Back to Journals » Research and Reports in Neonatology » Volume 13
Hydrops fetalis: Incidence, Etiologies, Management Strategies, and Outcomes
Authors Younge T , Ottolini KM, Al-Kouatly HB , Berger SI
Received 25 August 2023
Accepted for publication 21 November 2023
Published 20 December 2023 Volume 2023:13 Pages 81—92
DOI https://doi.org/10.2147/RRN.S411736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Tamiko Younge,1 Katherine M Ottolini,1 Huda B Al-Kouatly,2 Seth I Berger3
1Division of Neonatology, Children’s National Hospital, Washington, DC, USA; 2Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, PA, USA; 3Center for Genetic Medicine Research, Rare Disease Institute, Children’s National Hospital, Washington, DC, USA
Correspondence: Seth I Berger, Email [email protected]
Abstract: Hydrops fetalis describes a pathologic over accumulation of fluid affecting multiple organs of the fetus or neonate. It is a rare condition with numerous etiologies, including many rare diseases. Causes of hydrops fetalis vary regionally based on prevalence of inheritable conditions and availability of RhD immune globulin to prevent immune hydrops fetalis. In some instances, the etiology remains unknown despite extensive evaluation. Overall, the prognosis is extremely poor, but it is also extremely variable and full recovery is possible. Prenatal management includes evaluation to determine etiology, which can provide prognostic value for counseling and medical decision-making. Diagnostic work-up often includes genetic testing with increasing evidence supporting the use of exome sequencing. Depending on the etiology, targeted therapies may be available or in development. Postnatal management emphasizes supportive care as diagnostic work-up continues. Perinatal palliative care plays an essential role for families affected by this challenging condition.
Keywords: Hydrops fetalis, rare disease, prenatal diagnosis
Introduction
Hydrops fetalis is defined as excessive, pathologic fluid accumulation within two or more tissues or body cavities including the peritoneal cavity, pleural space, pericardial sac, and skin. Placentomegaly and polyhydramnios are also common findings but not part of the clinical diagnostic criteria.1,2 Various underlying disease processes can lead to the development of hydrops fetalis, including both immune and nonimmune-mediated etiologies. Immune hydrops fetalis, also known as erythroblastosis fetalis, occurs in the setting of hemolytic disease of the fetus and newborn (HDFN) which can lead to severe anemia. Nonimmune hydrops fetalis may occur as a result of a wide spectrum of underlying etiologies including cardiac abnormalities, aneuploidy, hemoglobinopathies, lymphatic malformations, and congenital infections, among others.3 Through this review, we aim to provide a comprehensive summary of hydrops fetalis epidemiology, pathophysiology, diagnosis, and management as a reference for maternal fetal medicine and neonatal providers and trainees.
Incidence
The overall incidence of hydrops fetalis has remained stable for the past five decades at an estimated 1 in 1500 to 3800 births, despite a significant decrease in the proportion of immune hydrops fetalis in high-income countries from 80% to less than 10% following the advent of maternal RhD immunoglobulin therapy in the 1960s.1 Nonimmune hydrops fetalis is estimated to affect 1 in 1700 to 3000 pregnancies but only 1 in 4000 live births due to the high incidence of intrauterine fetal demise and pregnancy termination.4 However, such estimates may be inaccurately high due to selection bias towards high-risk pregnancies in the literature. Larger population birth registry studies from the United States (California) and Sweden estimate a much lower rate of hydrops fetalis of 1.6 to 2.5 per 10,000 live births.5,6
Pathophysiology
Various aspects of fetal physiology contribute to the risk of interstitial fluid accumulation relative to lymphatic drainage which characterizes hydrops fetalis. The revised Starling equation describes how intravascular fluid balance is regulated by relative differences in oncotic and hydrostatic pressures between the intravascular and interstitial spaces (Figure 1).7,8 Fetal total body water and extracellular fluid content are higher than in the neonate and infant. This additional free fluid may contribute to the risk of developing hydrops fetalis.9 Additionally, the endothelial glycocalyx layer that lines blood and lymphatic vessels is more permeable when newly formed in the fetus, especially following inflammation or ischemia.7,9 The immature fetal lymphatic system, which drains excess fluid from interstitial to intravascular compartments, may not keep pace with fluid accumulation or may be damaged or obstructed.10,11 The compliant interstitial compartments in the fetus can also accommodate more fluid, leading to the accumulation seen in hydrops fetalis.7
 |
Figure 1 Revised Starling forces describe mechanisms of interstitial fluid accumulation in hydrops fetalis. Red text describes relative direction of different contributors to the equation. Green text describes specific fetal physiology that contribute to susceptibility and development of hydrops fetalis. Data from these studies.7–9 |
Etiologies of hydrops fetalis encompass failures in the above fluid regulation mechanisms. The four primary mechanisms that contribute to the development of hydrops fetalis are as follows: (1) increased capillary hydrostatic pressure, (2) reduced plasma oncotic pressure, (3) obstructed lymphatic flow, and (4) damaged peripheral capillaries.3,12 Although one feature typically predominates depending on the specific underlying etiology, the multiple mechanisms of fetal fluid regulation interact and contribute to fluid accrual (Figure 2). Additionally, multiple disease processes may coexist within the same fetus. For example, Trisomy 13 and 18 are associated with lymphatic flow disorders and congenital heart disease, both conditions can result in hydrops fetalis. In contrast, for some conditions, such as myotonic dystrophy, the precise mechanisms that lead to hydrops fetalis are unclear.
