Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Immunotherapy: Constructive Approach for Breast Cancer Treatment
Authors Anayyat U , Ahad F , Muluh TA , Zaidi SAA, Usmani F, Yang H, Li M, Hassan HA, Wang X
Received 6 June 2023
Accepted for publication 28 November 2023
Published 15 December 2023 Volume 2023:15 Pages 925—951
DOI https://doi.org/10.2147/BCTT.S424624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Pranela Rameshwar
Umer Anayyat,1 Faiza Ahad,1 Tobias Achu Muluh,1 Syed Aqib Ali Zaidi,1 Faiza Usmani,2 Hua Yang,1 Mengqing Li,1 Hammad Ali Hassan,3 Xiaomei Wang1
1Department of Physiology, School of Basic Medical Sciences, Health Sciences Center, Shenzhen University Medical School, Shenzhen University, Shenzhen, Guangdong, 518055, People’s Republic of China; 2Department of Biotechnology, University of Karachi, Karachi, Pakistan; 3Department of Cell Developmental and Integrative Biology, University of Alabama at Birmingham, Birmingham, AL, USA
Correspondence: Xiaomei Wang, Department of Physiology, School of Basic Medical Sciences, Health Sciences Center, Shenzhen University Medical School, Shenzhen University, Shenzhen, Guangdong, 518055, People’s Republic of China, Tel +86-13544234361, Email [email protected]
Abstract: A novel and rapid therapeutic approach is the treatment of human breast cancer by enhancing the host’s immune system. In initial findings, program death one (PD-1) and program cell death ligand one (PD-L1) showed positive results towards solid tumors, but tumor relapse and drug resistance are the major concerns. Breast cancer therapy has been transformed by the advent of immune checkpoint blockades (ICBs). Triple-negative breast cancers (TNBCs) have exhibited enduring responses to clinical usage of immune checkpoint inhibitors (ICBs) like atezolizumab and pembrolizumab. Nonetheless, a notable proportion of individuals with TNBC do not experience advantages from these treatments, and there is limited comprehension of the resistance mechanisms. Another approach to overcome resistance is cancer stem cells (CSCs), as these cells are crucial for the initiation and growth of tumors in the body. Various cancer vaccines are created using stem cells (dendritic, whole cell, bacterial) and focus primarily on targeting tumor-related antigens. The ultimate objective of cancer vaccines is to immunize the patients by active artificial immunity against cancer, though. In this review, we primarily focused on existing immunotherapeutic options, immune checkpoint blockers, the latest progress in understanding the molecular mechanisms underlying resistance to immune checkpoint inhibitors (ICBs), advanced strategies to overcome resistance to ICBs, cancer stem cell antigens and molecular markers, ongoing clinical trials for BCs and cancer vaccines for breast cancer.
Keywords: breast cancer, cancer stem cell, immunotherapy, cancer therapy
Introduction
Breast cancer is the deadliest cancer among women worldwide and the most prevalent cancer after lung cancer.1 Breast cancer can be divided into three subtypes based on the presence or absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal receptor2 (HER2), and instantaneous absenteeism of ER, PR, and HER2 is known as triple-negative breast cancer comprised of six subclasses. At present, conventional breast cancer therapeutic approaches used are chemotherapy, surgery, and radiotherapy.2 Among the array of emerging immune therapeutic approaches, immunotherapy is the focused approach for researchers worldwide as it embraces various approaches, including vaccines, adoptive cell therapies, oncolytic viruses, and markedly, immune checkpoint blockade3 Of these approaches, the FDA-approved the following strategies: programmed cell death receptor 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) for solid tumors in breast cancer.4
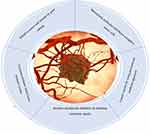
In the tumor microenvironment (TME), heterogeneous cells exhibit adaptable behavior that contributes to the formation, as well as the development of malignant tumors. This behavior resembles the characteristics of cancer. The tumor microenvironment is essential for the emergence of diseases, the evolution of heterogeneous diseases, the recurrence of diseases, and susceptibility to or resistance to treatments5 (Figure 1). Breast carcinoma patients account for up to 38% of all carcinoma in the world, and it is estimated to exist in 1 out of every 8 cancer diagnoses, according to the most recent WHO estimates. Globally, there will be 2.3 million additional instances and 685,000 deaths from it in 2020. According to worldwide demographic forecasts, this burden is expected to increase by a total of three million instances and 1 million fatalities every single year.6
Breast cancer development takes place by the abnormal growth of malignant cells in the epithelial linings of lobes or ducts of the breast. The primary tumor starts in the breast; due to invasive potential, it infiltrates into the lymph nodes. Lymphatic vessels are among the major contributors and reservoirs in metastasis. Cancer cells settle in the lymph nodes in the subcapsular sinuses, proliferate, and can eventually expand outside the capsule and destroy the lymph nodes. Intact lymph nodes have sinus veins, which are connected to blood vessels through lymph vessels and become a part of this circulatory system. Tumor cells reside at other secondary sites of the body and metastasize. Cases having metastatic breast cancer have the highest mortality rate, and tumor-associated lymphatic vessels have proved to be among the important factors for the early spread of cancer cells.7,8
Challenges in Cancer Treatment
In recent times, there have been two significant revolutions that have changed the procedure of cancer treatment: achievable alterations for tumor-causing cancers and immunotherapies for immune-related tumors. There are still major challenges in the two areas of cancer treatment. Only a small percentage of patients with specific types of tumors have pharmacologically curable genetic alterations, which limits the evidence available for their therapeutic impact in biomarker-based research studies. The use of next-generation sequencing technology for molecular preselection in clinical research is growing, but its widespread use in clinical practices is complicated by the issue of substantial clinical interpretation of genome data. The primary obstacle hindering the success of precise oncology could be surmounting tumor heterogeneity and acquired treatment resistance. To help us identify these subgroups and enhance the potential indicators of treatment provision and selection, immunological checkpoint impediments (programmed cell protein [PD-1/L1] and the monoclonal antigen a cytotoxic T lymphocyte apoptosis-1/ligand-1) are only used in a small number of patients.9 Initially, most of the instances illustrate the basic concept of carcinogenic addiction. In these tumor models, a single-driver mutation has unique biological properties and can drive the basic capacity of tumors, making cancer cells highly dependent on specific genetic changes to survive10 (Figure 2).
Cancer Immunosurveillance
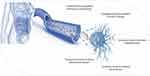
Cancer immunosurveillance is comprised of the recognition and destruction of the infected cells or aberrant cells depicting cancerous phenotype. This process detects the disruptions in tissue’s familiar homeostasis often triggered by infection or inflammation through the antigenic molecules expressed on their surfaces. However, tumor cells can escape this immunosurveillance mechanism by a variety of approaches that mask their malignant phenotype. Administering vaccinations based on cancer antigens can improve immunosurveillance.11 Paul Ehrlich was among the ones who conceived of the idea that the immune system could suppress the potential “activation” of carcinoma.12,13 The fact of cancer immune monitoring, which is a part of the more general procedure of cancer immunological sensibility, made that mechanism clear. This procedure eliminates cancers and records the immunological phenotypic of malignancies that will inevitably develop in an immunological host. A brief explanation of cancer and immune response is in (Figure 3).
Carcinoma immunohydration plays two distinct functions in preventing malignancies from being destroyed by the body’s natural defenses and improving the host’s defense against cancer.14 The immune system’s response against cancers has involved natural killer (NK) cells. They are lymphocytes that are a part of the innate immune system, and they can regulate the aberrant production of MHC class I molecules and cell stress indicators on the surface of autophagy cells. The Natural killer group 2D (NKG2D) receptor can recognize various molecules related to the first type of MHC. These molecules are believed to act as “immune signals” in malignant cells and enhance the application of NKG2D in mediated countermeasures Fields.15,16 As the fundamental processes responsible for the immune response’s association with the microenvironment of tumors become more fully understood, immunotherapy is more effectively used to treat cancer. Utilizing the immune system to better fight the disease is known as immunotherapy. Immunotherapy offers greater selectivity and less toxicity than traditional cancer treatments like chemotherapy and radiotherapy. Additionally, it provides long-lasting immunity via immunological memory. The development of numerous monotherapies and combination medicines that target particular cancer types is a result of the increased interest in immunotherapies17 (Figure 4).
