Back to Journals » International Journal of Nanomedicine » Volume 18
How Synthesis of Algal Nanoparticles Affects Cancer Therapy? – A Complete Review of the Literature
Authors El-Sheekh MM , AlKafaas SS , Rady HA, Abdelmoaty BE, Bedair HM, Ahmed AA, El-Saadony MT, AbuQamar SF , El-Tarabily KA
Received 22 June 2023
Accepted for publication 22 September 2023
Published 10 November 2023 Volume 2023:18 Pages 6601—6638
DOI https://doi.org/10.2147/IJN.S423171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Mostafa M El-Sheekh,1 Samar Sami AlKafaas,2 Hadeer A Rady,1 Bassant E Abdelmoaty,2 Heba M Bedair,1 Abdelhamid A Ahmed,3 Mohamed T El-Saadony,4 Synan F AbuQamar,5 Khaled A El-Tarabily5
1Botany Department, Faculty of Science, Tanta University, Tanta, 31527, Egypt; 2Molecular Cell Biology Unit, Division of Biochemistry, Chemistry Department, Faculty of Science, Tanta University, Tanta, 31527, Egypt; 3Plastic Surgery Department, Faculty of Medicine, Tanta University, Tanta, 31527, Egypt; 4Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, 44511, Egypt; 5Department of Biology, College of Science, United Arab Emirates University, Al Ain, 15551, United Arab Emirates
Correspondence: Synan F AbuQamar; Khaled A El-Tarabily, Department of Biology, College of Science, United Arab Emirates University, Al Ain, 15551, United Arab Emirates, Tel +971 3 713 6733 ; +971 3 713 6518, Fax +971 3 713 4927, Email [email protected]; [email protected]
Abstract: The necessity to engineer sustainable nanomaterials for the environment and human health has recently increased. Due to their abundance, fast growth, easy cultivation, biocompatibility and richness of secondary metabolites, algae are valuable biological source for the green synthesis of nanoparticles (NPs). The aim of this review is to demonstrate the feasibility of using algal-based NPs for cancer treatment. Blue-green, brown, red and green micro- and macro-algae are the most commonly participating algae in the green synthesis of NPs. In this process, many algal bioactive compounds, such as proteins, carbohydrates, lipids, alkaloids, flavonoids and phenols, can catalyze the reduction of metal ions to NPs. In addition, many driving factors, including pH, temperature, duration, static conditions and substrate concentration, are involved to facilitate the green synthesis of algal-based NPs. Here, the biosynthesis, mechanisms and applications of algal-synthesized NPs in cancer therapy have been critically discussed. We also reviewed the effective role of algal synthesized NPs as anticancer treatment against human breast, colon and lung cancers and carcinoma.
Keywords: algae, metabolites, cancer therapy, green synthesis, medical applications, nanoparticles
Introduction
Cancer is one of the most dangerous diseases and causes of death worldwide. More than one million cases of cancer and half a million deaths are reported each year.1–3 Some of the risk variables include family history, exogenous hormones, reproductive abnormalities, geographic location and age.4 Currently, chemotherapy is the most widely used approach for cancer treatment. Indeed, many drugs utilized in chemotherapy have undesirable side effects on healthy cells, multidrug resistance, and poor solubility.5–7 Therefore, it is crucial to develop alternative therapeutic approaches to treat cancer cells without harming healthy cells.
Efficient cancer therapy and cancer treatment substitute to chemotherapy are the subjects of unrelenting research efforts these days. Nanotechnology is the science/technology to synthesize, manipulate, control and manufacture nanoparticles (NPs) having size ranging from 1 to 100 nm.2,8–12 Although efforts to employ nanotechnology to cure cancer and enhance the effectiveness of medications are still at the developmental stage, NPs have been widely used in biomedical applications. It has been reported that metal NPs, such as copper (Cu), silver (Ag) and zinc oxide (ZnO), could be potentially used to treat cancer cells without affecting normal cells.7,9
There are two major methods used to synthesize NPs: (1) Top-down method that uses chemical and physical energy to break down larger structures into smaller components.3,13–15 Thermal decomposition, mechanical milling/ball milling, lithography, laser ablation and sputtering are the most common top-down approaches; and (2) bottom-up method is to generate nanomaterials from the reaction of atoms and other substances.16 Examples of bottom-up approaches are chemical vapor deposition, sol-gel process, spinning, pyrolysis, and biological synthesis.
Due to their nano-size,17 unique properties (eg, mass density)18 and surface charge,19 NPs can be linked with different ligands, such as RNA,20 DNA,21 aptamers,22 peptides23 and antibodies.24 This will facilitate the drug transportation of the modified NP to the action site to improve the pharmacokinetic characteristics and therapeutic efficacy against cancer.25 The use of NPs as an immunogenic cargo in traditional radio- and chemo-therapies has also been investigated.26 For instance, the biocompatibility of NPs is linked with the unconventional artificial antigen-presenting cells (aAPCs) and in vivo repositories of immunostimulatory molecules for sustained antitumor activity.27
Reactive oxygen species (ROS) can mediate apoptosis by regulating the expression of various pro-apoptotic proteins such as caspases or anti-apoptotic proteins [B cell lymphoma-2 (Bcl-2) and cellular FLICE-inhibitory protein (c-FLIP)].28 Other strategies of action of NPs in inducing apoptosis in cancer cells by protein regulation, immunological interventions, transcription inhibition and site-specific cytotoxicity. There is growing evidence that proteins engaged in signaling pathways are linked to the etiology, development and oncogenic activity of cancer cells may also be regulated by NPs.28,29
The apoptotic regulatory proteins can be downregulated by copper oxide (CuO) NPs.29 Selenium (Se) NPs at a concentration of 5 µg Se mL−1 affect the expression profile of apoptotic proteins. In addition, SeNPs can also cause alteration in the signaling pathways of the unfolded protein response (UPR).29 The expression pattern of the estrogen receptor (ER)-resident Se-proteins and Se-containing glutathione peroxidases, and thioredoxin reductases significantly increased after SeNP treatment on different cancer cell lines.30
SeNPs can also control pro-apoptotic proteins by activating Cx43 hemichannels.31 In MCF-7 cell lines that positively absorbed AgNPs, there was increased expression of -H2AX. This was followed by the release of Ag ions inside the cells and subsequently caused cell death.31 Gold (Au) NP conjugates can induce G1 cell cycle arrest and apoptosis induction in ER-positive human breast cancer cell lines, MCF-7.32
In addition to its role in improving the performance of biofuel cells and production of viable biofuels,33 nanotechnology is also involved in phyco-nanotechnology (phycochemicals produced from algae).10,34,35 Many algal species, including Chlorophyceae (green algae), Cyanophyceae (blue-green algae), Phaeophyceae (brown algae) and Rhodophyceae (red algae), are efficient in the synthesis of metal/metal oxide NPs.36–38 Algae are rich with secondary metabolites (alkaloids, flavonoids and terpenoids), pigments, vitamins and proteins that act as nano-biofactories.16,36,39 They also contain cytotoxic compounds, such as fucoidans, terpenoids, and laminarians, which have anticancer, antiproliferative, and antitumor activities.1,10,35,40 It is highly recommended to use algae for the green synthesis of NPs. This could be attributed to the simplicity, safety, low-cost, high energy efficiency, lack of external capping or reducing agents, and application in pharmaceutical and biomedical fields.40
Depending on the properties of the algal species, biosynthesis of NPs can be either extracellularly or intracellularly.41,42 Polysaccharides, proteins, enzymes, and reducing factors present in the algal culture and precipitating reducing metallic ions to nanomaterials have been proposed in extracellular metallic NP production.38,41,43–45
Henceforth, the current review highlights the potential capabilities of algae classes in the green synthesis of NPs and their role in cancer treatment. The mechanisms involved in this process along with their importance in cancer research are also discussed.