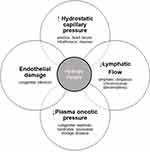 |
Figure 2 Contributing mechanisms to hydrops fetalis and examples of etiologies. For any given etiology, a specific mechanism may predominate but all mechanisms operate interconnectedly. |
Etiologies
Immune Hydrops Fetalis
Immune hydrops fetalis or erythroblastosis fetalis is the pathophysiologic outcome of progressive red blood cell destruction resulting in severe anemia that overwhelms the capacity of extramedullary tissue to compensate.1 The majority of cases are due to alloimmunization (antibody formation) in a Rhesus factor-D (RhD)-negative mother with an RhD-positive fetus causing HDNF; however, alloimmunization due to other red blood cell antigens is possible.1 Severe fetal anemia, defined as an estimated fetal hemoglobin less than five grams per deciliter, is associated with the development of hydrops fetalis.13 The mechanism is believed to be multifactorial including high-output cardiac failure, liver dysfunction, and fetal hypoxemia.1
Maternal treatment with RhD immunoglobulin effectively prevents exposure of the maternal immune system to the RhD antigen on fetal red blood cells.14 This prevents the maternal immune system from generating RhD antibodies that would transfer to the fetus and result in fetal hemolytic anemia. Maternal sensitization in countries where RhD immunoglobulin is accessible is rarely encountered. However, the disease burden of RhD alloimmunization remains high globally.15,16 Publication bias towards reports from high-income countries may underrepresent the ongoing burden of immune hydrops fetalis, given that its prevention is dependent upon access to RhD immunoglobulin. For example, a recent case series of 62 neonates with hydrops fetalis from Turkey reported that 28 cases (45.2%) were immune-mediated compared to the incidence of less than 10% reported elsewhere.1,17
Non-Immune Hydrops Fetalis
Knowledge of the relative predominance of specific causes of nonimmune hydrops fetalis is limited by the rarity of cases and lack of centralized registries to monitor and consolidate information. Based on published data, the most common etiologies of nonimmune hydrops fetalis are cardiovascular (20.1%), lymphatic dysplasia (15%), hematologic (9.3%), and chromosomal (9%) (Figure 3).18 A summary of diagnoses and their proposed primary mechanisms resulting in hydrops fetalis can be found in Table 1.
 |
Table 1 Summary of Diagnoses and Proposed Mechanism of Resultant Hydrops Fetalis Adapted from Swearingen 2020 |
 |
Figure 3 (A) Etiologies of nonimmune hydrops fetalis as described by Bellini et al 2009; Copyright © 2009 Wiley-Liss, Inc.19 and updated by Bellini et al 2015; © 2015 Wiley Periodicals, Inc.18 (B) Monogenic etiologies of nonimmune hydrops diagnosed by exome sequencing (as described by Al-Kouatly et al 2023; © 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.20). *Significant difference in proportion of cases with etiology between review in 2009 and 2015 (p<0.05). Abbreviations: NMSK, Neuromusculoskeletal; IEM, Inborn Errors of Metabolism; CV, Cardiovascular. |
Historically, the underlying cause has remained unknown in as many as 15 to 20% of cases of nonimmune hydrops fetalis, but diagnostic accuracy continues to evolve over time with improved testing capabilities including exome sequencing.18,20 Lysosomal storage diseases represent a substantial proportion of cases and may underlie 8% of nonimmune hydrops fetalis cases previously believed to be idiopathic.21 Geographical location and population characteristics also play a role in the prevalence of specific diagnoses. For example, in a recent review of 122 cases of nonimmune hydrops fetalis from Thailand, hematologic etiologies predominated at 38.5% mostly due to regional incidence of alpha thalassemia.22
Cardiovascular diseases, including structural heart defects, cardiomyopathies, arrhythmias, and arteriovenous malformations (AVM), are largely believed to result in hydrops fetalis secondary to increased central venous or hydrostatic pressure.3,23 This can occur in the context of high or low cardiac output. Hematologic conditions resulting in severe fetal anemia also lead to heart failure and increased hydrostatic pressure that then precipitates the findings of hydrops fetalis.24 Anemia and heart failure will also lead to tissue hypoxia resulting in endothelial damage.
Renal and gastrointestinal disorders may lead to hydrops fetalis secondary to hypoproteinemia leading to decreased plasma oncotic pressure.3 The mechanism of hydrops fetalis from lysosomal storage disease includes splenomegaly and hepatomegaly, leading to sequestration of fetal blood which lowers oncotic pressure.21 However, a study of fetuses of RhD alloimmunized pregnancies did not find a greater incidence of hydrops fetalis in those also affected by hypoalbuminemia.25 This suggests that hypoalbuminemia may play a more minor role in the pathophysiology of hydrops fetalis.
Lymphatic obstruction or dysplasia, often associated with chromosomal abnormalities and other syndromes, may cause fluid accumulation due to impaired drainage.10 Noonan syndrome is associated with both lymphatic abnormalities and congenital heart malformations including pulmonary stenosis, which may both contribute to the findings of hydrops fetalis, again illustrating how various mechanisms may compound each other.26 Congenital infections, such as Parvovirus, may lead to hydrops fetalis due to endothelial damage but also cardiomyopathy.27
Diagnosis
Prenatal
When hydrops fetalis is the presenting phenotype during pregnancy, the first step is to evaluate for the presence or absence of red blood cell alloimmunization to distinguish immune from nonimmune causes.4,28 Prenatal Doppler ultrasound to assess blood flow through the middle cerebral artery (MCA) aids in the diagnosis of fetal anemia. Anemia from both immune and non-immune causes is particularly important to identify, as it can be effectively managed with intrauterine transfusions. If fetal anemia is identified in the absence of alloimmunization, the Kleihauer–Betke test or liquid chromatography can be performed to evaluate for fetal maternal hemorrhage via the presence of fetal blood cells in the maternal circulation.1 However, recent retrospective cohort study suggests that this test has little association with fetal outcomes.29 Additionally, the Society of Maternal Fetal Medicine (SMFM) recommends serological testing for potential infectious causes of fetal anemia including parvovirus, toxoplasma, cytomegalovirus, and syphillis.4
If hydrops fetalis is determined to be nonimmune, detailed fetal imaging via ultrasound or magnetic resonance imaging (MRI) can help determine the presence of other anomalies and comorbidities that may direct further diagnostic evaluation. For example, fetal hepatomegaly can be associated with lysosomal storage disease in the setting of nonimmune hydrops fetalis.30 However, accurate phenotyping can be challenging in the prenatal setting due to the limitations of fetal imaging.31
The presence of structural abnormalities and specific phenotypic findings can further guide prenatal genetic diagnostic testing, which can include karyotype, chromosomal microarray, and gene-specific testing.4 Over 100 genetic conditions, many very rare, have been associated with nonimmune hydrops fetalis.32 Although stepwise evaluation of genetic etiologies of nonimmune hydrops fetalis may be a prudent and judicious use of resources, it may delay higher yield investigation of rarer causes of nonimmune hydrops fetalis. Yield for chromosomal microarray is estimated to be 6–14% beyond high-resolution karyotype.31 It is unlikely that both need to be performed, especially if sequentially, before proceeding to exome sequencing. If karyotype is unrevealing, exome sequencing is a reasonable next step as further findings of copy variations would be subsumed with sequencing.