For detecting and eliminating cancer cells, a series of complex natural mechanisms have been developed by the immune system. These mechanisms can stop the growth of malignancies, but they generate selective pressure on the cancerous cells, which help in the progression of resistant or non-immunogenic phenotype-containing tumors. Thus, the three phases of eradication, equilibrium, and escape make up the editing processes used by tumors to circumvent the body’s immune system.18
Host’s adaptive as well as innate immune systems identify tumor-related antigens during the elimination phase and react to them.19 It mostly depends on the propensity to guide the immune response. In cancer cells results in the production of new antigens (neoantigens) on the cell surface.20 Human leukocyte antigen class I (HLA-1) is present, presented, and used to treat these novel antigens as antigen-derived peptides. Neoantigens formed in the tumor microenvironment are displayed and processed as antigen-derived peptides by adhering to the antigen-presenting cells (APC) with human leukocyte antigen class II (HLA-II). The helper T-cell receptors identify the aforementioned complex, and as a result, the cytotoxic T-cells and B-cells are subsequently stimulated and matured. The stimulated T-cells cause the cancer cells to undergo apoptosis by releasing cytotoxic granules through the Fas cell surface death receptor (FAS) and activating the caspase cascade.21,22 Certain malignant cells with a robust and innocuous nature manage to evade the removal process and establish a stable state where they can continue to grow. When the immune system is integrated, the tumor’s growth can be entirely stopped, but the surviving malignant clones are subject to selective pressure.23
The malignant tumor cells egress as they thwart the tumor’s immunological response. Malignant cells that are resistant to therapies for cancer and the immune system escape and multiply rapidly to maintain their survival.24 The tumor will grow and spread to more places as a result of this. The absence or mutation of antigens,25 the manipulation of cytokine expression,26 alterations in APCs,27 dysfunction of the effector cells, alterations in HLA-1 expression,28 apoptosis-resistant tumor cells selection and the regulation of immune checkpoint proteins and other processes have all been proposed to explain the evolution of this escape.29–31
The maturation and activation of immune cells serve as a conduit for immunosurveillance. The two-signal paradigm of lymphocyte activation, in which a single antigen simultaneously binds and activates T-cells and antigen-presenting cells (APC), is used to activate lymphocytes. The co-stimulatory antigen interacts with both the HLA-II on APCs and the T-cell receptor expressed on T-cells. The balance between co-stimulatory impulses, inhibitory signals, and immunological checkpoints, which limit tissue damage, determines this immune response at various levels. The immunosuppression leading to immune escape by tumors is facilitated by the imbalance of inhibitory signals. Moreover, immune checkpoint receptor-ligand combinations and their inhibitors have proved to be associated with inducing and suppressing the lymphocyte-mediated immune response against cancer.32
Programmed Cell Death Protein 1 (PD-1, CD279), Programmed Cell Death Protein 1 Ligand (PD-L1, CD274), and Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4, CD152) have a crucial role in regulating and generating an immune response against cancer and proved to be an effective target for cancer immunotherapy. The PD-1 receptor is temporarily expressed as a membrane protein in different activated immune cells, whereas it is constitutively expressed in T-cells, representing an exhaustive phenotype. When PD-1 and its ligand PD-L1 are bound, the nearest activation elements of the T-cell receptor are dephosphorylated, which inhibits various T-cell signaling pathways. The synthesis of cytokines, survival, expansion, and T-cell effector capabilities are all decreased by this suppression. Therefore, it is possible to reestablish the eradication of cells that are cancerous by immune defenses by preventing PD-1 from interacting with PD-L1.33
Furthermore, T-cell activation is regulated by costimulatory signals, which are CTLA-4 (negative regulator) and CD28 (positive regulator), which are independent of the antigens. The CTLA-4 prevents the T-cell activation, whereas CD28 triggers the cytokines secretion and T-cell activation. CD86 and CD80 trigger both positive and negative regulators for T-cell differentiation and proliferation expressed on APCs. Negative regulation by CTLA-4 is characterized by the dephosphorylation of signaling proteins produced by T-cell receptors by an increased ratio of CTLA-4: CD80/86. Contrary to this, positive regulation is mediated by the increased production of growth cytokines in the presence of an elevated ratio of CD28:CD80/86. The binding affinity of CTLA-4 for CD80/86 is greater than CD28, which also induces their removal from the APCs. Preventing the binding of CTLA-4 with CD80/86 can impede immunosuppression and regaining the antitumor reaction of the body.1,34
Ever since the tumors have exploited various mechanisms to evade immunosurveillance, therefore, recent trends in cancer therapeutic research are more directed toward activating a patient’s natural immunity to recognize and destroy the malignant cells. Based on this, some of the developing modalities of cancer immunotherapy are adoptive cell immunotherapy, oncolytic viruses, anticancer vaccines, and tumor-targeting immunotherapies, which destroy the tumor and function at different levels of immunity. This will make cancer immunotherapy a therapeutic choice harmonizing with radiotherapy, chemotherapy, and surgery.35–38
Cancer Immunotherapy
Through the years, there have been notable improvements in cancer therapies. Cancer immunotherapy, as opposed to direct medicinal treatment of cancer cells, strengthens the host’s immune system to recognize particular cancer cells for efficient elimination. The escape mechanism of tumors, which can influence the immune system’s ability to reinvigorate the anti-tumor immunological activity and defeat the escape pathway, is being studied in the development of cancer treatment.39
Immunotherapy and Cytokines
Earlier cancer immunotherapy modifies immune cell function with specific cytokines. High doses of interleukin-2 (IL-2) and interferon (IFN)-2b, for instance, might have several different impacts.31
Immunotherapy and Nanoparticles
Most of the therapies used to combat cancer have a systemic mode of action rather than a specific one, which reduces their efficacy and facilitates absorption at non-specific sites. Penetration of immunotherapeutic drugs can be enhanced by mixing nanoparticles with immunomodulators. This will initiate immune cells and regulate the tumor microenvironment, thus enhancing anti-cancer immunity. Nanocarriers for immunomodulators can be natural (lipid-based nanoparticles, extracellular vehicles) and synthetic (polymeric nanoparticles, inorganic nanoparticles). Delivery systems of immunomodulators coupled with nanoparticles can be Peptide/ protein-based nanoparticle delivery or Nucleic acid-based nanoparticle delivery systems. In peptide/ protein-based delivery systems, whole antigenic protein or its subunit derived from tumor cells can be attached with nanocarriers via recombinant DNA technology. Peptide vaccines can be produced directly from APCs. These are to initiate the desired immune response and generate immunological memory. The Nucleic acid-based nanoparticles system is performed by pDNA, siRNA, ODN, and mRNA. Nucleic acids have limited bioavailability and susceptibility to degradation by internal and external nucleases; they must be protected by encapsulation in nanoparticles. Both mRNA and pDNA are the preferred choice for immunomodulation as they can produce desired immunogenic proteins and immunostimulant cytokines. Through multiple pre-clinical and clinical trials, they have proved to possess limited side effects and suitability reactions40,41 (Figure 5).
Immunotherapy and T-Cell Response
Natural killer (NK) cells can quickly eliminate several successive cells if they exhibit surface-level indications of oncogenic transformation. Natural killer cells are supported in their role as anticancer agents by the distinct characteristics of the immune system and their capacity to boost T cell and antibody responses. Activation, growth, and genetic alteration of NK cells in vitro can dramatically increase their anti-tumor activity and help them overcome resistance, even if tumors have developed a variety of defenses against the incursion of endogenous natural killer cells. Some of these techniques have been developed into platforms fit for use in clinical trials, supporting the injection of natural killer cells into those who have aggressive hematomas or solid tumors. These techniques have so far yielded great outcomes.42,43
Immunotherapy and Immune Checkpoints
The regulation of the immunosurveillance mechanism of cancer takes place at multiple steps, which is mediated by immune checkpoints, and co-stimulatory and inhibitory signals.
These regulators actively contribute to the suppression or progression of the immune system against abnormal growth. Over time, different therapies targeting positive and negative regulators of immune checkpoints have been developed. The CTLA4 has an inhibitory role in the T-cell activation. It is up-regulated in T cells, showing exhausted phenotypes and CD4+ T-cells. Blocking its interaction with CD80/86 may result in T-cell activation. The antagonists of CTLA4 were permitted through the Food and Drug Administration in 2011 and are commercially available as Yervoy® (ipilimumab, Bristol Myers Squibb). It has been used as a monotherapy and in combination for the treatment of advanced melanoma, advanced renal carcinoma, metastatic melanoma, and metastatic colorectal cancer.44
Immunotherapeutic strategies targetting cancer, such as ICB (immune checkpoint blockades) and CAR (chimeric antigen receptors) T-cell therapies, have demonstrated efficacy in yielding promising outcomes across diverse cancer types, specifically breast cancer.45 Immune checkpoint blockade clinical trials are going on, and some are approved for triple-negative breast cancer. Here, we will discuss immune checkpoint inhibitors in detail:
Pembrolizumab
Pembrolizumab, a humanized monoclonal antibody, was evaluated for its safety and anti-cancerous properties against PD1 in advanced triple-negative breast cancer, gastric cancer, head and neck cancer, and urothelial cancer. The overall survival rate for triple-negative breast cancer patients was median with higher PD-L1 expression.46
Atezolizumab
Atezolizumab (a monoclonal antibody) assessing for the safety and effectiveness of targetting PDL-1, as a single agent in patients with advanced solid and hematologic malignancies (NCT01375842), the overall response rate (ORR) stood at 10% for 116 patients with metastatic triple-negative breast cancer(mTNBC).47 Analysis of specific patient subgroups indicated that individuals receiving atezolizumab as their initial treatment exhibited a higher overall response of 24%, in contrast to those receiving it in second-line settings and beyond, where the overall response was 6%. The study highlighted a correlation between the efficacy of atezolizumab as a standalone treatment and increased level of PD-L1 positive immune cells.48
Nivolumab, Avelumab and Durvalumab (PD-1 and PD-L1 Inhibitors)
The TONIC Phase 2 trials (NCT02499367) explored the efficacy of nivolumab, a humanized monoclonal antibody targetting PD-1, across different treatment protocols. When administered after pretreatment with cisplatin and doxorubicin, nivolumab demonstrated an elevated overall rate of 23% and 35% respectively, as compared to 17% in metastatic triple-negative breast cancer patients without pre-treatment.49 In the JAVELIN Solid Tumor trial (NCT01772004), mTNBC patients received a human antibody, avelumab, against PD-L1. The study documented the overall response rate of 3.0%, with a slightly higher ORR of 5.2% observed within the subgroup of triple-negative breast cancer (TNBC) patients.50 The GeparNuevo Phase 3 clinical trial (NCT02685059) assessed the effectiveness of combining durvalumab, a monoclonal antibody targetting PD-L1, with chemotherapy as a treatment approach for primary TNBC.51
Ipilimumab (Anti-CTLA-4 Inhibitor)
In an initial investigation to assess the impact of preoperative ipilimumab, a monoclonal antibody targetting CTLA-4, in conjunction with cryoablation, a total of 19 breast cancer patients spanning various subtypes were subjected to treatment with cryoablation, ipilimumab or a combination of both.52 The main motive behind this research was that the tumor cryoablation process might support tumor-specific immunity by causing cell lysis, potentially augmenting the presentation of tumor antigens to immune cells.53 The researchers illustrated that cryo-immunotherapy treatment elevated not just Ki67+ T cells but also the ratio of Ki67+ effector T cells to Ki67+ regulatory T cells, as compared to cryoablation or ipilimumab administered alone.52 A separate study examined the efficacy of combining tremelimumab, a monoclonal antibody that targets CTLA-4, with exemestane in patients with hormone receptors (HR)- positive metastatic breast cancer. Out of the entire cohort of 26 patients, 11 individuals (42%) maintained a stable disease status for more than 12 weeks. Moreover, there was an increase in the count of inducible costimulator-expressing T cells, accompanied by a decrease in Foxp3+ regulatory T cells (Tregs) after the treatment.54
Combinatorial Treatments