Why to Synthesize Eco-Friendly NPs?
Plants and microorganisms can produce NPs in a safe, affordable, and environmentally friendly manner.46–48 Inorganic metallic ions can be drawn in and stored by plants and microorganisms from their environment. These characteristics make living organisms reduce the environmental pollution and speed up the recovery of heavy metals from industrial waste into less hazardous forms.47,49,50 The features of biological agents in using their biochemical processes to convert inorganic metallic ions to metal NPs have provided new and unexplored fields of research.51–53
Unicellular and multicellular organisms have been shown to be capable of producing inorganic external and intracellular compounds in the micro- and nano-range. Bacteria, actinobacteria, algae, molds, yeasts, plants and viruses can be used to synthesize NPs.53 Each organism has varying degrees of metabolic processing capacity available to produce NPs of specific metals/metal oxides. Because not all organisms can produce NPs, careful selection of appropriate biological entities is required,54,55 taking into consideration that organisms with the potential to accumulate heavy metals have better chances of producing metallic NPs.55–58 In addition, optimizing culture parameters of nutrition, light, pH, temperature mixing rate and buffer strength can increase the enzymatic activities of organisms to produce NPs.59,60
To start the production of NPs, biomaterials are mixed with precursors of noble metal salts.4 When NPs are produced from their metal salt predecessors, proteins, alkaloids, flavonoids, reducing sugars, polyphenols, and other compounds present in biomaterials serve as reducing and capping agents.61 However, the process underlying NPs formation in microorganisms still needs to be fully understood.46 In general, the potential biological pathway involved, the interactions and metabolic processes of a certain microorganism as well as the influence of environmental factors determine the ultimate size and morphology of NPs.46,62
How Actinobacteria Produce NPs?
Actinobacteria can produce metallic NPs either extracellularly or intracellularly, with extracellular production being the more prevalent method.63–66 Rhodococcus sp. reduced metallic Au ions intracellularly, although AuNPs were primarily reduced on the cell wall and cell membrane (not in the cytoplasm).63 During the biosynthesis process, mono-dispersed AuNPs with sizes of 5–15 nm were produced; these particles had no harmful effects on cells.63 Karthik et al65 successfully produced AgNPs by reducing silver nitrate (AgNO3) ions using Streptomyces sp. LK-3. Nitrate reductase is an active enzyme in the cellular nitrogen cycle that reduces nitrate to nitrite.67 The nicotinamide adenine dinucleotide (NADH)-dependent nitrate reductase enzyme is in charge of reducing Ag ions to metallic Ag by an electron transfer process, producing stable AgNPs as a result.
Similar nitrate reductase enzyme activity was found when reducing Au ions from aqueous solutions containing gold chloride (AuCl4) ions.68 During the electron transfer from NADH by NADH-dependent reductase, each Au ion receives an electron, reduced to Au, and subsequently creating stable AuNPs.69,70 The attributes of the produced NPs must be protected from agglomeration brought on by the high surface energy and must be prevented with appropriate stabilization.70 Interestingly, naturally produced NPs typically have stronger antibacterial activity than conventionally synthesized NPs. The increased antibacterial activity is assumed to be associated with the synergistic proteins that are in charge of capping and stabilizing NPs.71
How Bacteria Produce NPs?
Bacteria have emerged as rapidly developing research area in green nanotechnology. Bacterial species, such as Escherichia coli, Bacillus cereus, Acinetobacter sp., Klebsiella pneumonia, Lactobacillus sp., Corynebacterium sp. and Pseudomonas sp. can produce metallic NPs.72–75 It is known that bacteria can produce metallic NPs via extracellular or intracellular methods. Pseudomonas stutzeri AG259 was used to produce AgNPs by NADH-dependent reductase enzyme, which provides electrons to oxidize NADH to NAD+.76,77 Pseudomonas aeruginosa was used to decrease Au ions, which led to the extracellular production of AuNPs.78 Others have, however, demonstrated that biological enzymes are not involved.78 Several variables work together to reduce the number of NPs. The first factor is dependent on the particular organic functional groups in the cell wall, whereas the second is dependent on external conditions.79
E. coli can produce biodegradable biopolymers, ie, polyhydroxyalkanoates (PHAs), on a wide scale that have the potential to replace petrochemical-based plastics.80–82 Due to growing concerns about rising crude oil prices and environmental harm caused by plastics, PHAs have drawn more attention recently.81,82
Bacteria have defense systems to adapt extreme environmental conditions. Such mechanisms include, but not limited to, redox state changes, efflux mechanisms, intracellular metal precipitation and accumulation, and extracellular complex formation of high metallic ion concentrations.83
How Fungi Produce NPs?
Many research groups use fungi in the production of NPs. The biosynthetic capacity of fungi, including Aspergillus sp., Fusarium sp., and Penicillium sp., to produce both AgNPs and AuNPs has been documented.84–87 Fungi may also produce mono-distributed NPs of all sizes and chemical compositions.87 In comparison to bacteria, fungi have additional characteristics that help in the production of metallic NPs. They secrete tremendous amounts of proteins and enzymes per unit of biomass, resulting in larger levels of NPs.88
Several fungi have high intracellular metal absorption volumes, and the synthesized particles often have smaller sizes.89,90 During the biosynthesis of metallic NPs, the culture conditions might, however, have a substantial impact. The biomass of Trichothecium sp. was employed to generate extracellular NPs during the biological reduction of Au ions in stationary conditions.91 When the biomass was, however, mixed up, it tended to generate intracellular NPs.
How Plants Produce NPs?
Plants can hyper-accumulate and physiologically decrease metallic ions.92,93 Plants are more environmentally acceptable method for biologically generating metallic NPs and detoxifying applications. Plant extracts rich in proteins, carbohydrates, terpenoids, alkaloids and phenolics can also decrease metallic ions and stabilize them.94
Variations in the composition and quantity of active biomolecules among plants, as well as their subsequent interaction with aqueous metal ions, are the primary contributors to the diversity of sizes and shapes of generated NPs.95 The applied procedure for producing NPs is mixing plant extracts with a metal salt solution at room temperature. The salts are biochemically reduced, and the presence of NPs can be detected using the color change in the reaction mixture.95
As expanded, NPs can form a variety of morphologies such as cubes, spheres, triangles, hexagons, pentagons, rods and wires.96 The final stage of synthesis identifies the most stable and energy-efficient form of NPs. The content of plant extracts, metal salt concentration, reaction duration, pH and temperature of the reaction solution can substantially influence the quality, size and shape of the formed NPs.97,98
Because plant extracts are rich in bioactive compounds, they are essential for the fabrication and stability of NPs.94 Date pulp waste was used as an effective bio-reductant in the green production of ZnONPs for wastewater treatment as an alternative to traditional ways of NPs synthesis.99
How Algae Produce NPs?
Some algae can also be used to biologically produce metallic NPs in addition to the accumulation of heavy metals. For instance, the dried unicellular alga, Chlorella vulgaris, was used to produce tetra-chloroauric ions, which were attached to the algae and reduced to produce AuNPs. It was discovered that the tetrahedral, decahedral, and icosahedral-shaped NPs accumulated close to the cell surface.100 The proteins in C. vulgaris extract can also serve as a reducing agent, shape-controlling modifier and stabilizing agent for the production of Ag at room temperature.101
The brown alga Sargassum wightii can extracellularly synthesize Au, Ag, and Au/Ag bimetallic NPs.102,103 Kappaphycus alvarezii was also used to generate extracellular AuNPs,104 and the brown alga Fucus vesiculosus provided biomass during the biological reduction of Au.105 It has also been reported that Tetraselmis kochinensis has the ability to produce intracellular AuNPs.106 Castro et al107 have recently reported the red alga Chondrus crispus for the same purpose of biosynthesis of NPs. In their effort to bioremediate carcinogenic components, El-Sheekh et al108 have reported that sodium alginate, Sargassum latifolium extract and their AgNPs are effective and cheap adsorbent agents to remove malachite green dye from aqueous solutions.
