Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Impact of Delay Prior to Treatment in Ethiopian Children with Acute Lymphoblastic Leukemia
Authors Hailu A , Mekasha A, Hailu D, Fentie AM , Korones DN, Gidey AM
Received 8 February 2023
Accepted for publication 1 May 2023
Published 11 May 2023 Volume 2023:14 Pages 147—157
DOI https://doi.org/10.2147/PHMT.S406181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Abel Hailu,1 Amha Mekasha,1 Daniel Hailu,1 Atalay Mulu Fentie,2 David N Korones,3 Abdulkadir Mohammedsaid Gidey1
1Department of Pediatrics and Child Health, Addis Ababa University Addis Ababa, Addis Ababa, Ethiopia; 2Department of Pharmacology and Clinical Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pediatrics, University of Rochester, New York, NY, USA
Correspondence: Abel Hailu, Email [email protected]
Introduction: More than 85% of childhood malignancies occur in developing countries with less than a 30% cure rate as opposed to more than 80% cure rate in developed countries. This disproportionately significant difference might be due to delays in diagnosis, treatment initiation, lack of adequate supportive care, and treatment abandonment. We aimed to determine the impact of overall treatment delay on induction mortality of children with acute lymphoblastic leukemia treated at Tikur Anbessa specialized hospital (TASH).
Methods: A cross-sectional study was conducted among children who were treated from 2016 to 2019. Children with Down syndrome and relapsed leukemia were excluded from this study.
Results: A total of 166 children were included; most patients were males (71.7%). The mean age at diagnosis was 5.9 years. The median time interval from the onset of symptoms to the first TASH visit was 30 days and the median period from TASH’s first clinic visit to diagnosis was 11 days. The median time to initiate chemotherapy after diagnosis was 8 days. The total median time from the first onset of symptoms to chemotherapy initiation was 53.5 days. Induction mortality was 31.3%. High-risk ALL and patients with an overall delay between 30 and 90 days were more likely to experience induction mortality.
Discussion: Patient and healthcare system delay is high compared to most studies done and a significant association has been noted with induction mortality. Efforts to expand the pediatric oncology service in the country and efficient diagnostic and treatment approach need to be established to reduce mortality associated with overall delay.
Keywords: delay, acute lymphoblastic leukemia, induction mortality, Ethiopia
Introduction
Cancer is a public health, social and economic threat. More than 85% of worldwide childhood cancers are diagnosed in low- and middle-income countries (LMICs) such as Ethiopia.1 Despite a 65% decline in childhood cancer mortality from 1970 to 2016, cancer remains the leading cause of childhood death. The cure rate for childhood cancer in high-income countries has reached more than 80% but remains <30% in LMICs.2,3
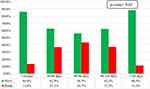 |
Figure 1 Association between overall treatment delay and survival status of patients at the end of induction treatment. |
Acute lymphoblastic leukemia (ALL) is the most common cancer in children and adolescents and accounts for more than 26% of all childhood cancers in the U.S.,4 and 30% in Europe and LMICs.5,6 The 5-year survival rate of childhood ALL among high-income countries is >90%, but it remains low in LMICs ranging from 40% to 70%.7,8 This disproportionately low cure rate in LMICs is because of presentation at an advanced stage due to delays in presentation and/or limited access to care, inadequate and/or lack of supportive care such as antibiotics, and a high rate of treatment abandonment.7,9
There is increasing attention within health care on the issue of waiting times and the potential effect of these waiting times on outcomes. Even though there is no universal agreement on the specific duration in the definition of delays, the literature defines delays as more than 30 days from the onset of symptoms to initial treatment.10
Generally, “delay” is broadly classified into patient delay (from the onset of symptoms to first healthcare visit) and healthcare system delay which is further sub-classified as diagnosis delay (from initial healthcare visit to confirmed diagnosis) and treatment delay (from confirmed diagnosis to treatment initiation). There are controversies regarding the impact of delay in diagnosis and initiation of treatment on survival rate among patients treated for ALL.10–12
Hence, this study aimed to describe the delay in treating children with ALL and to determine the impact on the early survival of these children. Early (induction mortality) is defined as death occurring within 42 days of initiation of induction treatment.13–16 Hence findings from this study might lay the foundation for possible ways to decrease the delay in diagnosis and treatment initiation.