Although targeted panels to detect monogenic causes of nonimmune hydrops fetalis are commercially available, these standard panels include many genes with poor quality of evidence for causal association with hydrops fetalis and do not evaluate many more recently described pathogenic variants. Additionally, many genes that can cause hydrops fetalis are not currently associated with human disease due to high rates of fetal demise.32 In contrast to targeted panels, exome sequencing represents a higher-yield, unbiased diagnostic approach for genetic causes of hydrops fetalis.33 In a study of 127 cases of unexplained nonimmune hydrops fetalis, whole-exome sequencing identified 37 (29%) diagnostic genetic variants.34 Al-Kouatly et al demonstrated that compared to narrower testing strategies such as biochemical testing and available panels, exome sequencing provided an incremental increase in diagnostic yield of over 50% and revealed a potential diagnosis in an additional 22.7% of cases with unknown cause.35 A similarly powerful yield was demonstrated by Mone et al who found an additional diagnostic yield of 25% for isolated and non-isolated nonimmune hydrops fetalis with whole-exome sequencing.36 A recently published meta-analysis of 445 cases of hydrops fetalis undergoing exome sequencing after negative standard diagnostic evaluation showed an incrementally higher yield of 37% regardless of associated prenatal structural malformations.20
Given the high-yield potential, exome sequencing may represent a diagnosis strategy preferable to microarray and gene-specific evaluation. While currently more expensive than these modalities, exome sequencing may nonetheless represent a more timely and overall more cost-effective approach.37,38 It should be noted that clinical judgement should continue to direct genetic testing as exome does not diagnose all known genetic etiologies of hydrops fetalis such as myotonic dystrophy, which is caused by a short tandem repeat expansion, or genetic changes due to deep intronic variants such as cryptic splice changes.39 In addition to genetic testing, amniotic fluid analysis for glycosaminoglycans and lysosomal enzymatic activity may aid in the diagnosis of lysosomal storage diseases or disambiguation of variants of uncertain significance in related genes.40 As enzyme replacement therapy is an active area of research, more treatment options may be available for this diagnosis in the future.41
Timely availability of an underlying etiology is especially crucial when considering pregnancy termination, which becomes more challenging with greater gestational age, and when considering directing care towards palliation rather than life prolongation in a diagnosis associated with poor outcomes. While many of the etiologies identified by exome sequencing are rare with a wide spectrum of potential phenotypes and outcomes, having an underlying diagnosis may have therapeutic value for families and, in general, counseling and medical decision-making are improved when an underlying etiology is known.
Postnatal Diagnostic Evaluation
Postnatal diagnostic evaluation follows a similar strategy to prenatal evaluation. Additional postnatal phenotypic data can help inform exome or genome analysis if not diagnosed prenatally. Despite the ability to perform more extensive evaluations, including physical exam, imaging, and biochemical testing, 15 to 25% of cases remain idiopathic after postnatal evaluation, sometimes even following an autopsy.18,19,42 Nonetheless, the diagnosis of heritable causes can inform future pregnancy planning.28,43–45
Management Strategies
Prenatal Management
Prenatal therapies are available depending on the etiology of hydrops fetalis. When severe anemia is the mechanism, fetal transfusions may be indicated (eg Rh alloimmunization, Parvovirus, congenital anemia, fetal maternal hemorrhage).4,46 The first intrauterine fetal transfusion was done for anemia secondary to Rh alloimmunization in 1984.47 The middle cerebral artery peak systolic velocity is monitored as a proxy to detect significant fetal anemia.48 The umbilical vein is the most desired site for transfusion, free loop of cord, intrahepatic portion of the hepatic vein, or umbilical artery can be alternatives but are associated with greater complications.1 Intraperitoneal transfusion is rarely performed unless the caliber of the umbilical vessels is prohibitive (eg less than 18 weeks gestation) or technical difficulties related to fetal position or location of placental cord insertion.1
Transfusion is more effective in preventing progression to hydrops fetalis than reversing signs that are already present.1 Outcomes have improved over time with an overall procedure-related fetal loss of 1% and emergency delivery rate within 24 hours of 1.8%.49 Neurodevelopmental outcomes of children with hemolytic disease treated with transfusion at a median age of 8.2 years demonstrated an overall rate of neurodevelopmental deficits (eg cerebral palsy, severe developmental delay, or deafness) of 4.8%.50
Recent case reports suggest a potential role for prenatal steroids in the treatment of congenital pulmonary airway malformations (CPAM) (also referred to as congenital cystic adenomatoid malformations (CCAM)) associated with hydrops fetalis.51,52 Cases that do not respond to steroids may benefit from fetal or early neonatal resection.53 Surgical management for CPAM and other intrathoracic obstructive lesions may be available at quaternary referral centers. Importantly, fetal intrathoracic surgery has been associated with improved survival for infants affected by hydrops fetalis in the setting of CPAM specifically.54