Potential Exposure and Hazard of Metal-Based NPs on Human
Metallic-based NPs are chosen for their synergistic effects, biocompatibility, and minimal cytotoxicity. The radiosensitivity effects of metallic NPs can be classified into three categories: physical, chemical and biological.109 Biological impact indicates cell damage, including cell cycle effects, DNA damage and cell death. Depending on the cellular and subcellular distribution of NPs, radiosensitivity may affect specific cellular compartments, such as the cell membrane, cytoplasm, nucleus, mitochondria and endoplasmic reticulum.110,111
NPs can be breathed, infused or injected into the body or the bloodstream. They can also infiltrate the body by passing through the outer layers of skin or tissue organs. For radiosensitivity, inert therapeutic NPs (eg, AuNPs) or therapeutic medicines (eg, cisplatin) can be used.112–114 Depending on the chosen approach and the tumor site, NPs can be delivered locally via surgical or nonsurgical procedures combined with NP/drug-loaded implants, or systemically via injection or inhalation.112,113
Cobalt (Co) and Cobalt Oxide (Co3O4) NPs
The investigation of CoNPs as a potential anticancer treatment is a result of their antioxidant properties to enable cancer therapeutic protection. CoNPs can be a promising nanomedicine for cancer therapy.115 Vodyashkin et al116 have reported the potential application of CoNPs from water purification cytostatic agents against cancer to theranostic and diagnostic agents. CoNPs are also effective neoplastic disease treatment agent. This could be attributed to their high surface area, high mass transfer, and magnetic characteristics. They are also hazardous to tumor cells and a useful vehicle for cytotoxic medicines.117 Studies have demonstrated that CoNPs made using the green approach exhibit activity against cancer cells and are highly cytotoxic to cancer cells.118
Human lymphocytes are exposed to oxidative stress from Co3O4 NPs produced through thermal breakdown, which damages DNA and results in inflammatory reactions.119 Induction of apoptosis is brought on by oxidative stress, which significantly contributes to toxicity. It has been suggested that Co2+ ions released from Co3O4NPs induce TNF-caspase-8-p38-caspase-3 in immune cells, which is the main source of injury.119
In Balb3T3 cells, Co3O4NPs caused cytotoxicity, morphological change and genotoxicity.120 Human peripheral leukocytes are affected in a genotoxic way by Co3O4NPs.121 These effects are most likely caused by the dissolution of Co2+ ions from NPs. Bare Co3O4NPs have an influence on human health because they are toxic to primary human immune cells. Surface alterations, like protein corona, could pave the way for using Co3O4NPs in various applications.119
Copper Oxide (CuO and Cu2O) NPs
With a low dose-rate of gamma radiation, CuNPs can suppress tumor via inducing oxidative state, stimulating apoptosis and inhibiting proliferation pathway.122 CuNPs are also involved in optical imaging and image-guided phototherapy, for ultrasound and magnetic resonance imaging (MRI) with high spatial resolution scan.
In addition, CuONPs have been tested on the human lung epithelial cell line A549 and shown significant impact on cytotoxicity, DNA damage and ROS production.122 CuONPs are found to induce toxicity at the biochemical, physiological, and tissue levels in the blue mussel (Mytilus edulis).123 The synthesized CuONPs have potential cytotoxic effects on breast cancer cells (MCF7 and MDA-MB231) and antiangiogenic effects on endothelial cells (EA.hy926).124
Cu2ONPs also have distinctive features in the field of nanoscale technology.125 Taherzadeh‑Soureshjani and Chehelgerdi126 have reported beneficial cytotoxic effects of the green synthesis of Cu2ONPs produced using the algae Cystoseira myrica on BC cell lines. By reducing angiogenesis and inducing apoptosis, C. myrica Cu2ONPs can be used as an additional medication in cancer treatment.126
Iron Oxide (FeO2), Hematite (Fe2O3) and Magnetite (Fe3O4) NPs
The green synthesis of FeNPs and their effect on cancer cells have been reported. For instance, FeNPs synthesized from Ulva lactuca (30–40 nm) have anticancer effects against HeLa and colorectal adenocarcinoma (DLD-1) cell lines, and anti-tumor activity against glioblastoma tumors (U87-Luc and GL-261).127,128 FeO2NPs have emerged as a candidate in drug delivery and cancer therapy.129 The ROS-induced oxidative stress is associated with FeO2NPs toxicity.130 Important factors such as particle surface, size distribution, zeta potential and surface coating may affect their magnetic properties.
Fe2O3NPs and Fe3O4NPs also have numerous biological and industrial uses.131 Haris et al132 used Oscillatoria limnetica extract as a substantial reducing and capping agent for Fe2O3NPs synthesis. Previous studies have shown that Fe2O3NPs exert anticancer activity against human cervical carcinoma (HeLa) and MCF7 cell lines and inhibit growth and proliferation of MCF7 cells.133 In vitro and in silico studies have demonstrated antioxidant effects and anticancer activities of the brown algaSpatoglossum asperum Fe3O4NPs (half-maximal inhibitory concentration (IC50) = 19.24 µg mL−1) against human glioblastoma cells (LN-18).48
Titanium Oxide (TiO2) NPs
Due to their high accumulation in cells causing modifications in gene expression, DNA damage, metabolic processes, homeostasis, inflammatory responses and lipid oxidation, TiO2NPs can be used as anticancer agents that can lead to necrosis or programmed cell death (PCD).134 For example, TiO2NPs have an anticancer effect against HepG2 tumor cells. TiO2NPs are promising solutions to eliminate tumor growth through light irradiation and ultrasound waves caused by ROS production or ablation by heating, achieving synergistic effects, promoting cancer regression, and even reaching immunological memory.14
Silica (SiO2) NPs
Silicon (SiO2) NPs offer new perspectives in biosensor, drug delivery and cancer therapy. In Calu-3 epithelial cells, amorphous SiO2NPs (10 nm) can cause inflammation and elevated levels of ROS that cause apoptosis and reduce cell survival in a time- and concentration-dependent manner with a lethal concentration (LC50) of 9.7 μg mL−1 after 24 h.135
Characterization of Algal-Mediated NPs
The characterization of algae-mediated NPs is conducted for more profound information on their synthesis and applications to comprehend the capability of NPs. Depending on the particle size dispersion, surface morphology, accumulation, zeta potential, size, delivery, wettability adsorption potential and state of the intelligent surface, isolation strategies of NPs can be determined.2,136,137 The most frequently used techniques for determining the size and shape of NPs are the scanning and transmission electron microscopes.137 Due to the surface plasmon resonance (SPR), metallic NPs have exceptional optical properties that can be observed by ultraviolet-visible (UV-Vis) spectroscopy between 190 and 1100 nm.138,139 This radiation interacts with the metals, advancing the electronic transition from the ground to a higher energy state, and a specific SPR band is obtained for a desirable size and shape of NPs that may reach up to 2–100 nm.139,140
The ingestion spectra for different materials are distinctive. AgNPs, AuNPs and ZnONPs range between 400–450, 500–550 nm and 350–390 nm, respectively.69,137,139–142 Depending on various features, SPR band positions may have blue- or red-shift.102,139,143 For instance, when the size of NPs decreases from 20 nm, SPR absorption band blue-shifts; however, if it is near 12 nm it strongly red-shifts.139
UV-Vis diffuse reflectance spectrometer (DRS) is a comprehensive method for measuring optical retention, delivery and reflectance.139 It is considered an excellent approach to find the band gaps of nanomaterials, which is necessary for estimating the conductance and photoactivity of the material.137,139,144 Based on the retention behavior of the analytes and the infrared (IR), Fourier transform infrared (FTIR) spectroscopy can recognize the functional groups of NPs; which their frequency normally ranges between 4000–400 cm−1 (Table 1).139 The correlation between the delivery spectra of the local fluid concentration and the reaction medium considers the biomolecules engaged in the interaction.139,145 Most normal utilitarian functional groups that attach to NPs are C=O, NH2 and SH.139,143
 |
Table 1 Advantages and Limitations of the Current Techniques Used for the Detection of NPs |
Other characterization approaches, such as X-ray photoelectron spectroscopy (XPS), may provide insights into the implementation of the generated NPs and their surrounding biomolecules (Table 1).145,167 Dynamic light scattering (DLS) spectroscopy estimates the surface charge, hydrodynamic breadth and circulation of NPs in the fluid-structure, and the zeta potential detects the particle strength.139,140
X-ray diffraction (XRD) characterize and acquire accurate information regarding the composition, crystal structure, and crystalline grain size of NPs.68,139 The composition of NPs can be determined by comparing the position and intensity of the peaks with the reference patterns of the International Centre for Diffraction Data (ICDD) database. However, it is not suitable for amorphous materials, and XRD peaks which are too broad for NPs with a size <3 nm.168 The Debye–Scherrer equation is mostly used to estimate the particle size from XRD data.139,169 The characterization techniques for the detection of NPs are presented in Table 1.