Study Setting and Design
The study was conducted at TASH where the country’s first and biggest dedicated pediatric oncology center is housed. The pediatric oncology-hematology unit of TASH has 39 beds for inpatient service and a separate outpatient clinic for ambulatory patients. On average, up to 500–600 new childhood cancer patients have enrolled annually. This study was conducted using a hospital-based cross-sectional study from March 1 to August 31, 2019.
Eligibility Criteria and Data Collection
All pediatric ALL patients (under the age of 14 years old) whose diagnosis was confirmed morphologically from 2016 to 2019 were included in the study. Patients with relapse, Down syndrome, and incomplete medical records were excluded from the study.
As we do not routinely do flow cytometry or cytogenetics for immunophenotyping and risk assessments, we classify ALL patients as standard and high-risk patients based on the 1993 NCI risk classification.,Those patients with WBC less than 50,000/uL and ages between 1 year and less than 10 years are treated as standard risk ALL.18 The rest including CNS positive are treated as high risk with four drugs regimen containing prednisolone, vincristine, L-asparaginase, doxorubicin, and intrathecal methotrexate. For CNS positive we add intrathecal cytarabine and hydrocortisone. For patients with standard-risk ALL, we omit doxorubicin.
A structured questionnaire was developed by reviewing similar studies10–13 to collect the data from the patient’s medical record. Trained final-year medical interns under close supervision of the principal investigator and collected data retrospectively.
Phone contact was used to extract sociodemographic data that was not available in the medical record. Measurements and responses were crosschecked for missed values, irregularities, inconsistencies, and corrective measures were taken as required.
Statistical Methods
Data were cleaned and coded before being entered and analyzed using Statistical Package for Social Sciences (SPSS) version 25. Descriptive statistics such as frequency, percentage, mean, and median with interquartile range (IQR) were used to summarize the data. Median with IQR was used to summarize patient and healthcare system delay times since the delay times were not normally distributed. Logistic regression was used to determine factors associated with induction mortality and delay in diagnosis. The multivariate logistic regression analysis included all variables that showed a significance level <0.2 in the univariate analysis.
Ethical Considerations
Ethical clearance was obtained from the Pediatrics and Child Health Department’s Research and Publications Committee of the School of Medicine, College of Health Sciences, and Addis Ababa University. Privacy and confidentiality of collected information were ensured at all levels using de-identification, password-protected computers, and storing of questionnaires in a lockable cabinet. Informed consent was done in accordance with the current revision of the Declaration of Helsinki.
Results
Clinical Characteristics
A total of 166 children were included in this study. The majority of the patients were males (71.1%). The mean age at diagnosis was 5.9 (± 3.1) years and 81.9% were below 10 years of age. As shown in Table 1, the most common presenting symptoms were fatigue (42.4%), fever (40.4%), and neck swelling (19.6%). Of those 79 patients with complete weight and height information, 16.5% had severe wasting. The respective median white blood cell count, hemoglobin, platelet count, and lactate dehydrogenase levels at presentation were 17,400/mm3(IQR: 5500–76,000/mm3), 7.0 g/dl (IQR:5.18.8 g/dl), 27,000/mm3(IQR:27–59,300/mm3 and 1070 (IQR: 633–1742). About 83.7% of patients were diagnosed and treated for neutropenic fever during the induction period.
 |
Table 1 Clinical Characteristics of Children Diagnosed with ALL at Presentation |
Fifty-two of the 166 children (31.3%) died during the induction period.