Fetal interventions for various causes of hydrops fetalis include fluid diversion such as thoracoamniotic shunt placement, thoracentesis, or paracentesis, which are available but not widely studied. Limited data, however, suggest improved survival after fetal intervention. In a review of 273 cases of nonimmune hydrops fetalis in the United Kingdom, 48 of 152 continued pregnancies underwent fetal intervention (including fluid diversion, antiarrhythmics, and laser treatment for chorioangioma) with associated improvement in survival but no difference in resolution of hydrops fetalis.42
In cases of cardiac etiology, monitoring cardiac status can assist with management, such as administration of maternal antiarrhythmics, and timing of delivery in the case of non-reassuring fetal testing due to worsening heart failure.55 Sustained tachycardia can progress to hydrops fetalis and maternal administration of antiarrhythmics with consideration of direct fetal administration of antiarrhythmic therapy can be considered to help establish sufficient sinus rhythm to allow resolution of hydrops fetalis.56 Maternal digoxin, flecainide, or sotalol are common first-line therapies for tachyarrhythmias, with the latter having decreased absorption in the setting of hydrops fetalis.57
As more than two-thirds of infants with hydrops fetalis are delivered preterm, there is a low threshold for the administration of antenatal steroids in affected pregnancies. When considering the timing of delivery, the risks associated with worsening hydrops fetalis must be weighed against potential morbidity from preterm birth, and there is no evidence that elective preterm delivery will improve outcomes.4,6,58 Timing and mode of delivery should be individualized based on underlying pathology and family’s goals of care. Decision on mode of delivery depends upon the underlying cause, condition of the fetus, and maternal complications.4
Postnatal Management
Management of a neonate with hydrops fetalis consists of supportive care while investigating for and treating the underlying etiology. When possible, delivery should occur at a center with a skilled neonatal resuscitation team. The delivery team should be prepared to intubate if necessary as neonates with hydrops fetalis can develop cardiorespiratory failure related to multiple underlying causes including cardiac dysfunction and poor perfusion, pulmonary hypoplasia, pleural effusions, or ascites.3 Preparations should be made for fluid diversion including thoracocentesis, paracentesis, and pericardiocentesis based on prenatal evaluations.3 If a neonate is born prematurely, surfactant deficiency can also contribute to high respiratory support needs.59
Intravascular depletion and severe anemia in certain cases may present as hemodynamic shock. Fluid resuscitation can be extremely challenging due to capillary “leak” from endothelial damage, and fluid boluses should be used judiciously. Fluid management is a balance of fluid restriction, as patients have excess of extracellular free water and sodium, with fluid resuscitation as patients are typically intravascularly depleted or have poor oxygen carrying capacity in the case of anemia. While fluid replacement with colloid such as albumin infusion makes intuitive sense to increase intravascular oncotic pressure, the use of albumin infusion remains controversial with potential harms such as further fluid overload, and without evidence for improvement in clinical outcomes.60
Vasoactive medications and stress-dose steroids can treat poor cardiac output. If the etiology is related to congenital heart disease or arrhythmia, patients should undergo evaluation by cardiology, cardiovascular surgery, and electrophysiology as appropriate. While prognosis is guarded for patients affected by hydrops fetalis, extracorporeal life support is not contraindicated. Analysis of cases from the Extracorporeal Life Support Organization (ELSO) database demonstrated survival after extracorporeal life support in 13 of 24 (54%) of infants with nonimmune hydrops fetalis with earlier initiation associated with greater odds of survival.61
Trial of octreotide can be considered in the setting of chylothorax and/or chylous ascites and suspected lymphatic flow disorder.62 In the event of specific genetic diagnoses, targeted therapies may be available, including enzyme replacement therapy for lysosomal storage disorders.21,30 RASopathies describe developmental conditions caused by mutations which activate RAS/MAPK signaling pathways including neurofibromatosis type 1 and Noonan syndrome.63 Emerging evidence indicates effective treatment of these conditions with mitogen-activated protein kinase (MEK) inhibitors, such as trametinib.64 Case reports indicate a potential role for MEK inhibitors in treating pediatric patients with severe lymphatic abnormalities and associated fluid accumulation. These findings suggest the possibility of therapy for neonates with hydrops fetalis secondary to RASopathies, although their utility in the neonatal setting remains unproven.65,66
Outcomes
Hydrops fetalis has an overall poor prognosis with significant risks to maternal and perinatal health. Accurate prognosis is challenging due to the variety of etiologies and association with other obstetrical complications (eg prematurity and cesarean delivery).4,6 While in-utero interventions are available for some indications and may affect outcomes, options are limited and poorly studied. Long-term outcomes and impacts are similarly difficult to define due to the multitude of underlying causes and low rates of survival.
