in pregnancy.6,7 Pregnancies in patients with SpA are similarly considered as high risk, with disease activity driving the high risks.8 Suggested obstetric complications include preeclampsia/eclampsia, gestational diabetes, gestational hypertension, and peri-partum complications, resulting in assisted or cesarean section (CS) delivery, and fetal complications include the risk of preterm birth and low birth weight (LBW) or small for gestational age (SGA). However, evidence for adverse maternal and fetal outcomes is derived from few retrospective cohort and case–control studies and shows conflicting results.8–15 Recent reviews and meta-analyses have suggested an increased risk of CS and preterm birth, but are not conclusive for SGA and preeclampsia.16–18 Further, with advances in biologic disease-modifying anti-rheumatic drugs (bDMARD) that allow for better disease control, fewer adverse pregnancy outcomes are expected.19 To our knowledge, few reports have described maternal and fetal outcomes in patients with SpA in the Middle East.
Aim
This retrospective study aimed to describe adverse maternal and fetal outcomes in a group of patients diagnosed with SpA and categorized according to axial involvement.
Patients and Methods
Data Collection
Electronic medical records were sequentially reviewed for all patients attending the Pregnancy and Rheumatic Disease Clinic, Hamad General Hospital, Doha, Qatar, between July 1, 2016, and September 1, 2022. Patients with confirmed pregnancy and autoimmune rheumatic disease typically attend the clinic once each trimester during pregnancy and once postpartum.
The clinic was established in 2005 and has been continually run by the same expert clinician during the study time period, providing standardized clinical care. Data were de-identified, and each pregnancy was recorded as a separate event and linked using a patient identity code.
ASAS criteria were used to diagnose peripheral SpA and axSpA, with axSpA confirmed by MRI of the sacroiliac joint. Patients with confirmed spondyloarthritis prior to pregnancy were included in the present study. Peripheral and axial diseases were included, as well as radiographic and non-radiographic diseases.
Outcomes
Data on maternal and fetal outcomes, including preeclampsia/eclampsia, gestational hypertension, and gestational diabetes and gestational age at delivery, birth weight, delivery mode, delivery complications, neonatal intensive care unit (NICU) admission, and any congenital abnormalities, respectively, were collected from electronic medical records. Pregnancies with lacking data on pregnancy outcomes in hospital medical records owing to delivery outside the institution were excluded from the analysis.
Approval
This study adheres to the principles of the Declaration of Helsinki and Good Clinical Practice and is within the laws and regulations of the Ministry of Public Health, Qatar. The study was approved by the Medical Research Center at Hamad General Hospital with institutional review board approval (MRC 01–22-835). Patient consent was not required by the review board for this retrospective study. All data was anonymized.
Data Analysis
Data were analyzed using Statistical Package for Social Sciences Version 29 (SPSS Inc., Chicago, IL, USA). Descriptive statistics of mean±standard deviations for continuous data and frequency with percentages for categorical data were calculated.
Results
During the study period (July 1, 2016, to September 1, 2022), 59 pregnancies, in 28 women, were identified (Figure 1 and Supplementary Table 1). Each pregnancy was treated as a separate independent event. Two pregnancies were excluded because of incomplete pregnancy data (delivery outside the hospital and, hence, not available on electronic medical records). One pregnancy had missing birth weight data but remained in the analysis for all other outcomes.
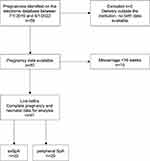 |
Figure 1 Flow chart showing inclusion of pregnancies in final analysis. Abbreviations: SpA, spondyloarthritis; axSpA, axial spondyloarthritis. |
Ten of the 57 pregnancies (18%) reported an early miscarriage (<16-week gestation). Nine miscarriages were observed in patients with peripheral SpA and one miscarriage in a patient with axSpA. Six patients who had miscarriages were on no treatment, and four miscarriages occurred in one mother who was on different treatment lines during the four pregnancies.
Descriptive Data
Forty-seven pregnancies resulted in live singleton births. Table 1 shows the maternal characteristics. Data were subdivided by maternal diagnosis into the axSpA and peripheral SpA groups for descriptive comparison (n=22 and n=25, respectively).
 |
Table 1 Maternal Characteristics for Pregnancies with Live Birth (n=47) |
All the patients were nonsmokers. The mean age of patients was 31.48 years and 29.64 years in peripheral SpA and axSpA patient groups, respectively. HLA-B27 was positive in seven patients (15%), all of whom had axSpA. The rate of pre-existing comorbidities was low, with a comorbidity noted in eight pregnancies (Table 1).