Inhibitors of poly (ADP-ribose) polymerase (PARP), such as niraparib, talazoparib, and olaparib, block the repair of single-strand DNA breaks, consequently inducing synthetic lethality in cancer with homologous recombination repair deficiencies.55 Approval for breast cancer patients with BRCA germline mutations has been granted to olaparib and talazoparib. PARP inhibitors (PARPi), by disrupting the DNA repair mechanisms, have been demonstrated to elevate neoantigens and tumor mutational burden, both of which are indicative of a strong response to immune checkpoint blockade (ICB) therapy.55 PARP inhibitors (PARPi) have been demonstrated to enhance PD-L1 expression in breast cancer by inhibiting GSK3, offering additional justification for combining PARPi with ICBs. Due to its high immunogenic potential, numerous clinical trials are currently underway to evaluate the effectiveness of combining PARP inhibitors with immune checkpoint blockers (ICBs) in patients with metastatic triple-negative breast cancer (mTNBC) (NCT02657889, NCT03167619).56
Hypothesized Molecular Mechanisms of Immune Checkpoint Inhibitor Resistance in TNBC
Immunotherapy holds an advantage over traditional treatment options in terms of treatment effect durability. Nonetheless, a limited fraction of patients can enjoy the enduring benefits due to low response rates and resistance.57 The development of resistance can be instigated by either tumor-extrinsic or intrinsic factors. Tumor-extrinsic pathways usually relate to the microenvironment of the tumor’s immune system, while tumor-intrinsic pathways are stimulated by a broader range of influences like abnormal oncogenic cues, disrupted immune regulatory points, and inflammatory or immune-damping proteins.57
Tumor Extrinsic Resistance Mechanism
Tumor-extrinsic elements encompass various components of the tumor microenvironment (TME), such as effector T cells, regulatory T cells, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and so forth. The preponderance of mechanisms causing resistance has been linked to T cells infiltrating the tumor. Scenery et al (2019), noted that the concurrent decline in T cell functionality due to aging restricts the impact of ICB treatment in TNBC by hindering IFN signaling and antigen presentation machinery.58 Moreover, stem-like CD8+ tumor-infiltrating lymphocytes (TILs) expressing Tcf1 and PD-1 foster the proliferation of specialized T cells within the tumor when exposed to ICB therapy. This indicates a potential function for these subsets as an innovative predictive indicator59 Tumor-infiltrating lymphocytes (TILs) comprising CD8+ cells with stem-like properties characterized by elevated TCF1 expression have been identified across different cancer varieties. These cells have been demonstrated to generate fully specialized CD8+ T cells, thereby forming a niche within the tumor that is susceptible to anti-tumor immune responses.60 In the case of triple-negative breast cancer (TNBC), drugs like cyclophosphamide and vinorelbine that trigger stem-like CD8+ T cells have shown enhancements in the effectiveness of PD-1 blockades. This suggests the involvement of stem-like CD8+ T cells in response to immune checkpoint blockers (ICBs).61
Tumor Intrinsic Resistance Mechanism
The abnormal triggering of oncogenic pathways has been linked to an immune-inactive tumor microenvironment (TME). A comprehensive analysis involving multiple omics data from individuals with triple-negative breast cancer (TNBC) has suggested the potential categorization of TNBC microenvironmental traits into three distinct clusters.62 The cluster designated as “immunologically active” (cluster 3) exhibited significant infiltration of immune cells and elevated expression of immune checkpoint molecules. In contrast, the cluster labeled as “innate-immune suppressed” (cluster 2), which demonstrated heightened mutation frequencies in the PI3K-AKT pathway, displayed diminished activity of innate immune cells and non-immune stromal cell infiltration.62 The cluster characterized as “immunologically inactive” (cluster 1), which showed minimal immune cell infiltration, was associated with MYC copy number amplification. Studies have indicated that MYC plays a role in orchestrating the lack of immunogenicity in the tumor microenvironment of triple-negative breast cancer (TNBC). At a mechanical level, MYC activates the transcription of DNMT1, an enzyme responsible for DNA methylation that inhibits the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway. The synergistic approach involving PD-1 inhibitors and decitabine, an inhibitor of DNA methyltransferase, demonstrated a significant and deep-seated anti-tumor impact in triple-negative breast cancer (TNBC) with overexpression of MYC.63 Stimulation of the Ras-mitogen-activated protein kinase (MAPK) pathway is linked to a decrease in MHC, PD-L1, and TIL levels within triple-negative breast cancer (TNBC). Consequently, utilizing MEK inhibitors alongside anti-PD1 antibodies resulted in synergistic inhibition of tumor growth in murine models.64
In a recent investigation, it was demonstrated that B7-H3, with abnormal glycosylation, contributes to fostering an immunosuppressive tumor microenvironment (TME) in TNBC. FUT8, a fucosyltransferase, glycosylates B7-H3, leading to the inhibition of T cell function within the TME and impeding NK cell infiltration into tumors. Remarkably, the fucosylation inhibitor 2F-Fuc significantly enhanced the effectiveness of anti-PD-L1 therapy in murine models of TNBC.65 Moreover, LINK-A, an extensive non-coding RNA, was evidenced to streamline the proteasome-induced breakdown of elements in the peptide-loading complex (PLC). This orchestration results in the establishment of steadfast peptide-MHC1 complexes, further amplifying the T-cell reaction. Employing locked nucleic acids to inhibit LINK-A augmented the stability of PLC elements, rendering tumors more responsive to immune checkpoint blockade (ICB) therapy.66
Immunity suppressors PD-1 and its ligand PD-L1. Their interaction may have resulted in terms of the inhibition of T-cell proliferation, survival, functioning, and generation of cytokines. By preventing their interaction, we can regain antitumor immune responses. Different PD-1 blockers are commercially available, such as Opdivo®, Keytruda®, and Libtayo®, which were approved by the FDA in 2014 and 2018, respectively. Commercially available PD-L1 inhibitors are Tecentriq®, Bavencio®, and Imfizi®, correspondingly approved for antitumor therapy in 2016 and 2017. The advanced stages of melanoma, non-small cell lung carcinoma, small cell lung carcinoma, renal cell carcinoma, Hodgkin lymphoma, head and neck squamous cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, colorectal carcinoma, B-cell lymphoma, cancer of the gastric tract, cancer of the cervical cavity, and Merkel cell carcinoma have all been treated with these drugs that have been approved for antagonist activity against PD-1 and PD-L1.67
Cancer Vaccine
The development of cancer vaccines for solid tumors is challenging. As solid tumors develop, the exertion is enforced through the immune system by selective pressure on cancer cells having differentiated malignant antigens. In the consortium, some malignant cells do not express tumorous antigens. Thus, they escape the immunosurveillance, become resistant, produce clones, and spread to distant locations. This phenomenon is called “immune liberation” and has functional similarity to selective stress caused by chemotherapy and hormone therapy. This clonal heterogeneity may lead to the molecular instability of solid tumors. This might provide the link to the high efficacy of cancer vaccines in animal models than medical situations.68–70
Vaccines against cancer stimulate the body’s immune system to find and destroy tumors. When exposed to tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs), they trigger type 1 CD+4 and CD+8 T cell replies.71,72 They may generate and amplify the adaptive immune response with minimal toxicity. This will develop immunologic memory, which can control and then eliminate the enduring ailment through retorting to TAA/TSA contact with time.73 Usage of cancer vaccines might be used to deterrence against oncogenic viruses (eg, human papillomavirus in cervical, for targeting early-stage tumor development (eg, human telomerase reverse transcript (hTERT) or p53); for treatment (expressed by known tumors targeting TAA (such as HER2). Cancer vaccines are used as a precautionary measure for disease prevention and control.74,75
Therapeutic Vaccines vs Preventive Vaccines
The immunotherapy for cancer can be further broken down into passive immunotherapy (or adaptive immunity), which includes antibodies, and proactive immunotherapy (such as vaccination therapy).76 Targeting tumor antigens with vaccination during active immunotherapy allows patients to elicit an immunological response and build immune memory.77 An innovative approach to tackling cancer is called vaccine-associated immunotherapy. Cancer cell migration can be stopped by tumor-related vaccinations that improve the immune surveillance.78–80
Preventive cancer vaccines produce stronger and broader responses to adjuvant CD+4 T1 (Th1) and CD+8 cytotoxic T lymphocytes (CTL) during the suppressed disease.81,82 Masses of human beings worldwide are affected by cancer, which continues to be the top cause of mortality despite recent improvements in diagnosis, prevention, and treatment. Several prophylactic cancer vaccines, including Gardasil (Merck), Cervarix (GlaxoSmithKline), and the curative vaccine Sipuleucel-T (Provenge), are in circulation thanks to the US Food and Drug Administration’s (FDA) authorization. The endorsement of such vaccines invigorated the cellular immunotherapy notion for cancer treatment. Adjuvants are an important part of effective vaccines, which can improve efficacy by enhancing specific immune responses to antigens. However, complexity challenges the designing of a safe, effective, and economically feasible adjuvant.83 Different adjuvant formulations are available as different administration methods. Restorative cancer vaccines have been tested for long on numerous diverse sorts of tumors containing several antigens (including co-mutated TAA and mutated patient-specific antigens). Effective cancer vaccines can provide concentrated antigens for class I and class II HLA molecules, thereby enhancing CD4 and CD8 T cell responses. Despite the use of adjuvants with cancer vaccines, suboptimal vaccine strategies and immune evading cancer microenvironment are the fundamental reasons for the failure to eradicate cancer.
The most effective vaccination bases comprise long-lasting synthesized peptides, DNA, and RNA vaccinations. To elicit a response from the immune system, the body receives DNA or RNA codes of certain antigens, which are then translated into the required antigenic protein. The treatment of chemotherapy, radiotherapy, indomethacin 2,3-dioxygenase (IDO) inhibitors, T cell checkpoint inhibitors, stimulators of particular tumor necrosis factors, inducers of receptor families, and inhibitors of undesirable cytokines are all examples of treatments that can lessen the immunosuppressive cancer microenvironment. The specificity of therapeutic vaccination in conjunction with this immunomodulation offers an alluring route for the development of future cancer treatments.84,85
Curative vaccines are applied options of active immunotherapy for malignancies, designed to treat advanced diseases using the patient’s immune system. Recent clinical trials have achieved encouraging results, which led to the US FDA approving the foremost curative cancer vaccine. This finding opens the door to increased anti-cancer efficacy and rationalization of future vaccines in addition to offering a new strategy for treating cancer. Numerous experimental and therapeutic vaccination methods are being assessed right now. Guo et al’s review discusses different curative cancer vaccines with their preclinical and clinical studies. Guo et al have also studied tumor-induced immunosuppression that impedes the effectiveness of therapeutic vaccines and potential approaches to counter these mechanisms to generate stronger and long-lasting immune responses against tumors.74
The immune basis from the randomized clinical trials of current cancer vaccination treatments (or “vaccine therapy”) has demonstrated the production of various proteins that were found to improve the survival rate. The release of prostatic acid phosphatase by the dendritic cells in the treatment of prostate cancer and tumor-derived auto shock proteins (gp96) are among them. Immunological monitoring of several clinical trials was unable to determine an alternative indicator of clinical outcome.86
Vaccines for Breast Cancer
In the past 20 years, breast cancer treatment has made significant progress, leading to a significant increase in disease-free survival.87 In particular, a growing understanding of the molecular basis of breast tumor formation has led to immunotherapy treatments in which antibodies are developed against tumor-associated antigens in the laboratory and used to inhibit specific cellular processes.77,87 Interest in immunotherapy continues to grow, and patients and physicians are excited and excited about the prospect of obtaining a breast cancer vaccine that can use the human immune function to search for and destroy cancer cells and prevent future progression of the illness. Compared to other treatments that can be used for breast cancer, this cancer vaccine will have significant advantages.79
Vaccines ought to be made to be preventive for illnesses with recognized pathogens, which are referred to as those brought on by viruses like influenza and polio, introducing immunogen into the organism before actual interaction with the human body. Disease. Cancer presents a more challenging position even though some malignancies, like cervical cancer, are linked to particular pathogens.88,89
Table 1 explains lists the antigens that have been examined as prospective candidates for breast cancer vaccinations. Although these antigens may only be expressed in fetal growth (carcinoembryonic antigen) or stem cells (hTERT) in normal cells, they are excessively expressed or altered in carcinoma of the breast cells (Table 1).