A specific but uncommon obstetrical complication of hydrops fetalis is mirror syndrome (Ballantyne’s syndrome) where the pregnant person develops symptoms that emulate fetal hydrops, in particular edema.67 Pathophysiology is poorly understood, but it may be a form of preeclampsia in which the hydropic placenta initiates a systemic inflammatory response causing an increase in trophoblastic debris into maternal blood.68 Mirror syndrome infers risks of postpartum hemorrhage, intensive care unit admission, heart failure, pulmonary edema, renal dysfunction, stillbirth, and neonatal death. A recent literature review suggests that the most common etiologies of fetal hydrops associated with mirror syndrome include structural cardiac malformations, alpha thalassemia, Rh isoimmunization, and nonimmune hydrops fetalis.67
Hydrops fetalis carries a high risk of perinatal mortality. Rates of miscarriage and stillbirth are high. A recent review of 361 cases of nonimmune hydrops fetalis identified during pregnancy in Austria found that 51 cases (14%) ended in miscarriage and 33 cases (9%) ended in stillbirth.69 In addition, families may elect to terminate a pregnancy with hydrops fetalis due to underlying etiology or high risk of mortality and suffering. Decision to terminate pregnancy in countries where abortion is accessible is common (40 to 50% of affected fetuses).22,42,69 As a comparison, in a cohort of 53 cases of nonimmune hydrops fetalis referred to a perinatal genetics program in Brazil, 4 pregnancies (7.5%) were legally permitted to be electively terminated.70
Numerous factors contribute to prognosis for infants born in the setting of hydrops fetalis. Earlier diagnosis (before 24 weeks gestation) has been associated with chromosomal abnormalities.22,42 In turn, earlier gestation at presentation has been associated with perinatal mortality.58,71–73 However, this may be more related to the high mortality associated with aneuploidy rather than an indication of the severity of hydrops fetalis. When omitting patients with chromosomal abnormalities, Sohan et al did not find a difference in mortality based on gestational age at presentation.74
The most significant factor for fetal and live birth prognosis is the underlying etiology. Mortality may be up to 92% among infants with underlying congenital heart disease given the severity of the disease associated with in-utero congestive heart failure.12 Better prognosis may be expected for infants with infectious etiologies and tachyarrhythmias.12,73 Etiology is especially important for determining if any corrective fetal or postnatal therapies are available. In general, fetal treatment is associated with improved survival, but studies are limited to case reports and smaller series.71,74,75
For live births, greater gestational age at delivery is associated with increased survivability.5,58,69,71,72,75,76 The likelihood of survival is improved in the absence of additional complications of prematurity. More severe illness (lower Apgar scores, acidosis, hypoalbuminemia) at delivery also seems associated with mortality.58,71,72,76 More fluid compartments involved and large for gestational age, perhaps indicating greater edema, have also been associated with higher risk of perinatal death.5,77
Among live births, those that survive the immediate neonatal period can survive and survival without major comorbidities is possible.5 Santo et al studied a cohort of 71 pregnancies affected by nonimmune hydrops fetalis and reported 25% survival with no morbidities among the total cohort or 17 out of 28 (61%) of neonates that survived beyond 28 days.73 They found three of 28 (11%) of survivors had significant neurodevelopmental delays and eight of 28 (29%) had other comorbidities.73 A more recent review of 100 pregnancies complicated by nonimmune hydrops fetalis found that of the 23 survivors beyond the neonatal period, 20 patients (87%) were well at long-term follow-up.78 In a Japanese cohort of 91 affected pregnancies, 33 patients of 51 live births (65%) survived to one-year old with 15 of the 33 (45%) reaching age-appropriate developmental milestones.79 Another Japanese cohort found normal development for 28 of 56 (50%) of infants born with nonimmune hydrops fetalis with follow-up data available at one year of age.80
Perinatal Palliative Care
Given the typically poor prognosis but variable and unpredictable outcomes in cases of hydrops fetalis, careful counseling is critical. Perinatal palliative care describes consideration and coordination of care across obstetrical and neonatal services that dually focuses on quality of life and respecting a family’s values.81 It is an essential component of care for any family in which the pregnancy or life of the infant may be limited. For a pregnancy or neonate affected by hydrops fetalis, palliative care should be provided concurrently with life-prolonging measures as well as when there is a transition to comfort care and allowing natural death.
The Society of Maternal Fetal Medicine describes cases of hydrops fetalis as generally falling into one of three categories: (1) cases in which fetal therapy is available, which require typically urgent referral and treatment to a specialized center such as fetal anemia for in-utero transfusion, fetal tachyarrhythmias for antiarrhythmic therapies, or intrathoracic masses for fetal surgery; (2) cases with a lethal prognosis, in which pregnancy termination or comfort care after delivery are generally recommended such as aneuploidy in the setting of complex congenital heart disease; and (3) cases in which the prognosis is likely poor but uncertain especially in cases where etiology is not yet determined.4 A similar categorization of cases is helpful in the context of postnatal counseling and decisions around direction of care. Early involvement of a specialized palliative care team is highly recommended to support families and primary care teams with discussions and decisions about goals and direction of care. An uncertain prognosis can be especially challenging for families and providers alike. A trial of therapies including invasive and intensive care is reasonable but ongoing discussions are important to guide care and families.
Conclusion
Hydrops fetalis may occur as a complication of many underlying diseases. While treatment with RhD immune globulin has reduced incidence of immune hydrops fetalis, the overall incidence has remained stable and RhD alloimmunization remains a significant cause of hydrops fetalis in areas of the world where treatment is less accessible. Both fetal and postnatal treatments may be available. As such, determining the underlying etiology is paramount to management and counseling. Many etiologies of hydrops fetalis are extremely rare, and some management decisions are time sensitive. A comprehensive evaluation may include exome sequencing to arrive at a diagnosis to guide management. Exome sequencing is becoming more rapid and less cost prohibitive, and within the context of appropriate genetic counseling, can provide earlier and more accurate diagnosis to aid in decision-making for families and clinicians. Despite such a guarded prognosis, survival and even survival without morbidity is possible. Prognosis is expected to continue to improve as diagnostic abilities, such as prenatal imaging and exome sequencing technologies, and therapies, such as fetal surgery and gene-specific therapies, continue to advance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Simmons PM, Magann EF. Immune and Nonimmune Hydrops Fetalis. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s Neonatal-Perinatal Medicine E-Book: Diseases of the Fetus and Infant.
2. Berger VK, Sparks TN, Jelin AC, et al. Non-Immune Hydrops Fetails. J Ultrasound Med. 2018;37(5):1185–1191. doi:10.1002/jum.14462
3. Swearingen C, Colvin ZA, Leuthner SR. Nonimmune Hydrops Fetalis. Clin Perinatol. 2020;47(1):105–121. doi:10.1016/j.clp.2019.10.001
4. Norton ME, Chauhan SP, Dashe JS. Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #7: nonimmune hydrops fetalis. Am J Clin Exp Obstet Gynecol. 2015;212(2):127–139.