Mechanisms of Algal -Mediated NPs Synthesis
Algae (also known as bio-nano factories) are recognized for their ability to hyper-accumulate heavy metal ions and convert them into more malleable forms, making them superior candidates for the biosynthesis of NPs.5,170,171 Their downstream processing methods are well-developed and cost-effective.34,38 Over the last decade, there has been a lot of interest in digesting algal biomass under catalytic conditions. Microalgae are single-celled colony-forming or filamentous photosynthetic microorganisms that are classified into various categories, including Chlorophyta, Charophyta, and Bacillariophyta. AuNPs, AgNPs, and platinum (Pt) NPs have been synthesized, purified and characterized from the filamentous blue-green alga, Plectonema organum.170
Compared to other microbial systems, algae have promising multi-purpose potential for the green biosynthesis of NPs and mass production of valuable commercial products (Figure 1).9,172–174 Green biosynthesis of NPs from algae can be performed in the following steps: (i) heat/boil algal extract in water or in an organic solution for a certain period of time, (ii) prepare the molar solutions of ionic metallic compounds, and (iii) incubate the algal solutions and ionic metallic compounds for a certain period of time under controlled conditions with or without stirring.41,175
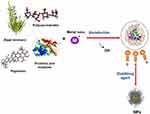 |
Figure 1 Green biosynthesis of nanoparticles using algae. Abbreviations: M, metal; NPs, nanoparticles. |
Algae secrete different enzymes responsible for metal bioreduction, which includes three main phases: activation, growth and termination. During the activation phase, metal ion reduction is followed by the nucleation of reduced atoms.176 The growth phase, which comprises the immediate coherence of small relative NPs into stable thermodynamic particles of huge size, is followed by the termination phase that includes the final shape and size of NPs. The metal bioreduction process is affected by various factors, including pH, temperature, substrate concentration, churning and static conditions.177,178
Several studies have reported that the location of the formed NPs and secondary metabolites determines whether the biosynthesis of NPs is achieved intracellularly or extracellularly.36,141,172 In a dose-dependent method, the intracellular biosynthesis mode involves biosynthesis of NPs depending on reductase enzymes within the algal cell.179 Algae produce reducing agents during their metabolic processes, such as NADPH or NADPH-dependent reductase.139,180 The pathway(s) showing the extracellular and intracellular synthesis of AuNPs is illustrated in Figure 2.
 |
Figure 2 Intracellular and extracellular pathways for the biosynthesis of AuNPs. Abbreviations: Au, gold; HAuCl4, chloroauric acid; NP, nanoparticles; Tyr, tyrosine; Trp, tryptophan; Cys, cysteine. |
Due to bioactive moieties involved in the bioreduction of the algal cell wall, biosynthesis of metallic NPs was more abundant in the cell wall than in the cytoplasm.139 At higher pH, secondary metabolites such as proteins and residual amino acids attached to the surface, have a role in capping and stabilization of NPs via NH2 (amine) groups.106,181 Vijayan et al176 stated that extracellular green biosynthesis of NPs occurs when metal ions become aggregated on the algal cell surface; whereas, secondary metabolites can reduce the metal ion on the algal cell surface. Extracellular mode of biosynthesis is more popular as NPs are easily purified, but pre-treatments of washing and blending algal biomass are required.145
Secondary metabolites act as reducing agents during the green biosynthesis of NPs.46 Secondary metabolites produced by many algal species include alkaloids, phenols, flavonoids, glycosides, glutathiones, terpenoids and phenazines (Table 2).182–184 Other biomolecules, such as polysaccharides, lipids, polyols, phycobiliproteins, organic acids, alcohol-based compounds and amino acids are also found in algae. The ability of algae to reduce metal ions and stabilize them into NPs in plants forms the basis of green synthesis of NPs (Figure 3).
 |
Table 2 Secondary Metabolites That Act as Reducing Agents for the Green Biosynthesis of NPs |
FTIR examination on the green-synthesized AgNPs from algal extracts revealed that biomolecules containing carboxyl, amine and hydroxyl functional groups are engaged in the reduction of Au ions.215 Thus, this has been successfully used to isolate flavonoids, terpenoids and chlorogenic acid. The flavonoid antioxidant, kaempferol, is used to generate and stabilize highly monodisperse (18.24 nm) spherical AuNPs.215,216 The presence of the C=O functional group in NPs has also been used to validate the potential of terpenoid fractions in the green production of ZnONPs. Furthermore, AuNPs were produced with chlorogenic acid as a reductant, and the FTIR spectra revealed an -OH functional group that was most likely involved in the synthesis.215
Factors Affecting NPs Synthesis
Various factors influence the characterization, synthesis and application of NPs. Numerous physical factors can manage algae-mediated biosynthesis of NPs, including pH, temperature, time, static condition, and substrate concentration,212,217,218 as the following:
pH
pH is an important factor that affects the extent and texture of the produced NPs.219 In general, algae require pH of 8.2 to 8.7, which is optimal alkaline for the growth and synthesis of NPs.220 Higher pH affects the reducing energy of functional groups and prevents aggregation of NPs.184 The reaction of the amine groups of surface-bound protein with leftover amino acids that caps and maintains NPs is mediated by basic pH.181
More functional groups bind at pH 3.0 and 4.0, and nucleate metal ions become more accessible at pH 2.0. The most attainable metal ions decrease many nucleation processes at pH 2.0, resulting in metal agglomeration.221 To study the effect of pH, alterations in the UV-Vis spectra of AgNPs were generated at pH 9.0, 7.0, and 4.0. At pH 9, the color intensity of the reaction mixture peaked. Although no reaction occurred at pH 3, mono-dispersive AgNPs were formed at pH 9.221
Temperature
Another important factor influencing NPs synthesis is temperature. A temperature of <100°C or ambient temperature is required for green technology. The temperature of the medium identifies the nature of NPs produced during the reaction.69 As confirmed by UV-Vis spectra, AgNPs synthesis is affected by temperatures of 25°C, 35°C and 45°C. In this case, an increase in the AgNPs formation rate was observed with increasing the temperature.222
Time
The duration of the incubation of the reaction medium has major effect on the form and quality of the produced NPs in green technology.223 Time dependence can also be influenced by the synthesis procedure, light exposure and storage conditions.224,225 Thus, NPs may aggregate, compress or expand as a result of long-term storage.226
Commonly Used Green Algal-Synthesized NPs
Algae are autotrophic (eukaryotic) protists that can be unicellular or multicellular. They produce natural biomolecules and NPs of varied shapes and sizes.143 The most widely researched algae for biosynthesis for the development of safer and more environmentally friendly NPs synthesis processes are brown, red, and green algae (Figure 4).143,227–229 Because algae grow quickly, are manageable and expand their biomass on average 10-fold faster than any plant species. Furthermore, they are frequently used to biosynthesize different metal/metal oxide NPs. Many strains of algae have been investigated for the green fabrication of different NPs.143
As part of their photosynthetic process, microalgae take-up carbon dioxide (CO2), capture sunlight and produce valuable molecules for renewable energy; thus, contributing to the “green” environment.228,229 Artificial photosynthesis is a chemical process that biomimics the natural photosynthesis process in order to fix CO2 in the atmosphere. This technology is currently being researched for large-scale production. Microalgae photosynthesis can provide the same benefits as artificial photosynthesis, in addition to the possibility of a wide range of microalgal products and applications.230 Figure 4 shows algae-mediated biosynthesis and the strategies employed to synthesize a wide spectrum of NPs.