 |
Table 1 Targeted Antigen from Experimental Production of Vaccines Against Breast Cancer |
Numerous cancer vaccines are now undergoing clinical testing. They may be molecular biology-based and focus on various antigens delivered as peptides, proteins, genetically altered plasmid DNA, viruses, or bacteria. They can also be based on cell biology and utilize dendritic cells obtained from patients, autologous tumor cells, tumor cell lysates, or allogeneic tumor cells. The various tumor vaccine formulations include dendritic cell vaccine, tumor cell vaccine, peptide plus adjuvants, plasmid DNA immunization, recombinant bacteria, recombinant viruses, heat shock protein, and exome-based vaccines. Breast cancer vaccines that are currently available and vaccines under clinical trials will be discussed as follows:
E75 (HER2/Neu 369–377, KIFGSLAFL), Nelipepimut-S/NeuVax
The E75-centered immunization stands as the extensively researched approach for addressing patients with breast cancer (BC). E75 comprises a peptide with a length of 9 amino acids sourced from the ECD (extracellular domain) of the HER2 receptor. This peptide functions as a prominent CTL epitope within the immune response and exhibits a strong binding affinity to human leukocyte antigens, particularly HLA-A2 and HLA-A3 molecules (HLA-restricted). E75 expression is detected in nearly 60–75% of the populace. Additionally, researchers have noted a possible cooperative impact when combining trastuzumab with the E75 vaccine. As a result, a phase IIb trial was initiated, involving multiple centers and adopting a randomized, single-blinded, controlled design. The trial enrolled breast cancer patients who were disease-free after concluding standard treatment for high-risk cases of HER2-low BC.96 Drawing from the current data, Nelipepimut-S is set to undergo comprehensive assessment in a Phase III clinical trial. This trial will explore its use in conjunction with trastuzumab for managing individuals diagnosed with triple-negative breast cancer (TNBC) within the adjuvant context following the completion of standard therapeutic protocols.97
GP2 (654–662, IISAVVGIL)
GP2 represents a subdominant epitope and exhibits limited binding affinity with the HLA-A2 molecule.98 A cancer vaccine (CV) centered around GP2 has been examined in a phase IIb trial featuring a prospective, randomized, placebo-controlled design. In the adjuvant scenario, GP2 coupled with GM-CSF was administered to breast cancer patients with positive lymph nodes or a high risk of lymph node involvement and whose tumors exhibited any level of HER2 expression.99
AE37 (HER2/Neu 776–790, GVGSPYVSRLLGICL)
AE37 is a vaccine targeting HER2, formulated using the AE36 hybrid peptide (aa 776–790). This hybrid peptide is extracted from the intracellular region of the HER2 protein and the central fragment of the MHC class II invariant chain. The AE37 vaccine, which consists of this hybrid peptide along with the GM-CSF immune-adjuvant, can effectively stimulate both CD8+ and CD4+ cells, both in laboratory conditions (in vitro) and within a living organism (in vivo).100
TPIV100 and Sargramostim
A Phase II clinical study is presently enrolling patients with HER2-positive breast cancer (BC) in stages II to III who have residual disease following neoadjuvant treatment (NCT04197687, refer to Table 1). TPIV is a vaccine designed with a multi-epitope approach to target HER2. Sargramostim, a recombinant GM-CSF, acts as an adjuvant. Patients are randomly assigned to receive standard-of-care (SoC) maintenance therapy using trastuzumab emtansine (T-DM1) and either TPIV100 or a placebo, along with sargramostim every 21 days for a maximum of 6 cycles, unless there is disease progression or intolerable adverse effects. Post the conclusion of T-DM1 maintenance therapy; patients receive two supplementary booster injections of TPIV100 and sargramostim at 3 and 12 months. The key objective of the research is to analyze invasive disease-free survival (iDFS). The study anticipates enrolling about 480 patients and is estimated to conclude in January 2025.70
MVF-HER-2 (597-626) and MVF-HER-2 (266-296)
A Phase I clinical study is currently assessing the safety and determining the optimal biological dose (OBD) for a combination of two HER2 B-cell peptide-based vaccines, resembling chimeric trastuzumab (MVF-HER-2, aa 597–626) and pertuzumab (MVF-HER-2, aa 266–296). These vaccines are emulsified with Montanide (ISA-720) and muramyl dipeptide derivative (nor-MDP), serving as adjuvants. The trial involves patients with breast, ovarian, or gastrointestinal cancer at the metastatic or unresectable stage, progressing after initial standard therapy.70
Whole Protein-Based Vaccines
Complete protein vaccines encompass epitopes from both HLA class I and II and are not HLA-restricted. A vaccine utilizing the HER2 ICD protein in combination with GM-CSF was examined in 25 patients at various stages (II, III, IV) of HER2-positive BC. Demonstrating a favorable safety profile, 89% of patients developed T-cell immunity specific to HER-2/neu ICD, and 82% also established immunoglobulin G antibody immunity against HER-2/neu. Notably, while heightened vaccine doses did not correlate with an augmented T-cell response, the high-dose vaccine group exhibited an earlier onset of detectable HER2/neu-specific immunity compared to the low-dose group (p = 0.003). The vaccine stimulated and enhanced HER2-specific cellular immunity for 9–12 months.101
Lapuleucel-T (APC8024) (Cell-Based Vaccine)
Vaccines based on cellular components are tailored to individual patients and represent a secure method for crafting personalized vaccines.102 In a phase I clinical trial, 18 individuals diagnosed with metastatic breast cancer characterized by HER2 overexpression were examined to assess the toxicity and immune response triggered by Lapuleucel-T (APC8024), an autologous active cellular immunotherapy (IO). This therapeutic vaccine, grounded in cell-based methodology, comprises peripheral blood mononuclear cells (PBMCs) previously activated with a recombinant fusion protein encompassing the ICD and ECD of HER2, in addition to GM-CSF. The vaccine was well-received and elicited specific anti-HER2 cellular immune responses. Notably, one patient exhibited a partial response (PR) that endured for 6 months, and an additional three patients experienced stable disease (SD) with a duration exceeding 1 year.103
VRP-HER2
A phase II clinical study is set to explore the immunogenicity, focusing on the quantity of tumor-infiltrating lymphocytes (TILs) and anti-HER2 antibodies, utilizing alphavirus-like replicon particles (VRPs) that carry self-amplifying replicon RNA encoding HER2 (NCT03632941). The initial stage of the trial involves assessing safety, wherein participants receive VRP-HER2 vaccinations in conjunction with pembrolizumab. Upon observing no dose-limiting toxicity, subjects are then distributed into three groups (VRP-HER2 alone, pembrolizumab alone, or VRP-HER2 combined with pembrolizumab). The combination regimen entails a three-dose vaccine administered every 2 weeks, complemented by a flat dose of pembrolizumab (200 mg every 3 weeks) over five administrations.104
Xenogeneic HER2/Neu DNA-Based Vaccine
A phase I clinical study (NCT00393783) is currently examining a genetic-based cancer vaccine (CV) incorporating a plasmid hosting a HER2-encoding gene derived from rats. The trial has enlisted individuals with stage III–IV HER2-positive breast cancer. The genetic-based vaccine is administered via intramuscular injections at four distinct dosage tiers (0.5 mg, 1 mg, 3 mg, or 6 mg) during weeks 1, 4, 7, 10, and 13, summing up to five injections. The principal focal point of the study is to determine dose-limiting toxicity (DLT), and the anticipated enrollment comprises 12 patients.70
pNGVL3-hICD (Plasmid-Based Vaccine)
Another early-phase clinical trial (NCT00436254) is evaluating the safety, acceptability, and immunogenicity of a plasmid-based vaccine named pNGVL3-hICD. This vaccine carries genetic data related to the Intracellular Domain (ICD) of HER2. The study welcomed 66 participants who were either at stage III–IV of HER2-positive breast cancer or at stage III–IV of ovarian cancer, with remission of metastases. Enrolled individuals were administered the pNGVL3-hICD vaccine combined with GM-CSF intradermally once a month for 3 months unless disease progression or unacceptable adverse effects were observed. The vaccine was administered at three distinct doses: 10 μg, 100 μg, and 500 μg. An analysis conducted at a median follow-up of 68 months post-vaccination highlighted that patients on the intermediate dosing schedule (100 μg) exhibited the most favorable overall survival (OS, p = 0.02) in comparison to the other two dosing schedules, all while maintaining a strong safety record.105
PVX-410 (PVX, OncoPep)
Two clinical trials, one in phase Ib and the other in phase II are presently investigating the potential of this vaccine in treating triple-negative breast cancer (TNBC) at both early (stage II–III) and metastatic stages. PVX-410 stands out as a groundbreaking tetra-peptide vaccine restricted to HLA-A2, encompassing three out of the four most frequently overexpressed antigens in TNBC. These antigens include X-box–binding protein 1, featuring two splice variants, syndecan-1 (CD138) and cell surface glycoprotein SLAM family member 7 (SLAMF7 or CD319). Research indicates that X-box–binding protein 1 plays a pivotal role in driving relapse and advancement in TNBC by governing the hypoxia-inducing factor 1α.106 Commencing in the year 2021, a clinical study is set to evaluate PVX-410 in individuals with metastatic triple-negative breast cancer (TNBC) who possess the HLA-A2 marker (NCT04634747). The vaccine will be delivered alongside pembrolizumab and chemotherapy, with progression-free survival (PFS) as the primary outcome. The study aims to enroll an estimated 53 participants, and the anticipated conclusion date is in April 2025.106
TSMA (Tumour-Specific Mutant Antigens-Based Synthetic Long Peptide Vaccine)
In the phase II clinical study, the potential of a peptide-centered vaccine targeting neoantigens specific to triple-negative breast cancer (TNBC) is being explored in the context of metastasis (NCT03606967). In one group, the vaccine is administered alongside chemotherapy, durvalumab, and tremelimumab. Conversely, the other group is subjected to the same triple therapy regimen but with a placebo substituting the vaccine. This placebo-containing regimen is accompanied by a poly-ICLC on specified days (1, 4, 8, 15, 22, 50, and 78) until either disease progression or unacceptable levels of toxicity. The central focus of this study is progression-free survival (PFS), with an estimated enrollment of 70 participants and an anticipated conclusion of the study in December 2021.106
p53MVA
TP53 gene mutations are prevalent in the majority of solid tumors, leading to an accumulation of oncogenic and potentially immunogenic p53 protein products within tumor cells.107 An engineered MVA virus expressing the wild-type TP53 transgene (p53MVA) has been developed as an immunotherapeutic approach. Strong p53-specific CD8+ T-cell responses were observed and were further boosted by anti-PD-1 treatment in preclinical models. Subsequently, a phase I clinical trial was devised to assess the safety and tolerability of the p53MVA vaccine in combination with pembrolizumab. Patients with diverse solid tumors, including TNBC that had not responded to standard treatment, were eligible, provided p53 involvement was confirmed through IHC or mutational analysis. A 3-at-risk rolling design was employed, with patients receiving 5.6×108 plaque-forming units (PFU) of p53MVA for three doses alongside 200 mg of pembrolizumab for seven doses every 3 weeks. Initial findings demonstrated clinical benefits linked to sustained p53-specific CD8+ T-cell responses, particularly notable in two cases of TNBC and head and neck squamous cell carcinoma.108
Top of Form
New Methods for Developing Therapeutic Breast Cancer Vaccines
Vaccines Based on Dendritic and Tumor Cell
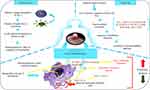
In the immune defense of the body, dendritic cells (DC) are potent antigen-presenting cells (APCs) that are crucial to the start and control of immune responses. Scientists have loaded tumor antigens into curative vaccinations for tumors using DC’s biological characteristics. Dendritic cells (DCs), which are skilled antigen-presenting cells (APCs), play a crucial role in the initiation and maintenance of efficient T-cell-mediated anti-tumor immunological reactions.109,110 It is crucial to comprehend how DCs regulate T cells’ anti-cancer resistance in the tumor microenvironment (TME), as tumor-induced immunosuppression continues to be a significant obstacle to immunotherapy for cancer. By ingesting, digesting, and presenting tumor antigens to T cells that are specific for those antigens, DCs control the immune response that fights cancer. Adjuvants must still be employed in DC vaccine techniques for maximum clinical success, even though tumor-associated antigens (TAA) are used in DC vaccines. TAA and adjuvants may be enclosed in self-polymerizing scaffolds, oncolytic viruses, or peptide/mRNA conjugates (such as polysaccharides, C-type lectin receptors, or nanoparticles). Because of its capacity to overcome the tumor’s immunosuppressive milieu and enable DCs-T cell immunological responses, adjuvant selection is crucial for boosting treatment efficacy. Xia et al demonstrated through an experiment that dendritic and tumor cells were found to be very effective in the prevention of the development of tumors.111 In a clinical experiment conducted by Avigan et al, dendritic/tumor fusion cell vaccines were administered by injection to thirty-two individuals with breast cancer and twenty-six individuals having renal cell carcinoma. From viable tumor tissue samples, tumor cells were isolated and suspended in a single-cell suspension.112 Leukocyte apheresis is used to isolate dendritic cells, which are then cultivated with GM-CSF, interleukin-4, and autogen. Polyethylene glycol is used to cultivate stem cells and cancer cells to create fusion cells.113 While the theory underpinning dendritic/tumor fusion cell vaccines is trustworthy, and these sparse research results are encouraging, the focus of this strategy is on the capacity to create enough fusion cells for several vaccines. Occasionally, cancer cells proliferate extremely gradually, which causes a tiny amount of biopsy material to be produced.114 Assuming enough cancer cells are present, the fusion cell’s production efficiency will be less than 45%.