5. Steurer MA, Peyvandi S, Baer RJ, et al. Epidemiology of Live Born Infants with Nonimmune Hydrops Fetalis—Insights from a Population-Based Dataset. J Pediatrics. 2017;187:182–188.e3. doi:10.1016/j.jpeds.2017.04.025
6. Whybra C, Källén K, Hansson SR, Gunnarsson R. Non-immune hydrops fetalis was rare in Sweden during 1997-2015, but cases were associated with complications and poor prognosis. Acta Paediatrica. 2020;109(12):2570–2577.
7. Bellini C, Hennekam RCM. Non-immune hydrops fetalis: a short review of etiology and pathophysiology. Am J Med Genet A. 2012;158A(3):597–605. doi:10.1002/ajmg.a.34438
8. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovascular Res. 2010;87(2):198–210.
9. Kim CR, Stonestreet B. 105: fluid Distribution in the Fetus and Neonate. In: Fetal and Neonatal Physiology.
10. Bellini C, Hennekam RCM, Boccardo F, Campisi C, Serra G, Bonioli E. Nonimmune idiopathic hydrops fetalis and congenital lymphatic dysplasia. Am J Med Genet A. 2006;140(7):678–684. doi:10.1002/ajmg.a.31100
11. Yurdakök M. Non-immune hydrops fetalis. J Pediatric Neonatal Individualized Med. 2014;3(2):56.
12. Randenberg AL. Nonimmune hydrops fetalis part II: does etiology influence mortality? Neonatal Netw. 2010;29(6):367–380. doi:10.1891/0730-0832.29.6.367
13. Whitecar PW, Moise KJ. Sonographic methods to detect fetal anemia in red blood cell alloimmunization. Obstet Gynecol Surv. 2000;55(4):240–250.
14. Practice Bulletin No. 181 Summary: prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130(2):481–483. doi:10.1097/AOG.0000000000002226
15. Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74 Suppl 1(Suppl 1):86–100.
16. Zipursky A, Paul VK. The global burden of Rh disease. Arch Dis Child Fetal Neonatal Ed. 2011;96(2):F84–F85. doi:10.1136/adc.2009.181172
17. Takci S, Gharibzadeh M, Yurdakok M, et al. Etiology and Outcome of Hydrops Fetalis: report of 62 Cases. Pediatrics Neonatol. 2014;55(2):108–113. doi:10.1016/j.pedneo.2013.07.008
18. Bellini C, Donarini G, Paladini D, et al. Etiology of non-immune hydrops fetalis: an update. Am J Med Genet A. 2015;167A(5):1082–1088. doi:10.1002/ajmg.a.36988
19. Bellini C, Hennekam RCM, Fulcheri E, et al. Etiology of nonimmune hydrops fetalis: a systematic review. Am J Med Genet A. 2009;149A(5):844–851. doi:10.1002/ajmg.a.32655
20. Al-Kouatly HB, Shivashankar K, Mossayebi MH, et al. Diagnostic yield from prenatal exome sequencing for non-immune hydrops fetalis: a systematic review and meta-analysis. Clin Genet. 2023;103(5):503–512.
21. Iyer NS, Gimovsky AC, Ferreira CR, Critchlow E, Al-Kouatly HB. Lysosomal storage disorders as an etiology of nonimmune hydrops fetalis: a systematic review. Clin Genet. 2021;100(5):493–503. doi:10.1111/cge.14005
22. Chainarong N, Muangpaisarn W, Suwanrath C. Etiology and outcome of non-immune hydrops fetalis in relation to gestational age at diagnosis and intrauterine treatment. J Perinatol. 2021;41(10):2544–2548.
23. Barros A, Freitas AC, Cabral AJ, et al. Giant placental chorioangioma: a rare cause of fetal hydrops. BMJ Case Rep. 2011;2011:bcr0220113880. doi:10.1136/bcr.02.2011.3880
24. Arcasoy MO, Gallagher PG. Hematologic disorders and nonimmune hydrops fetalis. Semin Perinatol. 1995;19(6):502–515.
25. Pasman SA, Meerman RH, Vandenbussche FPHA, Oepkes D. Hypoalbuminemia: a cause of fetal hydrops? Am J Clin Exp Obstet Gynecol. 2006;194(4):972–975. doi:10.1016/j.ajog.2006.02.028
26. Katz VL, Kort B, Watson WJ. Progression of nonimmune hydrops in a fetus with Noonan syndrome. Am J Perinatol. 1993;10(6):417–418. doi:10.1055/s-2007-994620
27. Yaegashi N. Pathogenesis of nonimmune hydrops fetalis caused by intrauterine B19 infection. Tohoku J Exp Med. 2000;190(2):65–82. doi:10.1620/tjem.190.65
28. Désilets V, De Bie I, Audibert F. No. 363-Investigation and Management of Non-immune Fetal Hydrops. J Obstetrics Gynaecology Canada. 2018;40(8):1077–1090.
29. Audette MC, Mclean K, Malkani N, Kingdom J, Sobel M. Diagnostic accuracy of Kleihauer-Betke (Kb) testing to predict fetal outcomes associated with fetomaternal hemorrhage: a retrospective cohort study. J Perinatol. 2022;42(1):91–96.
30. Al-Kouatly HB, Felder L, Makhamreh MM, et al. Lysosomal storage disease spectrum in nonimmune hydrops fetalis: a retrospective case control study. Prenat Diagn. 2020;40(6):738–745.
31. Mardy AH, Chetty SP, Norton ME, Sparks TN. A system‐based approach to the genetic etiologies of non‐immune hydrops fetalis. Prenatal Diagnosis. 2019;39(9):732–750.