Brown Algal-Mediated Biosynthesis of Metallic NPs
Brown algae consist of about 16 orders with ~285 genera and 1800 species. Their morphology can take different sizes and forms (from the smallest threads to 60-m long sea monsters). Giant kelp plays a major role in coastal marine ecosystems.231 Brown algae belonging to the order Fucales and family Sargassaceae contain important components of sterols (cholesterols, fucosterols, sulfated polysaccharides) and functional groups alginic acid, glucuronic acid, muramic acid and vinyl derivatives) may act as reducing and capping agents in biosynthesis of NPs.
Table 3 shows the different species of brown algae used to biosynthesize metal and metal oxide NPs. In general, CuNPs, AgNPs, and AuNPs are among the most frequently produced metal NPs from brown algae.193,195,232–234 Due to the physicochemical properties of AgNPs, which make them proper for various applications in industry and medicine, biosynthesis of AgNPs from algae has become popular and accounts for more than half of the published data.235,236
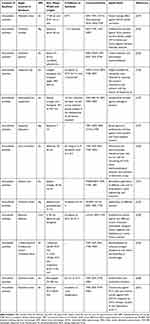 |
Table 3 Brown Algal-Biosynthesized Metal NPs |
Several species of brown algae, including Turbinaria conoides, Gelidiella acerosa, Sargassum polycystum, Desmarestia menziesii, Padina pavonica, and Cystophora moniliformis, have been reported to biosynthesize AgNPs.193,195,232–234 Extracellularly produced spherical AgNPs (96-nm) from T. conoides have high antibacterial effect against E. coli, P. aeruginosa, Staphylococcus epidermidis and Staphylococcus aureus, and antifungal effect against Candida albicans and Aspergillus niger.233 Turbinaria ornata and T. conoides are also reducing precursor agents of Ag salts used in AgNPs production.242,243 This could be attributed to their amines, polyamines, free hydroxyl, carbonyl groups and organic moieties.243
The widely produced AuNPs from different brown algal strains possess many medicinally relevant bioactivities, including anticoagulant, antifouling and antibacterial properties.167,232,244 For instance, the brown algal strain, Laminaria japonica, is characterized for its production of various NPs.244 T. conoides is one of the most common brown algal species that is also employed in AuNPs production.245 T. conoides can produce polydispersed, rectangular, spherical and triangular AuNPs. AuNPs are synthesized from chloroauric acid as a precursor of Au ions as well as T. conoides.
Brown algae can also biosynthesize metal oxides NPs, such as ZnONPs and TiO2NPs.246 The hexagonal ZnONPs, having a size of 35–57 nm and containing the bioactive functional groups (carbonyl, sulfate, amine and hydroxyl) can be biosynthesized from the dried seaweed powder of Sargassum muticum.247
Red Algal-Driven NPs Biosynthesis
Red algae (Rhodophyta) are largely used up as food source in many countries.248 Due to their reduction in stability, slow crystallization and self-aggregation by the red algae, research on biosynthesis of NPs from this group of seaweeds is still developing.103 Due to its role as reducing agent, Porphyra vietnamensis is a prominent red algal strain that has been involved in the fabrication of several NPs.249,250
Many species of red algae have been mentioned in the literature for their biosynthesis of AgNPs (Table 4), including Gracilaria dura, Gracilaria acerosa, Kappaphycus alvarezii, Kappaphycus sp. and Palmaria decipiens.251 Red algae-mediated AgNPs are cost-effective, environmentally friendly and efficient approach. Due to their spherical shape and tiny size (20–60 nm), AgNPs produced from red algal strains can be used in biomedical fields. For example, the extracellularly synthesized AgNPs from Gelidium amansii or Hypnea musciformis that possess anti-microfouling activities are of great interest in medical research.251,252
 |
Table 4 Red Algal-Biosynthesized Metal NPs |
In addition to AgNPs, species of red algae, including Chondrus crispus, Lemanea fluviatilis, Corallina officinalis, K. alvarezii, and Galaxaura elongata, are also linked to the biosynthesis of AuNPs.107 By using chloroauric acid, polydispersed crystalline AuNPs with a size of 5.9 nm have been produced from the marine red alga, L. fluviatilis.255,256 C. officinalis can also be used in the extracellular biosynthesis of spheroid AuNPs using phenol, carbonyl and hydroxyl functional groups as reducing agents.1 Furthermore, the red alga Gracilaria edulis has been successfully reported to synthesize the bimetallic AgNPs-AuNPs.257 These bimetallic NPs have been shown to have notable anticancer characteristics in human breast cancer cell lines.
Blue-Green Algal-Driven NPs Biosynthesis
Blue-green algae (Cyanophyta) contain three orders: Chroococcales, Chamaesiphonales, and Hormogoneales; of which Chroococcales separates into two families– Chroococcaceae and Entophysalidaceae.258,259 These two families can be distinguished from their ability to form colonies in their natural habitat, and are considered photoautotrophic, unicellular bacteria.258,259 Blue-green algae have been widely used to generate a wide range of NPs, as shown in Table 5.
 |
Table 5 Blue-Green Algal-Biosynthesized Metal NPs |
Spirulina platensis is the major contributor of AgNPs in blue-green algae. It contains rich nutritional substances, such as protein (60–70%), vitamins, β-carotene and essential fatty acids, to help reduce and cap NPs.202 The contribution of the production of spherical AgNPs (2–8 nm), S. platensis is widely used in the pharmaceutical industry, human health and food production. Other blue-green algal species producing different shapes and sizes of AgNPs have also been reported.266
S. platensis also plays an important role in the biosynthesis of AuNPs. S. platensis-mediated extracellular production of cubic, spherical and octahedral AuNPs has been reported to be linked with several groups, including peptides and proteins, to act as reducing agents.273 In Phormidium valderianum, an intracellular monodispersive triangle AuNPs was detected at 530 nm wavelengths and 1897 UV-Vis spectrometry absorbance.200,274 In addition, P. valderianum uses cytoplasmic metabolites as reducing agents in the extracellular biosynthesis of hexagonal, spherical and face-centered cubic (fcc; 24 nm) AuNPs.202 S. platensis is also known the biosynthesis of bimetallic NPs (core-shell AgNPs-AuNPs) and crystalline SiO2NPs.102,267
Micro Green Algal-Mediated Biosynthesis of NPs
The order Cladophorales that belongs to the micro green algae has been widely used in many industrial, pharmaceutical and biotechnological applications. Active compounds, such as phenols, alkaloids, flavonoids, sugars and functional groups, have been described as reducing and stabilizing agents in the biosynthesis of NPs.275 AgNPs are the most commonly in vitro-synthesized monometallic NPs from over 20 species of green microalgae producing them. Spherical (16 nm) form Chlorococcum humicola, cubical and hexagonal (24–55 nm) from Pithophora oedogonia, triangular (28 nm) from Chlamydomonas reinhardtii, and rectangular and rounded (1–15 nm) from both C. vulgaris and Enteromorpha flexuosa are examples of the ranges of AgNPs produced by green micro algae (Table 6).101,207,275,276
 |
Table 6 Micro Green Algal-Biosynthesized Metal NPs |
Recently, many studies have been published on green micro algae-driven production of AuNPs (Table 6). The micro algae, Pithophora crispa, has been extensively used for manufacturing AuNPs.206 Cyclic substances, carboxylic acids, peptides, and proteins make up most of the identified primary metabolites responsible for synthesizing metallic NPs from green micro algae.107,214,277
SiO2NPs, coming from C. vulgaris extract, are semiconductors employed as bio-indicators in numerous industrial wastes to detect harmful chemicals.203 In addition to SiO2NPs, biosynthesis of other semiconductor as well as metallic, bimetallic and metal oxide NPs is underway, with emphasis on early stages of production. The freshwater green alga, Chlamydomonas reinhardtii, has been implicated in the mediation of cadmium sulfide (CdS) bimetallic NPs that are highly used in photocatalysis, LEDs and biosensors.280 Table 6 shows that the commonly biosynthesized NPs coming from different species of micro green algae.