Purified Antigens as DNA Vaccine
To promote humoral and cellular immune responses against viral antigens, vaccines made from DNA were initially introduced at the beginning of the 1990s. Such vaccines manufacture antigens that are processed and transmitted utilizing MHC processes I and II and include the in vivo transfection of full-length cDNA molecules into target cells. By transcribing the full-length cDNA protein, numerous possible epitopes can be exploited for any one antigen. The cDNA is first cloned into a bacterial plasmid with promoter/promoter sequences and maybe with immunizing drugs to boost the possibility of a strong immunological reaction.115,116 Vaccines based on DNA seem effective in the breast cancer impediment, as Narayanan et al performed a series of trials to show the inhibitory effect of mammaglobin‐A cDNA on breast tumor cells. Issues affecting DC vaccine preparation and therapeutic efficacy also include the efficiency of tumor antigen loading; Immature DC has a strong ability to take and process antigens. As it matures, its ability to present antigens increases, and its ability to take antigens weakens. Therefore, the best time to load antigens is in the immature DC.
On the other hand, it is also very important to select the loading method of antigen. The methods currently used include co-incubation, virus transfection, electric shock pulse, immune adjuvant, etc. The efficiency and safety of reprinting are also different. MASCT technology adopts the method of enhancing antigen loading by immune adjuvant, and the antigen loading efficiency is more than 90%117 (Figure 6).
Whole-Cell Clipped DNA Fragments as DNA Vaccines
Whole-cell clipped DNA fragments have emerged as a promising new type of DNA vaccine. They are composed of small fragments of DNA that have been cut out of the genome of a pathogen and then cloned into a plasmid vector for delivery to a host organism. Once inside the host cell, these DNA fragments are processed and trigger an immune response that can protect against future infections.118
Whole-cell clipped DNA vaccines’ versatility and adaptability are among their key benefits. Because they are made from small, discrete fragments of DNA, they can be easily customized to target specific pathogenic proteins or antigens. This makes them potentially useful against a variety of infectious diseases, including those caused by rapidly evolving or mutating pathogens. Additionally, whole-cell clipped DNA vaccines have shown potential in pre-clinical findings and clinical research trials for their ability to induce potent and long-lasting immune responses. They have also been found to be safe and tolerated in humans, with a minimum of side effects.119,120
However, like any new technology, there are still some limitations and challenges associated with whole-cell clipped DNA vaccines. One major challenge is the issue of delivery. Because the DNA fragments must be delivered into the nucleus of host cells to be effective, they require specialized delivery systems, such as electroporation or gene guns. These methods can be complex and expensive, which may limit their widespread use.121
A Bacterium for the Development of Vaccine
The possibility of producing highly immunogenic HER-2 and neo antigens at APCs utilizing L. monocytogenes as a carrier is being investigated by research scholars at the University of Pennsylvania School of Medicine. Speculates that this may be due to a phenomenon called epitope proliferation, which develops immunity to important uses that are not part of the actual target area. It may be that the APC picks up the dead cell fragments and then offers a wide variety of approaches for the activation of the CD8 + T cell response122,123 (Figure 7).
Technical Difficulties with Cancer Vaccine Development
The investigational processes being used to create breast cancer vaccines remain in their infancy. It should be highlighted that the majority of scientists have not yet moved on to preliminary clinical research. The immune system is generally in good shape. Cancers control specific cell types and antigens as they grow and spread. This might assist in reducing the immunological reaction to the antigen. The is being constrained by certain circumstances, which researchers are working to manage.124,125
Why Breast Cancer Vaccine is Still an Unborn Child
According to Campoli et al, there are numerous plausible explanations for such a low rate of abyss. Regardless of whether the antigen is linked to a particular kind of tumor cell, the tumor cell may only express a small amount of the antigen, be positioned secretly, or somehow elude detection. As the tumor develops, the immune system can remove tumor antigens and alter their antigenic characteristics. The majority of tumor cells cannot express antigens otherwise. The expression of MHC proteins, along with other costimulatory proteins, which stimulate powerful immune reactions, can occur in tumor cells at very low numbers.126
Older recipients, who are frequently questioned for Phase 1/2 clinical trials, are the subject of additional queries. These patients cannot access the tumor’s cells since they have more metastatic tumors. This supports the idea that using vaccines as a treatment for hematological problems is effective.127 Additionally, as tumors spread, they may become more capable of releasing immunosuppressive substances into the surrounding tissue. Additionally, dendritic cell abnormalities and a decline in peripheral blood lymphocytes are linked to metastatic tumors.128
The majority of tumor antigens are automated, which can only be immunogenic at best; therefore, developing a successful vaccination may be challenging due to some or all of these limitations. As a result, the efficacy of peptide-based vaccinations, whether they contain or lack immunostimulatory additives, has been modest. There are several ways to increase the immune response, but the most important one is choosing the right antigen and ensuring that it is presented to the immune system in an effective way.129 There are various methods for preventing host elements that compromise the immune response.
Cancer Stem Cells and Vaccine
A subgroup of cancer cells known as cancer stem cells (CSCs) can self-renew and develop into different cell types inside the tumor. They are believed to be extremely important in the development, growth, and relapse of tumors. Therefore, targeting CSCs is an appealing approach to cancer treatment, and recent research has investigated the use of vaccines to elicit an immune response against CSC.130 The goal of cancer therapy using vaccines is to stimulate the immune system to identify and destroy tumor cells. Dendritic cell vaccines, peptide vaccines, and whole-cell vaccines are a few of the vaccine types that have been created to target CSCs.131
In dendritic cell (DC) vaccinations, DCs are taken from the blood of the patient and exposed to CSCs in a dish. The patient is then reinfused with the activated DCs to elicit an immunological response against CSCs. On the other hand, peptide vaccines employ particular peptides produced from CSC-associated antigens to elicit an immune response. CSCs or tumor cells are used in whole-cell vaccines as the source of the antigens that are exposed to the immune system.132,133
Notwithstanding the auspicious results from early clinical trials, several challenges need to be addressed in the development of CSC vaccines. One major challenge is the identification of specific CSC antigens that are universally expressed across different types of cancer. Another challenge is the heterogeneity of CSC populations within tumors, which makes it difficult to target all CSCs with a single vaccine. Additionally, there is a need to develop more effective adjuvants to increase the immune response to CSC vaccines.134
Cancer stem cells represent an attractive target for cancer treatment due to their role in tumor initiation, progression, and recurrence. Vaccines are a promising approach to stimulate an immune response against CSCs, and early clinical trials have demonstrated their safety and efficacy.133–136 However, further research is needed to overcome the challenges in the development of CSC vaccines and to determine their optimal use in cancer treatment.
Breast Cancer Stem Cells
Breast cancer stem cells (BCSCs) are a subset of cells inside a breast tumor that have been demonstrated to possess stem cell-like characteristics, such as the capacity to self-renew, differentiate, and start tumor growth.
- The ability of BCSCs to regenerate themselves and produce new BCSCs allows the tumor to develop and propagate.
- Differentiation: The heterogeneous nature of breast tumors is caused by the ability of BCSCs to differentiate into different cell types, including non-stem cancer cells.
- Tumor initiation: BCSCs are assumed to be in charge of tumor recurrence since they can start tumor genesis in vivo.
- Treatment resistance: BCSCs frequently develop resistance to standard cancer therapies, including chemotherapy and radiation therapy, which can speed up tumor growth and relapse.
- Molecular markers: BCSCs express specific molecular markers, such as CD44+/CD24-, ALDH1, and others, which have been used to identify and isolate these cells from breast tumors.137
BCSC markers are discussed in detail here.
CD44
CD44 was the initial efficacious surface marker utilized for CSC identification. Functioning as a cell receptor, CD44 orchestrates communication with the microenvironment by engaging with extracellular ligands like hyaluronan (HA). The interplay between CD44 and HA can trigger RhoA-specific guanine nucleotide exchange factor-mediated RhoA/Grb2-associated binder-1 signaling or c-Src kinase/Twist/miR-10b/RhoA signaling, which plays a role in activating the PI3K/AKT signaling pathway.138 Fascinatingly, Ghatak documented that hyaluronan (HA) can additionally impede the PI3K/AKT pathway by stimulating PTEN in TA3/St cells, providing insights into the “functional transition” of CD44 linked to diverse phenotypes138 Al-Hajj discovered that a minimal population of 100 cells displaying the EpCAM+CD44+CD24−/low phenotype could initiate the majority of tumors, housing a variety of non-tumorigenic cells, in NOD/SCID mice.139 The heightened CD44 expression could be attributed to the lack of p53 and plays a crucial role in the intrinsic resistance of breast cancer stem cells (BCSCs).139
Integrins
Like CD44, integrins are significant cell surface receptors engaging with extracellular ligands like fibronectin and laminin. They can form heterodimers with one another, facilitating cell adhesion to the extracellular matrix and engaging in bidirectional interactions with the microenvironment. CD29, CD49f, and CD61, encoding the β1, α6, and α3 subunits of heterodimeric integrin respectively, are extensively noted in breast cancers (BCs) and have been validated as potent markers for breast cancer stem cells (BCSCs). To illustrate, either CD29 or CD49f used in conjunction with CD24 was effective in pinpointing CSCs in BRCA1-mutant mouse primary mammary tumors.139 In individuals with BRCA1 mutations, the genetic expression profile of basal-like breast cancer closely resembled that of EpCAM+CD49f+ luminal progenitor cells found in normal mammary tissues. The EpCAM+CD49f+ cells isolated from these patients exhibited notably heightened clonogenic activity compared to those from non-mutated tissues, implying that luminal progenitors in BRCA1 mutation carriers might be implicated in early oncogenic occurrences. Correspondingly, the presence of CD49f+ cells within breast tumors correlated with a tendency for metastasis and reduced survival time among patients.140 In line with CD61’s function as an epithelial-mesenchymal transition (EMT) indicator, inhibiting CD61 eliminated the initiation of TGF-β signaling, crucial for preserving stemness, in mouse primary mammary tumor H6O5 cells.141
CD133
CD133, also referred to as Prominin-1, is a pentaspan and heavily glycosylated transmembrane protein that characterizes a wide range of stem cells, encompassing hematopoietic stem cells and endothelial progenitor cells. Despite the precise function of CD133 in BC remaining ambiguous, BC cells expressing CD133 certainly exhibit properties akin to CSCs. These cells demonstrate notable resilience against DNA-damaging substances and a heightened ability to initiate tumor growth in NOD/SCID mice.142 Additionally, BCSCs characterized by CD133 expression were observed to be notably concentrated in tumors of individuals with BC resistant to hormonal therapy (HT), fostering the self-renewal of luminal metastases in a manner not dependent on estrogen receptors (ER).143