Delay in Diagnosis and Induction Chemotherapy Initiation
The median time interval from the onset of symptoms to the initiation of chemotherapy (overall delay), from arrival to TASH to confirmed diagnosis (diagnosis delay), and confirmed diagnosis to chemotherapy initiation (treatment delay) was 53.5 days (IQR: 38–93.5 days), 11 days (IQR: 7–17 days) and 8.5 days (IQR: 3–13 days), respectively. Only 13.3% (n = 22) of patients started treatment within 30 days of symptom onset.
Causes of Delay
Patients coming from outside of Addis Ababa town and repeated bone marrow procedures were associated with patient delay and diagnostic delay, respectively (Table 2 and Table 3).
 |
Table 2 Association Between Patients’ Sociodemographic Status and Patient Delay |
 |
Table 3 Association Between the Number of Bone Marrow Aspiration Procedures and Diagnosis Delay |
Induction Mortality vs Overall Treatment Delay
As shown in Figure 1, children whose overall treatment delay was less than 30 days had a statistically significantly improved survival (86.4%) compared to those children whose overall treatment delay was 30–120 days (60%) (p=0.02). Similarly, survival in children with a delay of >120 days was better than in the 30–120-day delay group. As shown in Table 4, there were no statistically significant differences among the different groups of patients regarding overall treatment delay.
 |
Table 4 Patient Characteristics Among the Subgroup with Overall Treatment Delay |
Predictors of Induction Mortality
As shown in Table 5, after univariate logistic regression analysis, and screening for multivariable binary logistic regression, only 10 variables (one continuous variable is age in years at diagnosis and nine categorical variables) were included in the final model. In the final multivariable logistic regression model, only ALL risk status and diagnosis delay were significantly associated with induction mortality.
 |
Table 5 Predictors of Induction Mortality Among Ethiopian Children Treated for ALL |
After controlling all possible confounding variables, patients diagnosed with high-risk ALL were nearly four times (AOR: 3.63; 95% CI: 1.11–12.30, p-value=0.039) more likely to experience induction mortality compared to standard-risk ALL patients. Patients with an overall delay between 30 and 60 days and 61–90 days were 3.96 times (AOR: 3.96; 95% CI: 1.06–14.88; p-value=0.041) and 5.44 times (AOR: 5.44; 95% CI: 1.31–22.58; p value=0.020) more likely to die compared to patients with <30 days of overall delay.
Discussion
Delay in diagnosis and treatment has been described in many studies, with variable results according to the type of cancer studied. Generally, acute leukemia is diagnosed quickly compared to other pediatric cancers. This could be secondary to the early presentation of symptoms within the disease course. Our study had include 166 patients, included in the study and 71.1% of the patients were male. This finding is consistent across many studies. Reasons underlying sex differences in childhood ALL risk are still unknown, as it has not been extensively evaluated. But underlying genetic differences might have played a role.17 The median time interval from the onset of symptoms to the initiation of chemotherapy was 53.5 days. Compared to studies done in Brazil,10 Nicaragua,11 Bangladesh,19 Mexico,20 and Nigeria (Enugu),21 where the median overall delay lies between 30 and 35 days, our results showed greater delay. But it is comparable to studies done in Nigeria (Ibadan),22 and Kenya.23 As our hospital spans a large catchment area and is the major comprehensive cancer treatment center for the nation, longer travel time and the large volume of patients might have contributed to the longer overall delay compared to those studies showing less lag time.
The place of residence outside Addis Ababa showed a significant association with overall delay. But other variables such as age, sex, birth order, marital status, and types of major presenting symptoms showed no associations. Similar results had been reported in Bangladesh19 and Botswana24 but a positive association has been depicted in those studies done in Iraq,25 Brazil,10 and Central America.16
Healthcare system delays were further subclassified as diagnostic and treatment delays in our study. Our median diagnostic and treatment delay was 11 and 8.5 days, respectively. Compared to a study done in Egypt,26 our diagnosis delay is comparable, but the median treatment delay is longer than studies done in Egypt,26 Canada,27 and India.12 It is a challenge to compare our data with other studies regarding diagnostic delay as they defined “diagnosis delay” as the time from any first healthcare contact to cancer diagnosis; however, we defined it as the time interval to establish a diagnosis after the child has reached our cancer treatment facility. In addition, as it is a retrospective study, collecting data regarding the first healthcare contact was not possible.