The Role of Algal-Mediated NPs in Cancer Treatment
Due to the severe adverse effects of cancer on normal surrounding cells, many chemotherapeutic drugs diminish clinical efficacy. Alternatively, nanomedicine is the application of nanomaterials to achieve therapeutic benefits for screening, diagnosing, preventing and curing diseases.284–286 Because the chemically generated NPs cause high-tissue accumulation and lead to toxicity, scientists have started to pay more attention to the significant role of nanomedicine in drug delivery, especially algal-based nano drug delivery systems. Several treatment strategies to improve diagnostic accuracy, drug specificity, drug design, and drug delivery systems have been suggested.286,287 Due to their low toxicity and enhanced drug delivery as excellent nanocarriers, recent research has focused on NPs synthesized from microbial sources against various types of cancer, of which they are considered as excellent nanocarriers due to their low toxicity and enhanced drug delivery.3,10,27,34 Biosynthetic algal NPs are potential methods for chemotherapeutic drug target delivery. Biological NP-based drug delivery systems have shown many advantages in cancer therapy due to drug administration, precise tumor cell selectivity and reduction of side effects.3,36
Due to their low toxicity, biodegradability and large surface area, algal-based NPs are used in various fields of clinical biotechnology and targeted drug delivery for cancer.288–290 For instance, the nanodrug delivery systems based on different algal-polysaccharides (alginates, carrageenans, fucoidan, ulvan, and others) have been described.285 Algal polysaccharides are also associated with the production of pharmaceutical substances and drug delivery agents. Drug delivery to cancer cells is made easier and more effective by peptide-drug conjugates.291 For example, the anti-A20 leukemic-like cell line efficacy of phage Peptide P4 coupled with 2-chlorotrityl resin has been proven. Recently, AuNPs have been used as a potential candidate for delivering a variety of medications to their intended locations.292,293 These payloads include everything from tiny medicinal molecules to large macromolecules, including proteins, RNA, and DNA. Effective release of these payloads must be considered to provide effective therapy.294
Quantum dots are crystalline NPs used to determine the location of cancer cells in the body. AuNPs allow heat from infrared lasers to detect cancerous tumors. FeO2NPs are used to better diagnose tumors by MRI, immunoassays, tissue healing, and as efficient chemotherapeutic agents.295–297 When NPs are attached to the tumour, their magnetic properties improve computed tomography (CT) imaging.298,299
During NPs biosynthesis, no harmful chemicals are used to grow algae because it naturally contains secondary metabolites and biomolecules, making algal-mediated NPs potential candidates in many biomedical applications, including cancer treatment.27,300 Due to their cytotoxic and anticancer properties, AgNPs from different algal species are used as drug carriers to deliver anticancer drugs to malignant sites as well as anticancer agents on their own. The anticancer action of AgNPs is mediated by triggering PCD via double-strand DNA breaks, oxidative stress, and chromosomal instability.146 Cortese et al301 have tested hybrid clustered NPs (HCNPs) loaded with a colloidal suspension of AgNPs against leukemia KU812 cells. The binding of HCNPs with cancerous leukemic cells and the release of Ag+ ions have resulted in ROS production, allowing the cancerous leukemic cells to be killed. It has been stated in the literature that biogenically produced NPs outperform chemically synthesized NPs in destroying malignant cells.301 According to Al-Dulimi et al,302 biogenically produced NPs successfully destroy T-cell leukemia. Similarly, AgNPs produced from an aqueous extract of the macroalga, Gracilaria edulis, have shown anticancer effect against human PC3 cell lines and MCF-7 breast cancer cells.210 Table 7 summarizes the types of NPs synthesized from algal species against different cancer cells.
 |
Table 7 Algal-Mediated Biosynthesis of NPs and Their Role in Cancer Treatment |
Green NPs induce apoptosis by upregulating the expression of caspase-9, caspase 3 and Bax, caspase-8, and downregulating the expression of Bcl-2 and Bid to trigger death of cancer cells.314 Chitosan-coated Ag nanotriangles may act as a photothermal agent for a panel of human non-small-cell lung cancer cells (NCIH460).315 In addition, the brown alga (Sargassum vulgare) is used to make biological AgNPs with a size of 10 nm that can suppress the proliferation of malignant human myeloblastic leukemia cells HL60 and cervical cancer cells HeLa. It has been demonstrated that the green biosynthesized AuNPs have anticancer activity against A549 cell.316,317 Biological AuNPs have pivotal role in drug delivery and management of cancer cell.318
Different algae (eg, T. conoides, S. platensis, Galaxaura elongate) are used as bio-nanofactories to synthesize AuNPs.319,320 AuNPs synthesized from these “green” algal sources have anticancer effects against cancer cell lines HEK-293 and MCF-7.321 In general, AuNPs can be manipulated to absorb light efficiently at the near-infrared region, convert it into heat energy, and transmit it to the surrounding environment. This process is called photo-hyperthermia that is widely used to attenuate cancer cells, where Au nanorods are administered near the tumor region to destroy cancer cells without causing much damage to healthy neighboring cells.321 AuNPs, individually or combined with other treatment modalities, such as radio/chemotherapy, can induce hyperthermia or deliver the drug in the targeted region or cell to produce a synergetic effect, to facilitate cancer treatment. Rezaeian and co-workers322 have used a green approach to synthesize curcumin-coated AuNPs and performed in vitro studies to compare NP-mediated photothermal therapy and radiofrequency electric field hyperthermia on mouse colorectal cancer (CT26) cell lines. They concluded that NPs could considerably induce apoptosis using photothermal therapy and radiofrequency electric field hyperthermia.
Micro- and macroalgae are responsible for the production of antibodies, vaccines, growth factors, and some hormones used in medical biotechnology.323 The marine green alga (Ulva rigida), brown alga (C. myrica) and red alga (Gracilaria foliifer) can produce spherical AgNPs with a diameter of 12, 17 and 24 nm, respectively.324 AgNPs produced from these marine algae can be used as reducing and capping agents, and exhibit great selectivity and strong anticancer potential against malignant MCF-7 cells without generating cytotoxicity against Artemia salina.324
The aqueous extract of the red seaweed, Champia parvula, contains antioxidant, antibacterial and anticancer phytochemical components that help protect humans from diseases.325 This could be attributed to the characteristics of AgNPs found in C. parvula. It has been reported that AgNPs may have an effect on the induction necrosis and apoptosis through SubG1 cell cycle arrest.326–330 The induction of apoptosis can be attributed to the upregulation of caspase-8 and −3 to trigger the induction of Bid and tBid proteins, and the up-regulation of the apoptotic proteins Bax and Bak.329
The biosynthesized Cu2ONPs from the brown alga C. myrica (CM-Cu2ONPs) were evaluated for their cytotoxicity against breast cancer cell lines MDA-MB-231 and T47D.126 They concluded that Cu2ONPs could decrease angiogenesis and induce apoptosis, suggesting that CM-Cu2ONPs have the potential to be employed as a supplement in cancer therapy.