Treatment of SpA During Pregnancy
A high proportion (29 patients, 64% of pregnancies) received treatment: 18 patients (72%) with peripheral SpA and 11 patients (55%) with axSpA. Two patients were treated with a non-steroidal anti-inflammatory drug, which was discontinued at 25 weeks of gestation. Overall, 29 pregnancies (62%) received either a conventional synthetic disease-modifying anti-rheumatic drug (csDMARD), sulfasalazine, or a bDMARD, certolizumab; 17 of them (36%) received bDMARD throughout pregnancy and post-delivery. bDMARD was used as treatment throughout pregnancy in eight patients (32%) with peripheral SpA and nine patients (41%) with axSpA. csDMARD was used as treatment throughout pregnancy in 11 patients (50%) with peripheral SpA and in two patients (8%) with axSpA (Table 1).
Maternal Pregnancy Outcomes
Table 2 shows the maternal outcomes during pregnancy. Fifteen adverse maternal outcomes were reported: 4 in patients with peripheral SpA and 11 in patients with axSpA.
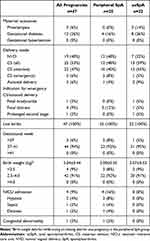 |
Table 2 Maternal and Fetal Outcomes for Pregnancies with Live Birth |
Fetal Outcomes
The majority, 44 pregnancies (94%) were term and only three (6%) were pre-term deliveries. CS was the most common delivery method (25 deliveries, 53%), with normal vaginal delivery accounting for 19 deliveries (40%). Assisted delivery was required in three (6%) cases. CS was performed on 12 patients (48%) with peripheral SpA and 13 patients (59%) with axSpA.
Birth weight was normal in 42 neonates (91%). The remaining four neonates were categorized as LBW, with two of them being delivered pre-term. Four neonates (9%) required admission to the NICU. One neonate was born with a congenital abnormality; however, no further data were available on this case.
To further explore the effects of treatment on pregnancy outcomes, we analyzed data from a subgroup of patients treated with bDMARD and csDMARD (Table 3). There were no suggestions of differences in the rate of adverse maternal and fetal outcomes (CS rate, gestational age at delivery, birth weight, NICU admission, or congenital abnormalities) between patients receiving bDMARD or csDMARD treatment.
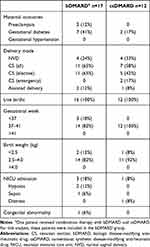 |
Table 3 Maternal and Fetal Outcomes for Pregnancies with a Live Birth: Sub-Group Analysis of Patients Treated with bDMARD or csDMARD During Pregnancy |
Discussion
In this study, we describe the maternal and fetal pregnancy outcomes in patients with axSpA and peripheral SpA. We showed low rates of adverse maternal or fetal pregnancy outcomes in patients with axSpA and peripheral SpA. To the best of our knowledge, few studies in the Middle East have reported on pregnancy outcomes in patients with SpA.
Two case–control studies have investigated pregnancy outcomes in patients with axSpA, with conflicting results.9,15 A small case–control study by Timur et al has shown no differences in route of delivery, birth weight, and NICU admission between patients with axSpA (n=20) and matched controls (n=40).15 Conversely, a larger study by Jakobsson et al showed increased risk of emergency CS, SGA, and preterm delivery in patients with axSpA (n=308) than in healthy controls (n=1082).9 In our study, we did not compare to healthy controls. However, the overall rate of adverse maternal and fetal outcomes was low.
A study of the Danish National Birth Register10 has found an increase in preterm birth and elective/emergency CS and no increase in SGA or preeclampsia when comparing pregnancies between patients with SpA and those without SpA. A similar study by Keeling et al analyzed data from the Alberta Pregnancy-Birth Cohort and found no increase in preterm birth or SGA, CS rate, or hypertensive disorders in patients with SpA compared with women with no inflammatory arthritis.20 In contrast, a recent meta-analysis has described a statistically significant increase in the rate of CS in axSpA.18 Nevertheless, there were non-significant trends towards increased prevalence of preeclampsia, IUGR and LBW, SGA, NICU admissions, and congenital abnormalities with axSpA.18
Rate of Cesarean Section in Spondyloarthritis
In this study, we report a high CS rate in patients with peripheral SpA and those with axSpA, explained by a high rate of elective CS. It is important to note that elective CS in our study population was primarily due to patient choice. This reported CS rate is high in comparison with that reported by Jakobsson et al (showing a CS rate of 28.9% in cases and 16.4% in controls),9 Morin et al (24.2% in cases and 17.5% in controls);19 and Keeling et al (29.1% in patients with SpA).20 Conversely, Timur et al have shown a similar high CS rate (55% in patients with axSpA).15
The rate of preterm delivery in our study was low and of a similar rate to that previously reported in axSpA.21 Literature is conflicting in this area, but review data suggest an overall increased risk of preterm delivery17 or a non-significant trend.18 Further, birth weight is influenced by gestation and potentially affected by a high elective CS rate. We were not able to calculate SGA since data on the sex of the neonates were not collected; thus, unadjusted birth weight data were used. However, in addition to a low rate of preterm delivery, our study found a low incidence of LBW in this population. Only two neonates who were delivered at term were classified as LBW.