The number of bone marrow aspiration procedures required to confirm the diagnosis is strongly associated with diagnosis delay. In our study, more than 1/3 of our patients required a BMA procedure more than once. Those patients who required bone marrow aspiration procedures 3 or more times were four times more likely to have a diagnosis delay compared to those who had once. Although the reason for doing repeated bone marrow aspirations was not addressed in this study, inconclusive results have been frequently noted in the pathologist’s report. Techniques of the procedure, inadequate experience as it is done by residents, quality of equipment, staining method, absence of appropriately sized needles for children below the age of 2 years, and patient condition, are likely reasons why the procedure might have been done multiple times. And even for those who required only a single bone marrow procedure once, a timely procedure could be difficult. There may have been delays because of the child’s condition. In our experience, children with severe thrombocytopenia, severe anemia, and respiratory problems are not cleared by anesthesia until deemed stable, thus resulting in further delays in diagnosis.
Another facet of health care system delay is a delay in starting treatment once the diagnosis is established. We found that patients needed to wait a median of 8.5 days to start the treatment and 25% of the patients had to wait more than 13 days in the emergency room before admission to the ward to start chemotherapy. Although reasons for treatment delay were not assessed in this study, we observed that most patients could not be admitted to the ward sooner because of a lack of bed availability. Further studies should be done to explore reasons for treatment delay.
Induction mortality in this study is high compared to most studies done. Reports vary depending on the setting and timing of the report. High-income countries report between 0.2% and 2% whereas low and middle-income countries report between 10% and 30%. Our report was on the higher end (33%). Though our study did not investigate the causes of death, high-risk patients died more frequently than standard-risk patients underscoring. The treatment-related mortality of doxorubicin which is well-proven in many studies shifting their treatment approach towards three-drug regimens till they improve their supportive care.15,28–38 Most studies emphasized examining the duration of lag time with its determinant factors.19–26 In our study, we also analyzed the impact of overall treatment delay on induction mortality.
We found an association between delay in diagnosis and treatment with early mortality of patients with ALL. One exception to this is, that the mortality rate for children whose delay was >120 days was lower than for shorter delays (30–120 days). One possible explanation is that these children may have had a more indolent disease.
Kulkarni et al showed that survival probability decreased as a symptom-to-diagnosis interval increased.39 Julian et al showed that those with a treatment delay >3 days after diagnosis had inferior overall survival and event-free survival.27 Mecneide et al found no association between overall delay and mortality. This study took 30 days as a cut-off to define delay.10
Agrawal et al showed that a delay of more than seven days after presentation to the hospital has no association with worse outcomes.12 Wahl et al demonstrated a weekend delay in the initiation of chemotherapy showed an increased trend of bacteremia and intensive care unit admissions but no impact on mortality or relapse.38 Most of these studies looked at shorter delay time than we examined; hence that may explain why fewer noted increased mortality with delay. Other studies of the impact of delay on mortality looked at mortality beyond the induction period.39–41
To strongly consider delay as a cause for early mortality, cytogenetics, immunophenotype, causes of death, and course of the patient were not assessed. And how exactly delay is associated with increased mortality remains to be proven. It is difficult to conclude the death is sole because of the delay as high-risk patients were more likely to die. As the research is retrospective, it suffers from missing data. Most of this study’s clinical and laboratory findings are initial values. Subsequent changes were not reflected. Therefore, we recommend a prospective study for further analysis.
In conclusion, there is a strong association between overall delay and induction mortality. Some potential areas of intervention can improve the delays associated with timely presentation and early initiation of treatment. Education of provincial practicing physicians on signs and symptoms of acute leukemia, improvement in the process of obtaining bone marrow samples, and working with the pathology team to determine how to improve diagnostic quality of samples and with the hospital level to improve bed space and equipment availability will reduce the overall treatment delay.