Microalgal colloidal suspensions of NPs have antiproliferative and apoptotic effects on various cancers.299 For example, sulfated polysaccharide such as fucoidan extracted from F. vesiculosus, Sargassum henslowianam, Cladosiphon fucoidan and Coccophora longsdorfii inhibits angiogenesis and metastasis through the down-regulation of kinase activity and activation of caspase-3/7 in the human lymphoma cell line, melanoma, human colon cancer, breast cancer, lung carcinoma, and human promyelocytic leukemia.331 Another microalgal metabolite, mono-acyl glycerides extracted from Skeletonema marinoi, can induce selective apoptosis through caspase-3/7 activation in colon cancer cell lines (HCT-116) and hematological cancer cell lines (U-937), without induction of apoptosis in normal cells.330 Some microalgal lipids (eg, polyunsaturated fatty acids) also have anticancer properties against cervical and breast cancer. Phycocyanin, which is a phycobiliprotein found in the microalgal species Arthronema africanum, Porphyra haitanensis and S. platensis, inhibits the growth of human hepatocellular carcinoma, lung/colon cancer and leukemia cells.330
Therefore, algal-mediated AgNPs may serve as an important baseline for the development of new nanodrugs for cancer therapy and microbial infections. The role of different green synthesized NPs against different types of cancer along with the mechanisms associated to control/inhibit cancer cells are illustrated in Figure 5.
 |
Figure 5 The mechanism of green synthesized nanoparticles in inhibiting cancer in human. Abbreviations: ROS, reactive oxygen species; Au, gold. |
The Future Prospective
Green chemistry is a concept that aims to reduce waste and byproducts, as well as the use of dangerous chemicals and energy needs, by combining renewable and natural resources.167,286,321 The combination of green chemistry with nanotechnology is a great trending strategy used in different fields of research.179,332 There are many challenges in green synthesis of NPs; among those is to obtain homogeneously dispersed NPs. Due to their distinctive morphological and physiochemical features, NPs have been utilized in many fields, including communication, space, medicine and agriculture.95,170,273 Green nanotechnology using algae is biocompatible, bioavailable and biosafe.7,34,67,132 There are several applications of green NPs in biomedical fields, including disease detection, cancer therapies, imaging, drug delivery, tissue engineering and treatment.172,244,256 Application of NPs in biological entities or biomedical applications, mainly cancer research, is crucial and should come from green precursors.14,48,292
Due to their secondary metabolites, algae have a great ability to synthesize green NPs.27,41,127,299 Algal nanotechnology has grown into a distinct field known as phyco-nanotechnology that can successfully provide a variety of applications.241,252,259 Many investigations have been undertaken on the production of NPs utilizing macroalgae (seaweed) extracts, where others have demonstrated that microalgae can produce metal NPs.1,276
Due to their appealing properties, algae have been proposed as model organisms for the processing of bio-nanomaterials.1,44,103 Because it is difficult to obtain NPs of desired shape and size through mechanical crushing, algal-synthesized NPs could be a feasible and more sustainable alternative for the future.9,35,142 This could be attributed to the fact that numerous factors, including temperature, pH, the type of capping agent, and the quantity of active chemicals, may be important in determining the size and morphology. Algal-mediated route to biogenic NPs offers a promising source of potential anticancer agents. For instance, algal-mediated AgNPs have been of great interest in cancer treatment due to their unique physiochemical properties.19,40,210
On the other hand, the selection of algal strains, the slow process of synthesizing NPs, poor morphological characteristics of NPs, low yield of NPs and high level of aggregation of NPs hinder the commercialization of green algal-synthesized NPs.38,173 The lack of understanding of the mechanism of biosynthesis can also be added as a limiting factor in the use of algae in green biosynthesis of NPs. Although there are some examples of types and quantities of NPs that have been previously produced from algae, future research may focus on algal-mediated NPs made of carbon (C), SiO2NPs, ZnO and other metals/metal oxides. With emerging characterization methods/technologies, controlled and comparative algal-based NP biosynthesis is now possible to improve the properties of algal-mediated NPs for commercial applications.
Future research should be considered on the factors affecting the uptake kinetics in order to increase the yield of algal-based NPs for commercial use as well. Further studies to establish new types of green synthesis of C-NPs, ZnONPs, palladium (Pd) NPs, and SiNPs using algal extracts are also required. It is also important to develop new technologies to help produce large quantities of algal-synthesized NPs with high efficacy to satisfy the biomedical applications for targeted effects against cancer cells without affecting normal cells. In addition, there is a big gap in the knowledge within the scientific community regarding the physiochemical characteristics of NPs produced using traditional technologies and those of algal origin. The role of biomolecules as reducing and capping agents during algae-mediated biosynthesis of NPs must also be elucidated.
Extensive research on identifying the proteins and enzymes involved in the formation of algal-mediated NPs should be on top of our priorities. Rapid, simple, cost-effective and environmentally safer procedures for alga-mediated synthesized NPs should be taken into account. Future research on the size, distribution and chemical composition of algal-derived NPs should be assessed. In general, the application of nanobiotechnology using algae is still in its infancy and needs further investigation.333 In addition to in vitro studies, in vivo testing of algal-synthesized NPs is a vital part of safety assessment and is a regulatory requirement before a drug can progress into clinical trials.334
Funding
This review was funded by Abu Dhabi Award for Research Excellence—Department of Education and Knowledge (Grant #: 21S105) to K El-Tarabily, and Khalifa Center for Biotechnology and Genetic Engineering—UAEU (Grant #: 31R286) to S. AbuQamar.
Disclosure
The authors report no conflict of interest in this work.
References
1. El-Kassas HY, El-Sheekh MM. Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 2014;15:4311–4317. doi:10.7314/apjcp.2014.15.10.4311
2. Diab T, Alkafaas SS, Shalaby TI, Hessien M. Dexamethasone simulates the anticancer effect of nano-formulated paclitaxel in breast cancer cells. Bioorg Chem. 2020a;99:103792. doi:10.1016/j.bioorg.2020.103792
3. Diab T, Alkafaas SS, Shalaby TI, Hessien M. Paclitaxel nanoparticles induce apoptosis and regulate txr1, cyp3a4 and cyp2c8 in breast cancer and hepatoma cells. Anticancer Agents Med Chem. 2020b;20:1582–1591. doi:10.2174/1871520620666200504071530
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
5. AlKafaas SS, Diab T, Shalaby T, Hessien M. Dexamethasone improves the responsiveness of hepatoma cells for both free and solvent containing paclitaxel in vitro. Egypt J Biochem Mol Biol. 2019;37:1–2.