32. Quinn AM, Valcarcel BN, Makhamreh MM, Al-Kouatly HB, Berger SI. A systematic review of monogenic etiologies of nonimmune hydrops fetalis. Genetics Med. 2021;23(1):3–12. doi:10.1038/s41436-020-00967-0
33. Wagner T, Fahham D, Frumkin A, et al. The many etiologies of nonimmune hydrops fetalis diagnosed by exome sequencing. Prenat Diagn. 2022;42(7):881–889. doi:10.1002/pd.5977
34. Sparks TN, Lianoglou BR, Adami RR, et al. Exome Sequencing for Prenatal Diagnosis in Nonimmune Hydrops Fetalis. N Engl J Med. 2020;383(18):1746–1756.
35. Al-Kouatly HB, Makhamreh MM, Rice SM, et al. High diagnosis rate for nonimmune hydrops fetalis with prenatal clinical exome from the Hydrops-Yielding Diagnostic Results of Prenatal Sequencing (HYDROPS) Study. Genetics Med. 2021;23(7):1325–1333. doi:10.1038/s41436-021-01121-0
36. Mone F, Eberhardt RY, Hurles ME, et al. Fetal hydrops and the Incremental yield of Next generation sequencing over standard prenatal Diagnostic testing (FIND) study: prospective cohort study and meta-analysis. Ultrasound Obstet Gynecol. 2021;58(4):509–518. doi:10.1002/uog.23652
37. Avram CM, Caughey AB, Norton ME, Sparks TN. Cost-Effectiveness of Exome Sequencing versus Targeted Gene Panels for Prenatal Diagnosis of Fetal Effusions and Non-Immune Hydrops Fetalis. Am J Obstet Gynecol MFM. 2022;4(6):100724.
38. Wei X, Zhou X, Zhou J, et al. The Value of Exome Sequencing in Thoracoamniotic Shunt for Severe Pleural Effusion with Fetal Hydrops: a Retrospective Clinical Study. Fetal Diagn Ther. 2022;49(3):138–144. doi:10.1159/000521212
39. Stratton RF, Patterson RM. DNA confirmation of congenital myotonic dystrophy in non-immune hydrops fetalis. Prenat Diagn. 1993;13(11):1027–1030.
40. Gort L, Granell MR, Fernández G, Carreto P, Sanchez A, Coll MJ. Fast protocol for the diagnosis of lysosomal diseases in nonimmune hydrops fetalis. Prenat Diagn. 2012;32(12):1139–1142. doi:10.1002/pd.3972
41. Fukui K, Amari S, Yotani N, et al. A Neonate with Mucopolysaccharidosis Type VII with Intractable Ascites. AJP Rep. 2023;13(1):e25–e28.
42. Sileo FG, Kulkarni A, Branescu I, et al. Non-immune fetal hydrops: etiology and outcome according to gestational age at diagnosis. Ultrasound Obstetrics Gynecol. 2020;56(3):416–421. doi:10.1002/uog.22019
43. Lallemand AV, Doco-Fenzy M, Gaillard DA. Investigation of nonimmune hydrops fetalis: multidisciplinary studies are necessary for diagnosis--review of 94 cases. Pediatr Dev Pathol. 1999;2(5):432–439.
44. Rodríguez MM, Chaves F, Romaguera RL, Ferrer PL, de la Guardia C, Bruce JH. Value of autopsy in nonimmune hydrops fetalis: series of 51 stillborn fetuses. Pediatr Dev Pathol. 2002;5(4):365–374. doi:10.1007/s10024-001-0260-6
45. Bellini C, Fulcheri E, Rutigliani M, et al. Immunohistochemistry in non-immune hydrops fetalis: a single center experience in 79 fetuses. Am J Med Genet A. 2010;152A(5):1189–1196. doi:10.1002/ajmg.a.33191
46. Troìa L, Al-Kouatly HB, McCurdy R, Konchak PS, Weiner S, Berghella V. The Recurrence Risk of Fetomaternal Hemorrhage. Fetal Diagn Ther. 2019;45(1):1–12. doi:10.1159/000491788
47. Rodeck CH, Kemp JR, Holman CA, Whitmore DN, Karnicki J, Austin MA. Direct intravascular fetal blood transfusion by fetoscopy in severe Rhesus isoimmunisation. Lancet. 1981;1(8221):625–627. doi:10.1016/S0140-6736(81)91549-X
48. Oepkes D, Seaward PG, Fpha V, et al.; DIAMOND Study Group. Doppler ultrasonography versus amniocentesis to predict fetal anemia. N Engl J Med. 2006;355(2):156–164. doi:10.1056/NEJMoa052855
49. Johnstone-Ayliffe C, Prior T, Ong C, Regan F, Kumar S. Early procedure-related complications of fetal blood sampling and intrauterine transfusion for fetal anemia. Acta Obstet Gynecol Scand. 2012;91(4):458–462. doi:10.1111/j.1600-0412.2011.01353.x
50. Lindenburg IT, Smits-Wintjens VE, van Klink JM; LOTUS study group. Long-term neurodevelopmental outcome after intrauterine transfusion for hemolytic disease of the fetus/newborn: the LOTUS study. Am J Obstet Gynecol. 2012;206(2):141.e1–8. doi:10.1016/j.ajog.2011.09.024
51. Tsao K, Hawgood S, Vu L, et al. Resolution of hydrops fetalis in congenital cystic adenomatoid malformation after prenatal steroid therapy. J Pediatric Surgery. 2003;38(3):508–510. doi:10.1053/jpsu.2003.50089
52. Fortes IMLP, Junior JRB. Use of Corticosteroids in Prenatal Treatment of Congenital Pulmonary Adenomatoid Malformation: integrative Review. Rev Bras Ginecol Obstet. 2022;44(03):304–310. doi:10.1055/s-0041-1741517
53. Peranteau WH, Boelig MM, Khalek N, et al. Effect of single and multiple courses of maternal betamethasone on prenatal congenital lung lesion growth and fetal survival. J Pediatric Surgery. 2016;51(1):28–32. doi:10.1016/j.jpedsurg.2015.10.018
54. Vu L, Tsao K, Lee H, et al. Characteristics of congenital cystic adenomatoid malformations associated with nonimmune hydrops and outcome. J Pediatric Surgery. 2007;42(8):1351–1356. doi:10.1016/j.jpedsurg.2007.03.039
55. Huhta JC. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol. 2004;25(3):274–286. doi:10.1007/s00246-003-0591-3
56. Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular and Stroke Nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242.