Acknowledgment
We express our gratitude to the College of Health Sciences management of Addis Ababa University for allowing the research to be done and The Aslan Project, USA. for facilating the work to go smoothly . We also thank Dr. Kaleab Debebe and Dr. Miskir Tesfaye who participated in the data collection and clearing process.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final. Approval of the version to be published; have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. World Health Organization. WHO Global Initiative for Childhood Cancer – An Overview. Vol. 5. World Health Organization; 2020.
2. Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731.
3. Force LM, Abdollahpour I, Advani SM, et al. The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019;20(9):1211–1225.
4. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood, and adolescent cancer statistics. CA Cancer J Clin. 2014;64(2):83–103.
5. Gupta S, Howard SC, Hunger SP, et al. Treating childhood cancer in low- and middle-income countries; 2008:121–146.
6. Gatta G, Rossi S, Foschi R, et al. Survival and cure trends for European children, adolescents, and young adults diagnosed with acute lymphoblastic leukemia from 1982 to 2002. Haematologica. 2013;98(5):744–752.
7. Tandon S. Acute leukemia treatment in low - and middle - income countries: is it time for tailored therapy? Cancer Res Stat Treat. 2020;3:18–19.
8. Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–2539.
9. Friedrich P, Lam CG, Itriago E, et al. Magnitude of treatment abandonment in childhood cancer. PLoS One. 2015;10(9):1–15.
10. Lins MM, Amorim M, Vilela P, et al. Delayed diagnosis of leukemia and association with morbid-mortality in children in Pernambuco, Brazil. J Pediatr Hematol Oncol. 2012;34(7):271–276. doi:10.1097/MPH.0b013e3182580bea
11. De Angelis C, Pacheco C, Lucchini G, et al. The experience in Nicaragua: childhood leukemia in low income countries—the main cause of late diagnosis may be “medical delay”. Int J Pediatr. 2012;2012:5 pages. doi:10.1155/2012/129707
12. Agrawal V, Kayal S, Ganesan P, Dubashi B. Chemotherapy delays are associated with inferior outcome in acute lymphoblastic leukemia: a retrospective study from a tertiary cancer center in South India. Indian J Med Paediatr Oncol. 2021;42(1):51–60.
13. Rubnitz JE, Lensing S, Zhou Y, et al. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude Experie Cancer. 2004;101:1677–1684.
14. Hargrave DR, Hann IM, Richards SM, et al. Progressive reduction in treatment related deaths in Medical Research Council childhood lymphoblastic leukemia trials from 1980 to 1997 (UKALL VIII, X and XI). Br J Haematol. 2001;112:293299.
15. Prucker C, Attarbaschi A, Peters C, et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster Study Group. Leukemia. 2009;23:1264–1269.
16. Gupta S, Bonilla M, Valverde P, et al. Treatment-related mortality in children with acute lymphoblastic leukemia leukemia in Central America: incidence, timing and predictors. Eur J Cancer. 2012;48(9):1363–1369.
17. Singh SK, Lupo PJ, Scheurer ME, et al. A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine. 2016;95(46):pe5300. doi:10.1097/MD.0000000000005300
18. Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18–24. PMID: 8558195. doi:10.1200/JCO.1996.14.1.18
19. Begum M, Islam M, Akhtar M, Karim S. Evaluation of delays in diagnosis and treatment of childhood malignancies in Bangladesh. South Asian J Cancer. 2016;5:192–193.
20. Fajardo-Gutiérrez A, Sandoval-Mex AM, Mejía-Aranguré JM, Rendón-Macías ME, Martínez-García Mdel C. Clinical and social factors that affect the time to diagnosis of Mexican children with cancer. Med Pediatr Oncol. 2002;39(1):2531.
21. Chukwu BF, Ezenwosu OU, Ikefuna AN, Emodi IJ. Diagnostic delay in pediatric cancer in Enugu, Nigeria: a prospective study. Pediatr Hematol Oncol. 2015;32(2):164–171.