ALDH1
ALDH1, an enzyme dependent on NAD(P)+, facilitates the oxidation of aldehydes within cells to carboxylic acids. In research by Ginestier, ALDH1 emerged as a common marker in both normal and malignant mammary stem cells. Their findings strongly suggested that ALDH1 functioned as an independent prognostic indicator significantly linked to reduced survival rates in BC patients.144 In vitro investigations confirmed that MDA-MB-231 cells, characterized by CD44+CD24−ALDH1+, and MDA-MB-468 BCE cells, marked by CD44+CD133−ALDH1+, demonstrated heightened tumorigenic and metastatic capabilities in comparison to ALDH1lowCD44low cancer cells.145 Recently, Marcato elucidated that the ALDH activity observed in BCSCs predominantly relied on ALDH1A3 rather than ALDH1A1, offering deeper insights into the precise target within BCSCs. This finding differs from observations in prostate CSCs, where ALDH1A1 plays a more critical role. The distinction can be attributed to the fact that mammary epithelial cells express a considerably lower basal level of ALDH1A1 compared to ALDH1A3. The strong correlation between heightened ALDH1A3 expression and BC metastasis in patients further underscores the significance of ALDH1A3.144
CXCR4
CXCR4 is a receptor found on the cell membrane that responds to chemokines. Stromal cell-derived factor 1 (SDF-1), also known as CXCL12, is the exclusive activator of CXCR4. The signaling pathway triggered by SDF-1/CXCR4 plays a crucial role in promoting the migration of BCSCs with CXCR4 expression to sites of metastasis. Disrupting CXCR4 through antibody neutralization or knockout significantly impeded the growth of orthotopically transplanted breast tumors and hindered their targeted metastasis to lymph nodes and lungs.144 In a recent investigation, it was observed that activation of the SDF-1/CXCR4 signaling pathway led to heightened phosphorylation of 60 proteins linked to migration or invasion in CD44+CD24− BCSCs. These proteins could potentially serve as crucial agents in sustaining BCSCs triggered by CXCR4.144
ABCG2
ABCG2 (also known as breast cancer resistance protein) exhibits high expression levels in numerous chemoresistant BC cell lines. This transmembrane pump shields BC cells from harm by reducing the intracellular accumulation of cytotoxic drugs, a behavior more pronounced in BCSCs. The CD44+CD24−/low cells from MCF-7, MDA-MB-231, and SK-BR-3 BC cell lines showed significantly elevated ABCG2 expression compared to non-stem cells.146 Likewise, these BCSCs demonstrated overexpression of various multidrug resistance-associated proteins and P-glycoprotein Fields.147 These findings find substantial support in pharmacodynamic studies suggesting that a broad spectrum of chemotherapeutic drugs can be expelled from cells by these transporters.148
ANTXR1
ANTXR1, recognized as a specific marker for tumor endothelial cells, plays a role in tumor angiogenesis. Chen revealed heightened expression of ANTXR1 on the cell surface of CD44+CD24− and ALDH1+ TMD231 BC cells. Their findings demonstrated that ANTXR1 overexpression activated vital genes related to cell proliferation, DNA replication, and the Wnt signaling pathway, endowing these BC cells with increased tumorigenic and metastatic capabilities. The identification of ANTXR1 facilitated the sorting of a subset of malignant BCSCs.149
EpCAM
EpCAM also referred to as epithelial-specific antigen (ESA) or CD326, is identified as a marker for epithelial tumors and is linked to invasive BCs. Recent studies have shed light on its dual role in either promoting or impeding epithelial cell-cell adhesion, underscoring its significance in cancer cell migration and metastasis. The detection of EpCAM+ cells allows for the isolation of a subset of circulating tumor cells (CTCs) or disseminated tumor cells (DTCs) from the peripheral blood of BC patients. These EpCAM+ cells encompass a subpopulation of BC cells that initiate metastasis, giving rise to bone, lung, and liver metastases in immunocompromised mice and offering insights into predicting unfavorable metastatic behavior in BC patients.150
PROCR
PROCR functions as a counterpart in preserving the equilibrium of tissue factor-mediated procoagulant effects by engaging with coagulation proteases like protein C. It has been validated as an exclusive marker for cancer stem cells (CSCs) in triple-negative breast cancer. In a study by Hwang-Verslues, it was highlighted that MDA-MB-361 and MDA-MB-231 cells marked by PROCR demonstrated a 2-fold and 9-fold surge in colony-forming efficiency, respectively, in comparison to cells lacking PROCR.151
GD2
GD2 is primarily a b-series ganglioside prominently expressed on the cellular membrane. A minority of GD2+ cells isolated from the MDA-MB-231 cell line demonstrated the ability to generate mammospheres and initiate tumors with as few as 10 cells in immunocompromised mice. The majority of GD2+ cells isolated from human mammary epithelial cells expressing the H-Ras oncogene exhibited the CD44+CD24− phenotype.152 GD3 synthase (GD3S) is intricately involved in the biosynthesis of GD2 and is linked to the EMT program in breast cancer. The expression of GD3S was significantly elevated in GD2+ breast cancer stem cells (BCSCs). Knocking down GD3S notably diminished GD2 expression and disrupted their migratory capabilities and ability to generate mammospheres, implying that the formation of GD2+ BCSCs might be correlated with EMT mediated by GD3S.153
Research is currently being done to determine the potential involvement of BCSCs in the development, spread, and resistance to therapy for breast cancer. Targeting BCSCs is considered to be a promising tactic for enhancing the effectiveness of breast cancer treatment and lowering the risk of tumor recurrence.154 To completely comprehend the nature of BCSCs and their function in breast cancer, however, a lot more study is required.
Discussion and Conclusion
Tumor immune evasion pertains to the scenario in which tumor cells evade detection and assault by the body’s immune system through diverse mechanisms, allowing them to survive and multiply within the organism. The principal mechanism by which breast cancer accomplishes immune evasion is through an imbalance in the expression of immune checkpoint proteins. Cytotoxic T lymphocyte antigen 4 (CTLA4), as well as programmed cell death protein 1 (PD-1) and programmed cell death protein-ligand 1 (PD-L1), are vital immune checkpoints in the context of breast cancer. Immunotherapeutic agents targeting these immune checkpoints obstruct their inhibitory effect on immune cells, reinvigorate T-cells, eradicate cancer cells, and reinstate the body’s ability to counter tumors. Currently, immune checkpoint inhibitors represent a significant advancement in breast cancer immunotherapy and hold promise as a novel avenue for breast cancer treatment.155
A novel theory, the cancer stem cell hypothesis, suggests new cancer treatment targets and has the potential to significantly alter how diseases are managed.156 In the overall scheme of the development of breasts and as a potential source of BCSCs, the biology of breast growth cells must be considered. Breast cancer, tumor recurrence, and tumor metastasis have all been linked to altered mammary stem cells. They have also developed into crucial targets for cancer immunotherapy. Facts have shown that current cancer treatment methods can most effectively kill the most discriminating cancer cells, but not cancer cells that cause tumor recurrence. Future treatment will require effective targeting of cancer cells to achieve significant medical remission of the disease. The target BCSC antigen must be further defined to achieve efficacious pointing of the BCSC compartment, thus avoiding contact with normal stem cells but destroying the location of the cancer stem cells. New therapies by themselves do not usually fully achieve the best results and must be further developed and integrated with existing treatments. After the differentiated tumor tissue is removed, therapies targeting BCSC may be used. This will make it easier for the immune system to eliminate the few cancer stem cells that are still alive. The treatment of breast cancer metastasis and recurrence may be made more appealing by targeting BCSCs. It can significantly raise the clinical eradication rate as well as the quality of life for breast cancer patients when utilized as part of a multimodal treatment strategy.
Immune checkpoint inhibitors (ICBs) have exhibited encouraging outcomes in treating triple-negative breast cancer (TNBC). Nevertheless, a significant portion of patients do not exhibit an initial response to ICBs or eventually develop resistance. Presently, numerous active clinical trials are investigating the potential clinical advantages of integrating ICBs with interventions like chemotherapy, targeted molecular therapies, and cancer vaccines.
There have been significant scientific advancements in the search for a breast cancer vaccine, but more work remains. Most of the developed curative vaccines so far have either not progressed beyond preclinical testing or have only produced modest therapeutic effects in human subjects. The idea of creating vaccinations for prevention is still an intriguing one. In contrast to cervical cancer, which has been linked to the infections HPV-16 and HPV-18, breast cancer has not been linked to any infectious pathogens.
Consequently, it is doubtful that an infectious disease preventive vaccination will be created shortly. Preventive vaccinations against recognized tumor-related antigens are the second tactic. These vaccinations have successfully undergone testing on volunteers who had mild or no disease, and the early results are encouraging, with the majority of patients producing antigen-specific immune responses. The use of these vaccines in patients who have been freed from the disease through conventional therapies, including surgery, chemotherapy, and radiation, may soon become a reality to prevent disease recurrence. A long-term objective is to immunize individuals with effective preventive measures before the condition worsens.157,158
Abbreviations
PD-1, Program death one; PD-L1, Program cell death ligand one; CSCs, Cancer stem cells; TME, Tumor microenvironment; NK, Natural killer; MHC, Major histocompatibility complex; NKG2D, Natural killer group 2D; CTLA4, Cytotoxic T-Lymphocyte-Associated Antigen 4; APCs, Antigen-presenting cells; IL-2, Interleukin-2; IFN, Interferon; TAAs, Tumor-associated antigens; TSAs, Tumor-specific antigens; BCSCs, Breast cancer stem cells.
Author Contributions
The author played a substantial role in the review, whether in the conception, design, or all of these aspects. They all participated in creating, revising, and providing critical input to the article. Additionally, they collectively approved the final version for publication and reached a consensus on the journal in which the review was submitted. Furthermore, all authors are committed to taking responsibility for every aspect of the work.
Funding
This research was supported by the National Natural Science Foundation of China under grant No. 81772002 and Shenzhen Science, Technology, and Innovation Commission under grant No. JCYJ20170818143334365 and No. JCYJ20170818092553608.
Disclosure
The authors declare that they have no competing interests.
References
1. García-Aranda M, Redondo M. Immunotherapy: a challenge of breast cancer treatment. Cancers. 2019;11(12):1822. doi:10.3390/cancers11121822
2. Liu JP, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805(1):35–42.
3. Adams S, Gatti-Mays ME, Kalinsky K, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019;5(8):1205–1214. doi:10.1001/jamaoncol.2018.7147
4. Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi:10.1001/jamaoncol.2016.1061
5. Arneth B. Tumor microenvironment. Medicina. 2019;56(1):15. doi:10.3390/medicina56010015
6. Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi:10.1016/j.breast.2022.08.010
7. Fatima N, Liu L, Hong S, et al. Prediction of breast cancer, comparative review of machine learning techniques, and their analysis. IEEE Access. 2020;8:150360–150376. doi:10.1109/ACCESS.2020.3016715
8. Mohammed SI, Torres-Luquis O, Zhou W, et al. Tumor-draining lymph secretome en route to the regional lymph node in breast cancer metastasis. Breast Cancer. 2020;57–67.