Consideration of Treatment with bDMARD
Recently, consistent treatment with bDMARD throughout pregnancy, for maternal and fetal outcomes, has been highlighted in the literature,19,22,23 with bDMARD use not being associated with adverse outcomes.22 A Swedish cohort study has shown decreased rates of elective CS, preeclampsia, preterm delivery, and infant infection in patients with axSpA. It inversely showed an increased use of bDMARD indicating that treatment with bDMARD and good disease control may reduce pregnancy complications in these women.19
Critically, we did not find any suggestions of increased adverse maternal or fetal outcomes in patients treated with bDMARD compared to patients treated with csDMARD during pregnancy.
The use of bDMARD has increased in recent years. In contrast to other studies, we report a high rate (36%) of consistent treatment with bDMARD throughout pregnancy; Meissner et al and Pons et al showed bDMARD usage of 27.5% and 8%, respectively.22,24 Owing to this change in the treatment of SpA, Morin et al have further suggested that older study results are not directly comparable with the more recent results,19 further supporting the need for up-to-date studies on outcomes in patients with SpA.
The miscarriage rate in the present study was 18%. We were unable to draw any further relevant conclusions because of repeated miscarriages in the same woman, potentially skewing our results. A similar analysis has shown a miscarriage rate of 7.5% in patients with axSpA.22 This outcome is an important consideration for future studies to fully understand whether this risk is related to the disease, the treatment of the disease, or unrelated causes.
Strengths of the Study
This study is one of the few to report on pregnancy outcomes of patients with SpA in the Middle East. Due to potential differences in the disease presentation and severity in different areas of the world, local data are important to guide clinical practice. Moreover, the importance of HLA-B27 during pregnancy is not well established. Our results show that 32% of the patients with axSpA were positive for HLA-B27, which is lower than that reported in European patients.4
The high rate and consistent use of bDMARD throughout pregnancy make the data from this study both reliable and clinically relevant.
Limitations of the Study
The major limitations of this study were the lack of a healthy control group, the relatively small sample size and the lack of disease activity measures. The small sample size, combined with the small expected differences between patients with axSpA and those with peripheral SpA, did not allow us to statistically compare these patient groups.
Our population included 47 pregnancies in 26 patients, and we report that the number of births varied considerably between one and seven births per woman, resulting in the sample size being insufficient to allow adjustment for within-person effects.
Conclusions
In this retrospective study, we demonstrate a high rate of favorable pregnancy outcomes in a sample of patients from the Middle East. Despite the high rate of bDMARD use in this population, the study size was not sufficiently large to draw conclusions, however, there was no evidence of adverse pregnancy outcomes. The study reports a high rate of CS, attributed to maternal choice. Larger, up-to-date studies that allow for multivariate analysis of treatment effects and disease activity measures during pregnancy are warranted.
Abbreviations
ASAS, Assessment of Spondyloarthritis International Society; axSpA, axial spondyloarthritis; CS, cesarean section; bDMARD, biologic disease-modifying anti-rheumatic drug; csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; HLA-B27, human leukocyte antigen B27; NICU, neonatal intensive care unit; NSAID, non-steroidal anti-inflammatory drug; NVD, normal vaginal delivery; SD, standard deviation; SpA, spondyloarthritis.
Data Sharing Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Data were collected by the research coordinator Hadeel Ashour of the Department of Rheumatology, Hamad Medical Corporation. Connect Communications (Dubai, UAE) provided medical writing support.
Funding
The study was approved by the Hamad Medical Corporation Medical Research Center without funding.
Disclosure
The authors report there are no competing interests to declare.
References
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet Lond Engl. 2017;390(10089):73–84.
2. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatol Oxf Engl. 2014;53(4):650–657. doi:10.1093/rheumatology/ket387
3. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi:10.1136/ard.2009.108233
4. Ziade NR. HLA B27 antigen in Middle Eastern and Arab countries: systematic review of the strength of association with axial spondyloarthritis and methodological gaps. BMC Musculoskelet Disord. 2017;18(1):280. doi:10.1186/s12891-017-1639-5
5. Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. gender differences in axial spondyloarthritis: women are not so lucky. curr Rheumatol rep. 2018;20(6):35. doi:10.1007/s11926-018-0744-2
6. Cornish J, Tan E, Teare J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56(6):830–837. doi:10.1136/gut.2006.108324
7. Sim BL, Daniel RS, Hong SS, et al. Pregnancy outcomes in women with rheumatoid arthritis: a systematic review and meta-analysis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2023;29(1):36–42.