22. Brown BJ, Ajayi SO, Ogun OA, Oladokun R. Factors influencing time to diagnosis of childhood cancer in Ibadan, Nigeria. Afr Health Sci. 2009;9(4):247–253.
23. Njuguna F, Martijn H, Langat S, et al. Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya. Pediatr Hematol Oncol. 2016;:33(3):186–199.
24. Carpenter K, Slone AK, Scheuer M, Mehta PS, Slone JS. Factors influencing diagnostic delays of pediatric cancers in Botswana. Pediatr Blood Cancer. 2020;2022:e28182.
25. Yadalla W, Al-Jadiry MF, Faraj SA, et al. Delay in diagnosis of cancer in Iraq: implications for survival and health outcomes at Children’s Welfare Teaching Hospital in Baghdad. J Glob Health. 2021;5:e2021047.
26. Suzy A, Shaimaa K, Ahmed M, Ashraf F. Delays in diagnosis and treatment among children with cancer: Egyptian perspective. EMHJ. 2017;23:6.
27. Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204–209.
28. Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938–2948.
29. Mostert S, Sitaresmi MN, Gundy CM, Veerman AJ. Influence of socioeconomic status on childhood acute lymphoblastic leukemia treatment in Indonesia. Pediatrics. 2006;118(6):e1600–e1606.
30. Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61–73.
31. Seif A, Fisher B, Li Y, et al. Patient and hospital factors associated with induction mortality in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(5):846–852. doi:10.1002/pbc.24855
32. O’Connor D, Bate J, Wade R, et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood. 2014;124(7):1056–1061).
33. Nakagawa S, Kato M, Imamura T, et al. InHospital management might reduce induction deaths in pediatric patients with acute lymphoblastic leukemia: results from a Japanese cohort. J Pediatr Hematol Oncol. 2021;43(2):39–46. doi:10.1097/MPH.000000000000192
34. Khan M, Naseem L, Manzoor R, Yasmeen N. Mortality analysis in children during induction therapy for acute lymphoblastic leukemia. Int J Cancer. 2013;132:427–436.
35. Maaz A, Mahmood T. High infection-related mortality in Pakistani children with acute lymphoblastic leukaemia during remission induction chemotherapy: review of data from a single institution. JCancer Allied Spec. 2016;2(4):6.
36. Wolf J, Flynn P, Gaur A, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65(11):1790–1798.
37. Fadoo Z, Nisar I, Yousuf F, et al. Clinical features and induction outcome of childhood acute lymphoblastic leukemia in a lower/middle-income population: a multi-institutional report from Pakistan. Pediatr Blood Cancer. 2015;62:1700–1708.
38. Abdul Mabood S, Fouda AE, Boujettif F, Mansour A. Treatment outcomes of children with acute lymphoblastic leukemia in a middle-income developing country: high mortalities, early relapses, and poor survival. J Pediatr. 2020;96:108--–16.
39. Wahl SK, Gildengorin G, Feusner J. Weekend delay in initiation of chemotherapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;34(1):e8–e11.
40. Suarez A, Pina M, Nichols-Vinueza D, et al. A strategy to improve treatment-related mortality and abandonment of therapy for childhood ALL in a developing country reveals the impact of treatment delays. Pediatr Blood Cancer. 2015;62:1395–1402.
41. Loh AH, Aung L, Ha C, Tan AM, Quah TC, Chui CH. Diagnostic delay in pediatric solid tumors: a population-based study on determinants and impact on outcomes. Pediatr Blood Cancer. 2012;58(4):561–565.
42. Kulkarni KP, Marwaha RK, Trehan A, Bansal D. Survival outcome in childhood ALL: experience from a tertiary care center in North India. Pediatr Blood Cancer. 2009;53:168–173.
43. Amelia Y, Anna C, Kahlia F, et al. Treatment delay and the risk of relapse in pediatric acute lymphoblastic leukemia, Pediatric. Hematol Oncol. 2017;34:38–42.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