9. Finn OJ. The Dawn of vaccines for cancer prevention. Nature Rev Immunol. 2018;18(3):183–194. doi:10.1038/nri.2017.140
10. Silverstein AM. Paul Ehrlich’s Receptor Immunology: The Magnificent Obsession. Elsevier; 2001.
11. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3(11):991–998. doi:10.1038/ni1102-991
12. Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943. doi:10.1038/onc.2008.267
13. Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39(1):11–25. doi:10.1002/eji.200838899
14. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi:10.1146/annurev.immunol.22.012703.104803
15. Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Exp Rev Clin Immunol. 2014;10(1):41–62. doi:10.1586/1744666X.2014.865519
16. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature13954
17. Kennedy LB, Salama AK. A review of cancer immunotherapy toxicity. Ca A Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
18. Yang Z, Ma Y, Zhao H, et al. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(2):e1590. doi:10.1002/wnan.1590
19. Klein G. Tumor antigens. Ann Rev Microbiol. 1966;20(1):223–252. doi:10.1146/annurev.mi.20.100166.001255
20. Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. Science. 1991;254(5035):1153–1160. doi:10.1126/science.1957167
21. Chaudhuri S, Cariappa A, Tang M, et al. Genetic susceptibility to breast cancer: HLA DQB* 03032 and HLA DRB1* 11 may represent protective alleles. Proc Natl Acad Sci. 2000;97(21):11451–11454. doi:10.1073/pnas.97.21.11451
22. Wang Z, Cao YJ. Adoptive cell therapy targeting neoantigens: a frontier for cancer research. Front Immunol. 2020;11:176. doi:10.3389/fimmu.2020.00176
23. Teixeira MR, Pandis N, Heim S. Cytogenetic clues to breast carcinogenesis. Genes Chromosomes Cancer. 2002;33(1):1–16. doi:10.1002/gcc.1206
24. Gil Del Alcazar CR, Huh SJ, Ekram MB, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discovery. 2017;7(10):1098–1115. doi:10.1158/2159-8290.CD-17-0222
25. Criscitiello C. Tumor-associated antigens in breast cancer. Breast Care. 2012;7(4):262–266. doi:10.1159/000342164
26. Nicolini A, Carpi A. Immune manipulation of advanced breast cancer: an interpretative model of the relationship between immune system and tumor cell biology. Med Res Rev. 2009;29(3):436–471. doi:10.1002/med.20143
27. VanKlompenberg MK, Bedalov CO, Soto KF, et al. APC selectively mediates response to chemotherapeutic agents in breast cancer. BMC Cancer. 2015;15(1):1–14. doi:10.1186/s12885-015-1456-x
28. Guđmundsdóttir I, Gunnlaugur Jónasson J, Sigurđsson H, et al. Altered expression of HLA class I antigens in breast cancer: association with prognosis. Int j Cancer. 2000;89(6):500–505.
29. Li B, Geng R, Wu Q, et al. Alterations in immune-related genes as potential marker of prognosis in breast cancer. Front Oncol. 2020;10:333. doi:10.3389/fonc.2020.00333
30. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nature Rev Drug Discov. 2020;19(3):200–218. doi:10.1038/s41573-019-0052-1
31. van der Burg SH. Correlates of Immune and Clinical Activity of Novel Cancer Vaccines. in Seminars in Immunology. Elsevier; 2018.
32. Rong L, Li R, Li S, et al. Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncol Lett. 2016;11(1):500–504. doi:10.3892/ol.2015.3841
33. Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112(9):1421–1427. doi:10.1038/bjc.2015.124
34. Mao H, Zhang L, Yang Y, et al. New insights of CTLA-4 into its biological function in breast cancer. Current Cancer Drug Targets. 2010;10(7):728–736. doi:10.2174/156800910793605811
35. Behranvand N, Nasri F, Zolfaghari Emameh R, et al. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. 2022;71(3):507–526.
36. Sanz-Garcia E, Zhao E, Bratman SV, et al. Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: current research, opportunities, and challenges. Sci Adv. 2022;8(4):eabi8618. doi:10.1126/sciadv.abi8618
37. Elez E, Chianese C, Sanz‐García E, et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS -mutant metastatic colorectal cancer. Mol Oncol. 2019;13(9):1827–1835. doi:10.1002/1878-0261.12547
38. Gao Q, Feng J, Liu W, et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Delivery Rev. 2022;188:114445. doi:10.1016/j.addr.2022.114445
39. Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1(2):138–147. doi:10.1002/biot.200500044
40. Park W, Heo Y-J, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22(1):1–10. doi:10.1186/s40824-018-0133-y
41. Gross S, Erdmann M, Haendle I, et al. Twelve-year survival and immune correlates in dendritic cell–vaccinated melanoma patients. JCI Insight. 2017;2(8). doi:10.1172/jci.insight.91438
42. Till SJ, Francis JN, Nouri-Aria K, et al. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113(6):1025–1034. doi:10.1016/j.jaci.2004.03.024
43. Gp D, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998.
44. Marin-Acevedo JA, Soyano AE, Dholaria B, et al. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):1–25. doi:10.1186/s13045-017-0552-6
45. Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52(1):55–81. doi:10.1016/j.immuni.2019.12.018
46. Kim H, Choi J-M, Lee K-M. Immune checkpoint blockades in triple-negative breast cancer: current state and molecular mechanisms of resistance. Biomedicines. 2022;10(5):1130. doi:10.3390/biomedicines10051130
47. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74–82. doi:10.1001/jamaoncol.2018.4224
48. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi:10.1016/S1470-2045(19)30689-8
49. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nature Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4
50. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi:10.1007/s10549-017-4537-5
51. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–1288. doi:10.1093/annonc/mdz158
52. McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22(23):5729–5737. doi:10.1158/1078-0432.CCR-16-0190
53. Sabel MS, Nehs MA, Su G, et al. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90(1):97–104. doi:10.1007/s10549-004-3289-1
54. Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–3494. doi:10.1158/1078-0432.CCR-10-0505
55. Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Aspects Med. 2013;34(6):1217–1256. doi:10.1016/j.mam.2013.01.006
56. Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi:10.1158/1078-0432.CCR-16-3215
57. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443–455. doi:10.1016/j.ccell.2020.03.017
58. Sceneay J, Goreczny GJ, Wilson K, et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 2019;9(9):1208–1227. doi:10.1158/2159-8290.CD-18-1454
59. Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195–211. e10. doi:10.1016/j.immuni.2018.12.021
60. Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–470. doi:10.1038/s41586-019-1836-5
61. Falvo P, Orecchioni S, Hillje R, et al. Cyclophosphamide and vinorelbine activate stem-like CD8+ T cells and improve anti-PD-1 efficacy in triple-negative breast cancer. Cancer Res. 2021;81(3):685–697. doi:10.1158/0008-5472.CAN-20-1818
62. Xiao Y, Ma D, Zhao S, et al. Multi-omics profiling reveals distinct microenvironment characterization and suggests immune escape mechanisms of triple-negative breast cancer. Clin Cancer Res. 2019;25(16):5002–5014. doi:10.1158/1078-0432.CCR-18-3524
63. Wu S-Y, Xiao Y, Wei J-L, et al. MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer. 2021;9(7):e002528. doi:10.1136/jitc-2021-002528
64. Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22(6):1499–1509. doi:10.1158/1078-0432.CCR-15-1125
65. Huang Y, Zhang H-L, Li Z-L, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672. doi:10.1038/s41467-021-22618-x
66. Hu Q, Ye Y, Chan L-C, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nature Immunol. 2019;20(7):835–851. doi:10.1038/s41590-019-0400-7
67. Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi:10.1016/j.molmed.2014.10.009
68. Bin Umair M, Akusa FN, Kashif H, et al. Viruses as tools in gene therapy, vaccine development, and cancer treatment. Arch Virol. 2022;167(6):1387–1404. doi:10.1007/s00705-022-05432-8
69. Gardella B, Gritti A, Soleymaninejadian E, et al. New Perspectives in Therapeutic Vaccines for HPV: a Critical Review. Medicina. 2022;58(7):860. doi:10.3390/medicina58070860
70. Corti C, Giachetti PPMB, Eggermont AMM, et al. Therapeutic vaccines for breast cancer: has the time finally come? Eur. J. Cancer. 2022;160:150–174. doi:10.1016/j.ejca.2021.10.027
71. Banday AH, Jeelani S, Hruby VJ. Cancer vaccine adjuvants–recent clinical progress and future perspectives. Immunopharmacol Immunotoxicol. 2015;37(1):1–11. doi:10.3109/08923973.2014.971963
72. Luo W, Yang G, Luo W, et al. Novel therapeutic strategies and perspectives for metastatic pancreatic cancer: vaccine therapy is more than just a theory. Cancer Cell Int. 2020;20(1):1–10. doi:10.1186/s12935-020-1147-9
73. Melief CJ, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Investig. 2015;125(9):3401–3412. doi:10.1172/JCI80009
74. Guo C, Manjili MH, Subjeck JR, et al. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi:10.1016/B978-0-12-407190-2.00007-1
75. Zhang L, Xu L, Wang Y, et al. A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chin Chem Lett. 2022;33(8):4089–4095. doi:10.1016/j.cclet.2022.01.071
76. Shah S, Famta P, Tiwari V, et al. Instigation of the epoch of nanovaccines in cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;15:e1870.
77. Debien V, De Caluwé A, Wang X, et al. Immunotherapy in breast cancer: an overview of current strategies and perspectives. NPJ Breast Cancer. 2023;9(1):7. doi:10.1038/s41523-023-00508-3
78. Zhu S-Y, Yu K-D. Breast cancer vaccines: disappointing or promising? Front Immunol. 2022;13:190.
79. Huang L, Rong Y, Tang X, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Molecular Cancer. 2022;21(1):1–19. doi:10.1186/s12943-022-01515-x
80. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi:10.1056/NEJM200103153441101
81. Srivastava PK. Therapeutic cancer vaccines. Curr Opinion Immunol. 2006;18(2):201–205. doi:10.1016/j.coi.2006.01.009
82. Mittendorf EA, Peoples GE, Singletary SE. Breast cancer vaccines: promise for the future or pipe dream? Cancer. 2007;110(8):1677–1686. doi:10.1002/cncr.22978
83. Parkin DM. The global health burden of infection‐associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi:10.1002/ijc.21731
84. Chung MA, Luo Y, O’Donnell M, et al. Development and preclinical evaluation of a Bacillus Calmette-Guérin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003;63(6):1280–1287.
85. Islam MR, Islam F, Nafady MH, et al. Natural small molecules in breast cancer treatment: understandings from a therapeutic viewpoint. Molecules. 2022;27(7):2165. doi:10.3390/molecules27072165
86. Bednarek AK, Sahin A, Brenner AJ, et al. Analysis of telomerase activity levels in breast cancer: positive detection at the in situ breast carcinoma stage. Clin Cancer Res. 1997;3(1):11–16.
87. Disis ML, Cecil DL. Breast cancer vaccines for treatment and prevention. Breast Cancer Res Treat. 2022;191(3):481–489. doi:10.1007/s10549-021-06459-2
88. Hodge JW. Carcinoembryonic antigen as a target for cancer vaccines. CII. 1996;43(3):127–134. doi:10.1007/s002620050313
89. Beckwith DM, Cudic M. Tumor-Associated O-Glycans of MUC1: Carriers of the Glyco-Code and Targets for Cancer Vaccine Design. in Seminars in Immunology. Elsevier; 2020.