57. Ebenroth ES, Cordes TM, Darragh RK. Second-line treatment of fetal supraventricular tachycardia using flecainide acetate. Pediatr Cardiol. 2001;22(6):483–487. doi:10.1007/s002460010279
58. Huang HR, Tsay PK, Chiang MC, Lien R, Chou YH. Prognostic factors and clinical features in liveborn neonates with hydrops fetalis. Am J Perinatol. 2007;24(1):33–38. doi:10.1055/s-2006-958158
59. Taha DK, Lorch SA. Nonimmune Hydrops. In: Gleason CA, Sawyer T, editors. Avery’s Diseases of the Newborn.
60. Shalish W, Olivier F, Aly H, Sant’Anna G. Uses and misuses of albumin during resuscitation and in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2017;22(5):328–335. doi:10.1016/j.siny.2017.07.009
61. Bealer JF, Mantor PC, Wehling L, Tunell WP, Tuggle DW. Extracorporeal life support for nonimmune hydrops fetalis. J Pediatr Surg. 1997;32(11):1645–1647. doi:10.1016/S0022-3468(97)90474-7
62. Murphy JH. Nonimmune Hydrops Fetalis. NeoReviews. 2004;5(1):e5–e15. doi:10.1542/neo.5-1-e5
63. Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170(1):17–33. doi:10.1016/j.cell.2017.06.009
64. de Blank PMK, Gross AM, Akshintala S, et al. MEK inhibitors for neurofibromatosis type 1 manifestations: clinical evidence and consensus. Neuro Oncol. 2022;24(11):1845–1856.
65. Dori Y, Smith C, Pinto E, et al. Severe Lymphatic Disorder Resolved With MEK Inhibition in a Patient With Noonan Syndrome and SOS1 Mutation. Pediatrics. 2020;146(6):e20200167. doi:10.1542/peds.2020-0167
66. Li D, March ME, Gutierrez-Uzquiza A, et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat Med. 2019;25(7):1116–1122. doi:10.1038/s41591-019-0479-2
67. Biswas S, Gomez J, Horgan R, et al. Mirror syndrome: a systematic literature review. Am J Obstet Gynecol MFM. 2023;5(9):101067. doi:10.1016/j.ajogmf.2023.101067
68. Graham N, Garrod A, Bullen P, Heazell AEP. Placental expression of anti-angiogenic proteins in mirror syndrome: a case report. Placenta. 2012;33(6):528–531. doi:10.1016/j.placenta.2012.02.016
69. Reischer T, Muth B, Catic A, et al. Clinical Course and Outcome of Non-Immune Fetal Hydrops in Singleton Pregnancies. J Clin Med. 2022;11(3):702. doi:10.3390/jcm11030702
70. Moreno CA, Kanazawa T, Barini R, et al. Non-immune hydrops fetalis: a prospective study of 53 cases. Am J Med Genet A. 2013;161A(12):3078–3086. doi:10.1002/ajmg.a.36171
71. Huang YY, Chang YJ, Chen LJ, et al. Survival of Hydrops Fetalis with and without Fetal Intervention. Children. 2022;9(4):530.
72. Czernik C, Proquitté H, Metze B, Bührer C. Hydrops fetalis--has there been a change in diagnostic spectrum and mortality? J Matern Fetal Neonatal Med. 2011;24(2):258–263. doi:10.3109/14767058.2010.483522
73. Santo S, Mansour S, Thilaganathan B, et al. Prenatal diagnosis of non-immune hydrops fetalis: what do we tell the parents? Prenat Diagn. 2011;31(2):186–195.
74. Sohan K, Carroll SG, De La Fuente S, Soothill P, Kyle P. Analysis of outcome in hydrops fetalis in relation to gestational age at diagnosis, cause and treatment. Acta Obstetricia et Gynecologica Scandinavica. 2001;80(8):726–730. doi:10.1034/j.1600-0412.2001.080008726.x
75. Derderian SC, Jeanty C, Fleck SR, et al. The Many Faces of Hydrops. J Pediatr Surg. 2015;50(1):50–54.
76. Abrams ME, Meredith KS, Kinnard P, Clark RH. Hydrops Fetalis: a Retrospective Review of Cases Reported to a Large National Database and Identification of Risk Factors Associated With Death. Pediatrics. 2007;120(1):84–89. doi:10.1542/peds.2006-3680
77. Kim SA, Lee SM, Hong JS, et al. Ultrasonographic severity scoring of non-immune hydrops: a predictor of perinatal mortality. J Perinat Med. 2015;43(1):53–59. doi:10.1515/jpm-2013-0208
78. Deng Q, Fu F, Yu Q, et al. Nonimmune hydrops fetalis: genetic analysis and clinical outcome. Prenatal Diagnosis. 2020;40(7):803–812. doi:10.1002/pd.5691
79. Ota S, Sahara J, Mabuchi A, Yamamoto R, Ishii K, Mitsuda N. Perinatal and one-year outcomes of non-immune hydrops fetalis by etiology and age at diagnosis. J Obstet Gynaecol Res. 2016;42(4):385–391. doi:10.1111/jog.12922
80. Fukushima K, Morokuma S, Fujita Y, et al. Short-term and long-term outcomes of 214 cases of non-immune hydrops fetalis. Early Human Dev. 2011;87(8):571–575. doi:10.1016/j.earlhumdev.2011.04.015
81. Miller RS. Perinatal Palliative Care: ACOG COMMITTEE OPINION, Number 786. Obstetrics Gynecol. 2019;134(3):e84–e89. doi:10.1097/AOG.0000000000003425
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