8. Zbinden A, van den Brandt S, Østensen M, Villiger PM, Förger F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatol Oxf Engl. 2018;57(7):1235–1242.
9. Jakobsson GL, Stephansson O, Askling J, Jacobsson LTH. Pregnancy outcomes in patients with ankylosing spondylitis: a nationwide register study. Ann Rheum Dis. 2016;75(10):1838–1842. doi:10.1136/annrheumdis-2015-207992
10. Mørk S, Voss A, Möller S, Bliddal M. Spondyloarthritis and outcomes in pregnancy and labor: a nationwide register-based cohort study. Arthritis Care Res. 2021;73(2):282–288.
11. Park EH, Lee JS, Kim YJ, et al. Pregnancy outcomes in Korean women with ankylosing spondylitis. Korean J Intern Med. 2021;36(3):721–730. doi:10.3904/kjim.2019.144
12. Ostensen M, Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983;26(9):1155–1159. doi:10.1002/art.1780260915
13. Zhou Q, Bian XM, Liu JT. Management of pregnancy with ankylosing spondylitis. Chin Med Sci J Chung-Kuo Hsueh Ko Hsueh Tsa Chih. 2012;27(1):46–49.
14. Smith CJF, Bandoli G, Kavanaugh A, Chambers CD. Birth outcomes and disease activity during pregnancy in a prospective cohort of women with psoriatic arthritis and ankylosing spondylitis. Arthritis Care Res. 2020;72(7):1029–1037. doi:10.1002/acr.23924
15. Timur H, Tokmak A, Türkmen GG, Inal H A, Uygur D, Danışman N. Pregnancy outcome in patients with ankylosing spondylitis. J Matern-Fetal Neonatal Med off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2016;29(15):2470–2474.
16. Hamroun S, Hamroun A, Bigna JJ, Allado E, Förger F, Molto A. Fertility and pregnancy outcomes in women with spondyloarthritis: a systematic review and meta-analysis. Rheumatol Oxf Engl. 2022;61(4):1314–1327. doi:10.1093/rheumatology/keab589
17. Mokbel A, Lawson DO, Farrokhyar F. Pregnancy outcomes in women with ankylosing spondylitis: a scoping literature and methodological review. Clin Rheumatol. 2021;40(9):3465–3480. doi:10.1007/s10067-021-05588-9
18. Maguire S, O’Dwyer T, Mockler D, O’Shea F, Wilson F. Pregnancy in axial spondyloarthropathy: a systematic review & meta-analysis. Semin Arthritis Rheum. 2020;50(6):1269–1279. doi:10.1016/j.semarthrit.2020.08.011
19. Morin M, Frisell T, Stephansson O, Hellgren K. Temporal trends in adverse pregnancy outcomes in axial spondyloarthritis in Sweden: a cohort study. Lancet Rheumatol. 2023;5:e121–e129. doi:10.1016/S2665-9913(23)00001-2
20. Keeling SO, Bowker SL, Savu A, Kaul P. A Population-level analysis of the differing effects of rheumatoid arthritis and spondyloarthritis on peripartum outcomes. J Rheumatol. 2020;47(2):197–203. doi:10.3899/jrheum.181320
21. Ostensen M, Ostensen H. Ankylosing spondylitis--The female aspect. J Rheumatol. 1998;25(1):120–124.
22. Pons M, Dougados M, Costedoat-Chalumeau N, et al. Pregnancy rates and outcomes in early axial spondyloarthritis: an analysis of the DESIR cohort. Joint Bone Spine. 2021;88(2):105075. doi:10.1016/j.jbspin.2020.09.007
23. Redeker I, Strangfeld A, Callhoff J, Marschall U, Zink A, Baraliakos X. Maternal and infant outcomes in pregnancies of women with axial spondyloarthritis compared with matched controls: results from nationwide health insurance data. RMD Open. 2022;8(2):e002146. doi:10.1136/rmdopen-2021-002146
24. Meissner Y, Strangfeld A, Molto A, et al. Pregnancy and neonatal outcomes in women with axial spondyloarthritis: pooled data analysis from the European network of pregnancy registries in rheumatology (EuNeP). Ann Rheum Dis. 2022;81(11):1524–1533. doi:10.1136/ard-2022-222641
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