90. Runnebaum IB, Nagarajan M, Bowman M, et al. Mutations in p53 as potential molecular markers for human breast cancer. Proc Natl Acad Sci. 1991;88(23):10657–10661. doi:10.1073/pnas.88.23.10657
91. Watson MA, Dintzis S, Darrow CM, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59(13):3028–3031.
92. Sahin U, Türeci Ö, Chen Y-T, et al. Expression of multiple cancer/testis (CT) antigens in breast cancer and melanoma: basis for polyvalent CT vaccine strategies. Int J Cancer. 1998;78(3):387–389. doi:10.1002/(SICI)1097-0215(19981029)78:3<387::AID-IJC22>3.0.CO;2-2
93. Xia J, Tanaka Y, Koido S, et al. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170(4):1980–1986. doi:10.4049/jimmunol.170.4.1980
94. Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10(14):4699–4708. doi:10.1158/1078-0432.CCR-04-0347
95. Tang D-C, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356(6365):152–154. doi:10.1038/356152a0
96. Clifton GT, Hale D, Vreeland TJ, et al. Results of a randomized phase IIb trial of nelipepimut-S+ trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer. Clin Cancer Res. 2020;26(11):2515–2523. doi:10.1158/1078-0432.CCR-19-2741
97. Chick RC, Clifton GT, Hale DF, et al. Subgroup analysis of nelipepimut-S plus GM-CSF combined with trastuzumab versus trastuzumab alone to prevent recurrences in patients with high-risk, HER2 low-expressing breast cancer. Clin Immunol. 2021;225:108679. doi:10.1016/j.clim.2021.108679
98. Ayoub NM, Al-Shami KM, Yaghan RJ. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer. 2019;11:53–69. doi:10.2147/BCTT.S175360
99. Patel S, McWilliams D, Fischette CT, et al. Final five-year median follow-up safety data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, Phase IIb study evaluating the use of HER2/Neu peptide GP2+ GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2-positive. J Clin Oncol. 2021;39:542. doi:10.1200/JCO.2021.39.15_suppl.542
100. McCarthy PM, Clifton GT, Vreeland TJ, et al. AE37: a HER2-targeted vaccine for the prevention of breast cancer recurrence. Exp Opinion Investig Drugs. 2021;30(1):5–11. doi:10.1080/13543784.2021.1849140
101. Disis ML, Schiffman K, Guthrie K, et al. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein—based vaccine. J Clin Oncol. 2004;22(10):1916–1925. doi:10.1200/JCO.2004.09.005
102. Arab A, Yazdian-Robati R, Behravan J. HER2-positive breast cancer immunotherapy: a focus on vaccine development. Arch Immunol Therapiae Exp. 2020;68(1):1–18. doi:10.1007/s00005-019-00566-1
103. Park JW, Melisko ME, Esserman LJ, et al. Treatment with autologous antigen-presenting cells activated with the HER-2 –based antigen lapuleucel-T: results of a Phase I study in immunologic and clinical activity in HER-2–overexpressing breast cancer. J Clin Oncol. 2007;25(24):3680–3687. doi:10.1200/JCO.2006.10.5718
104. Larocca C, Schlom J. Viral vector–based therapeutic cancer vaccines. Cancer J. 2011;17(5):359. doi:10.1097/PPO.0b013e3182325e63
105. Disis ML, Coveler AL, Higgins D, et al. A Phase I Trial of the Safety and Immunogenicity of a DNA-Based Vaccine Encoding the HER2/Neu (HER2) Intracellular Domain in Subjects with HER2+ Breast Cancer. American Society of Clinical Oncology; 2014.
106. Criscitiello C, Corti C, Pravettoni G, et al. Managing side effects of immune checkpoint inhibitors in breast cancer. Critical Rev Oncol. 2021;162:103354. doi:10.1016/j.critrevonc.2021.103354
107. Bykov VJ, Eriksson SE, Bianchi J, et al. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi:10.1038/nrc.2017.109
108. Chung VM, Kos F, Hardwick N, et al. A Phase 1 Study of p53MVA Vaccine in Combination with Pembrolizumab. American Society of Clinical Oncology; 2018.
109. Jeng L-B, Liao L-Y, Shih F-Y, et al. Dendritic-Cell-vaccine-based immunotherapy for hepatocellular carcinoma: clinical trials and recent preclinical studies. Cancers. 2022;14(18):4380. doi:10.3390/cancers14184380
110. Ke Y, Zhu J, Chu Y, et al. Bifunctional fusion membrane‐based hydrogel enhances antitumor potency of autologous cancer vaccines by activating dendritic cells. Adv Funct Mater. 2022;32(29):2201306. doi:10.1002/adfm.202201306
111. Xia D, Li F, Xiang J. Engineered fusion hybrid vaccine of IL-18 gene-modified tumor cells and dendritic cells induces enhanced antitumor immunity. Cancer Biother Radiopharm. 2004;19(3):322–330. doi:10.1089/1084978041424990
112. Avigan D, Rosenblatt J, Kufe D. Dendritic/Tumor Fusion Cells as Cancer Vaccines. in Seminars in Oncology. Elsevier; 2012.
113. Narayanan K, Jaramillo A, Benshoff ND, et al. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J Natl Cancer Inst. 2004;96(18):1388–1396. doi:10.1093/jnci/djh261
114. Liao F, Zhang J, Hu Y, et al. Efficacy of an ALDH peptide-based dendritic cell vaccine targeting cancer stem cells. Cancer Immunol Immunother. 2022;71(8):1959–1973. doi:10.1007/s00262-021-03129-6
115. Chopra A, Kim TS, O‐Sullivan I, et al. Combined therapy of an established, highly aggressive breast cancer in mice with paclitaxel and a unique DNA‐based cell vaccine. Int j Cancer. 2006;118(11):2888–2898. doi:10.1002/ijc.21724
116. Hsu C, Kavathas P, Herzenberg LA. Cell-surface antigens expressed on L-cells transfected with whole DNA from non-expressing and expressing cells. Nature. 1984;312(5989):68–69. doi:10.1038/312068a0
117. Kavathas P, Herzenberg LA. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci. 1983;80(2):524–528. doi:10.1073/pnas.80.2.524
118. Karim AM, Eun Kwon J, Ali T, et al. Triple-negative breast cancer: epidemiology, molecular mechanisms, and modern vaccine-based treatment strategies. Biochem Pharmacol. 2023;212:115545. doi:10.1016/j.bcp.2023.115545
119. Chen Y, Hu D, Eling DJ, et al. DNA vaccines encoding full-length or truncated Neu induce protective immunity against Neu-expressing mammary tumors. Cancer Res. 1998;58(9):1965–1971.
120. Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. doi:10.1111/j.1600-065X.2010.00980.x
121. Liu MA, Wahren B, Hedestam GBK. DNA vaccines: recent developments and future possibilities. Human Gene Therapy. 2006;17(11):1051–1061. doi:10.1089/hum.2006.17.1051
122. Toussaint B, Chauchet X, Wang Y, et al. Live-attenuated bacteria as a cancer vaccine vector. Exp Rev Vaccines. 2013;12(10):1139–1154. doi:10.1586/14760584.2013.836914
123. Tangney M, Gahan CG. Listeria monocytogenes as a vector for anti-cancer therapies. Curr Gene Therapy. 2010;10(1):46–55. doi:10.2174/156652310790945539
124. Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng. 2012;109(6):1443–1460. doi:10.1002/bit.24493
125. Marra F, Cloutier K, Oteng B, et al. Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. Pharmacoeconomics. 2009;27(2):127–147. doi:10.2165/00019053-200927020-00004
126. Campoli M, et al. Mechanisms of tumor evasion. In: Tumor Immunology and Cancer Vaccines; 2005:61–88.
127. Dougan M, Dranoff G. Immune therapy for cancer. Ann Rev Immunol. 2009;27(1):83–117. doi:10.1146/annurev.immunol.021908.132544
128. Bonassi S, Znaor A, Ceppi M, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28(3):625–631. doi:10.1093/carcin/bgl177
129. Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int Immunol. 2016;28(7):319–328. doi:10.1093/intimm/dxw027
130. Pan Q, Li Q, Liu S, et al. Concise review: targeting cancer stem cells using immunologic approaches. Stem Cells. 2015;33(7):2085–2092. doi:10.1002/stem.2039
131. Zhou L, Lu L, Wicha MS, et al. Promise of cancer stem cell vaccine. Hum Vaccin Immunother. 2015;11(12):2796–2799. doi:10.1080/21645515.2015.1083661
132. Teitz-Tennenbaum S, Wicha MS, Chang AE, et al. Targeting cancer stem cells via dendritic-cell vaccination. Oncoimmunology. 2012;1(8):1401–1403. doi:10.4161/onci.21026
133. Yu J, Sun H, Cao W, et al. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11(1):3. doi:10.1186/s40164-022-00257-2
134. Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. doi:10.1038/s41392-020-0110-5
135. Akbar Samadani A, Keymoradzdeh A, Shams S, et al. Mechanisms of cancer stem cell therapy. Clin Chim Acta. 2020;510:581–592. doi:10.1016/j.cca.2020.08.016
136. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):20. doi:10.1186/s12929-018-0426-4
137. Velasco-Velázquez MA, Homsi N, De La Fuente M, et al. Breast cancer stem cells. Int j Biochemistry Cell Biol. 2012;44(4):573–577. doi:10.1016/j.biocel.2011.12.020
138. Bourguignon LY, Wong G, Earle C, et al. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. 2010;285(47):36721–36735. doi:10.1074/jbc.M110.162305
139. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–3988. doi:10.1073/pnas.0530291100
140. Ye F, Qiu Y, Li L, et al. The presence of EpCAM - /CD49f + cells in breast cancer is associated with a poor clinical outcome. J Breast Cancer. 2015;18(3):242–248. doi:10.4048/jbc.2015.18.3.242
141. Lo P-K, Kanojia D, Liu X, et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin–TGFβ signaling. Oncogene. 2012;31(21):2614–2626. doi:10.1038/onc.2011.439
142. Wright MH, Calcagno AM, Salcido CD, et al. Brca1 breast tumors contain distinct CD44+/CD24-and CD133+ cells with cancer stem cell characteristics. Br Cancer Res. 2008;10(1):1–16. doi:10.1186/bcr1855
143. Vassilopoulos A, Wang R-H, Petrovas C, et al. Identification and characterization of cancer initiating cells from BRCA1 related mammary tumors using markers for normal mammary stem cells. Int J Bio Sci. 2008;4(3):133. doi:10.7150/ijbs.4.133
144. Marcato P, Dean CA, Pan D, et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32–45. doi:10.1002/stem.563
145. Croker AK, Goodale D, Chu J, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2009;13(8b):2236–2252. doi:10.1111/j.1582-4934.2008.00455.x
146. Sun M, Yang C, Zheng J, et al. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater. 2015;28:171–182. doi:10.1016/j.actbio.2015.09.029
147. Zhu Y, Yu F, Jiao Y, et al. Reduced miR-128 in breast tumor–initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res. 2011;17(22):7105–7115. doi:10.1158/1078-0432.CCR-11-0071
148. Bai X, Chen Y, Hou X, et al. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab. Rev. 2016;48(4):541–567. doi:10.1080/03602532.2016.1197239
149. Chen D, Bhat-Nakshatri P, Goswami C, et al. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer Res. 2013;73(18):5821–5833. doi:10.1158/0008-5472.CAN-13-1080
150. Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature Biotechnol. 2013;31(6):539–544. doi:10.1038/nbt.2576
151. Hwang-Verslues WW, Kuo W-H, Chang P-H, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One. 2009;4(12):e8377. doi:10.1371/journal.pone.0008377
152. Battula VL, Shi Y, Evans KW, et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Investig. 2012;122(6):2066–2078. doi:10.1172/JCI59735
153. Liang Y-J, Wang C-Y, Wang I-A, et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget. 2017;8(29):47454. doi:10.18632/oncotarget.17665
154. Zeng X, Liu C, Yao J, et al. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol Res. 2021;163:105320. doi:10.1016/j.phrs.2020.105320
155. Zhang W, Kong X, Ai B, et al. Research progresses in immunological checkpoint inhibitors for breast cancer immunotherapy. Front Oncol. 2021;11:582664. doi:10.3389/fonc.2021.582664
156. Lu H, Chen I, Shimoda LA, et al. Erratum: chemotherapy-Induced Ca2+ release stimulates breast cancer stem cell enrichment (Chemotherapy-Induced Ca^ 2^+ release stimulates breast cancer stem cell enrichment). Cell Rep. 2021;34(1):108605. doi:10.1016/j.celrep.2020.108605
157. Liu Q, Hodge J, Wang J, et al. Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics. 2020;10(18):8365. doi:10.7150/thno.45395
158. Gilani RA, Phadke S, Bao LW, et al. Retraction: UM-164: A Potent c-Src/p38 Kinase Inhibitor with in vivo Activity Against Triple-Negative Breast Cancer. AACR; 2020.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.