6. Somu P, Paul S. Supramolecular nanoassembly of lysozyme and α-lactalbumin (apo α-LA) exhibits selective cytotoxicity and enhanced bioavailability of curcumin to cancer cells. Colloids Surf B. 2019;178:297–306. doi:10.1016/j.colsurfb.2019.03.016
7. Acharya D, Satapathy S, Somu P, Parida UK, Mishra G. Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol Trace Elem Res. 2021;199:1812–1822. doi:10.1007/s12011-020-02304-7
8. Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O’Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667. doi:10.1016/S1470-2045(06)70793-8
9. El-Sheekh M, Alwaleed EA, Kassem WMA, Saber H. Antialgal and anticancer activities of the algal silver nanoparticles against the toxic cyanobacterium Microcystis aeruginosa and human tumor colon cell line. Environ Nanotechnol Monit Manag. 2020;14:100352. doi:10.1016/j.enmm.2020.100352
10. Sargazi S, Laraib U, Er S, et al. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials. 2022;27:1102. doi:10.3390/nano12071102
11. Abd Elkodous M, El-Husseiny HM, El-Sayyad GS, et al. Recent advances in waste-recycled nanomaterials for biomedical applications: waste-to-wealth. Nanotechnol Rev. 2021;10:1662–1739. doi:10.1515/ntrev-2021-0099
12. Abdelsalam IM, Ghosh S, AlKafaas SS, et al. Nanotechnology as a tool for abiotic stress mitigation in horticultural crops. Biologia. 2023;78:163–178. doi:10.1007/s11756-022-01251-z
13. Das RK, Pachapur VL, Lonappan L, et al. Biological synthesis of metallic nanoparticles: plants, animals, and microbial aspects. Nanotechnol Environ Eng. 2017;2:18. doi:10.1007/s41204-017-0029-4
14. Zhang D, Ma XL, Gu Y, Huang H, Zhang GW. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front Chem. 2020;8:799. doi:10.3389/fchem.2020.00799
15. Rajamohan R, Ashokkumar S, Lee YR. Environmental free synthesis of biologically active Cu2O nanoparticles for the cytotoxicity. J Mol Struc. 2023;1271:134081. doi:10.1016/j.molstruc.2022.134081
16. Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020;13:223–245. doi:10.1080/17518253.2020.1802517
17. Setyawati MI, Tay CY, Bay BH, Leong DT. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano. 2017;11:5020–5030. doi:10.1021/acsnano.7b01744
18. Tay CY, Setyawati MI, Leong DT. Nanoparticle density: a critical biophysical regulator of endothelial permeability. ACS Nano. 2017;11:2764–2772. doi:10.1021/acsnano.6b07806
19. Wang J, Zhang L, Peng F, Shi X, Leong DT. Targeting endothelial cell junctions with negatively charged gold nanoparticles. Chem Mater. 2018;30:3759–3767. doi:10.1021/acs.chemmater.8b00840
20. Ganbold T, Han S, Hasi A, Baigude H. Receptor-mediated delivery of therapeutic RNA by peptide functionalized curdlan nanoparticles. Int J Biol Macromol. 2019;126:633–640. doi:10.1016/j.ijbiomac.2018.12.152
21. Ge H, Wang D, Pan Y, et al. Sequence‐dependent DNA functionalization of upconversion nanoparticles and their programmable assemblies. Angew Chem Int Ed Engl. 2020;59:8133–8137. doi:10.1002/anie.202000831
22. Guan B, Zhang X. Aptamers as versatile ligands for biomedical and pharmaceutical applications. Int J Nanomed. 2020;Volume 15:1059–1071. doi:10.2147/IJN.S237544
23. Jia X, Guo M, Han Q, et al. Synergetic tumor probes for facilitating therapeutic delivery by combined-functionalized peptide ligands. Anal Chem. 2020;92:5650–5655. doi:10.1021/acs.analchem.0c00440
24. Marques AC, Costa PJ, Velho S, Amaral MH. Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J Control Release. 2020;320:180–200. doi:10.1016/j.jconrel.2020.01.035
25. Bakshi S, Zakharchenko A, Minko S, Kolpashchikov DM, Katz E. Towards nanomaterials for cancer theranostics: a system of DNA-modified magnetic nanoparticles for detection and suppression of RNA marker in cancer cells. Magnetochemistry. 2019;5:24. doi:10.3390/magnetochemistry5020024
26. Das M, Shen L, Liu Q, Goodwin TJ, Huang LJ. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol Ther. 2019;27:507–517. doi:10.1016/j.ymthe.2018.11.012
27. González-Ballesteros N, Diego-González L, Lastra-Valdor M, et al. Immunostimulant and biocompatible gold and silver nanoparticles synthesized using the Ulva intestinalis L. aqueous extract. J Mate Chem. 2019;7:4677–4691. doi:10.1039/C9TB00215D
28. Kim U, Kim C-Y, Lee JM, et al. Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol Oncol Res. 2020;26:977–984. doi:10.1007/s12253-019-00643-y
29. Khan S, Ansari AA, Khan AA, Abdulla M, Al-Obaid O, Ahmad R. In vitro evaluation of cytotoxicity, possible alteration of apoptotic regulatory proteins, and antibacterial activity of synthesized copper oxide nanoparticles. Colloids Surf B. 2017;153:320–326. doi:10.1016/j.colsurfb.2017.03.005
30. Varlamova EG, Goltyaev MV, Mal’tseva VN, et al. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int J Mol Sci. 2021;22:7798. doi:10.3390/ijms22157798
31. Turovsky EA, Varlamova EG. Mechanism of Ca2+-dependent pro-apoptotic action of selenium nanoparticles, mediated by activation of Cx43 hemichannels. Biology. 2021;10:743. doi:10.3390/biology10080743
32. Bhowmik T, Gomes A. Down–regulation of cyclin–dependent kinase-4 and MAPK through estrogen receptor mediated cell cycle arrest in human breast cancer induced by gold nanoparticle tagged toxin protein NKCT1. Chem Biol Interact. 2017;268:119–128. doi:10.1016/j.cbi.2017.03.009
33. Dabirian E, Hajipour A, Mehrizi AA, et al. Nanoparticles application on fuel production from biological resources: a review. Fuel. 2023;331:125682. doi:10.1016/j.fuel.2022.125682
34. El-Sheekh MM, Deyab M, Hassan NI, Seham E, Abu Ahmed SE. Bioadsorption of Fe (II) ions from aqueous solution using Sargassum latifolium aqueous extract and its synthesized silver nanoparticles. Int J Phytoremediation. 2022;14:1–14. doi:10.1080/15226514.2022.2145000
35. Pitchai P, Subramani P, Selvarajan R, Sankar R, Vilwanathan R, Sibanda T. Green synthesis of gold nanoparticles (AuNPs) using Caulerpa racemosa and evaluation of its antibacterial and cytotoxic activity against human lung cancer cell line. Arab J Basic Appl Sci. 2022;29:351–362. doi:10.1080/25765299.2022.2127510
36. Chaudhary R, Nawaz K, Khan AK, Hano C, Abbasi BH, Anjum S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules. 2020;10:1498. doi:10.3390/biom10111498
37. Gheda S, El-Sheekh M, Abou-Zeid A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pac J Trop Med. 2018;11:583–589. doi: 10.4103/1995-7645.244523
38. El-Sheekh MM, Shabaan MT, Hassan L, Morsi HH. Antiviral activity of algae biosynthesized silver and gold nanoparticles against herps simplex (HSV-1) virus in vitro using cell-line culture technique. Int J Environ Health Res. 2022b;32:616–627. doi:10.1080/09603123.2020.1789946
39. Borowitzka MA. High-value products from microalgae their development and commercialization. J Appl Phycol. 2013;25:743–756. doi:10.1007/s10811-013-9983-9
40. de Arruda MCS, da Silva MROB, Cavalcanti VLR, et al. Antitumor lectins from algae: a systematic review. Algal Res. 2023;70:102962. doi:10.1016/j.algal.2022.102962
41. Mukherjee A, Sarkar D, Sasmal S. A review of green synthesis of metal nanoparticles using algae. Front Microbiol. 2021;12:693899. doi:10.3389/fmicb.2021.693899
42. Ahmed A, Usman M, Ji Z, et al. Nature-inspired biogenic synthesis of silver nanoparticles for antibacterial applications. Mater Today Chem. 2023;27:101339. doi:10.1016/j.mtchem.2022.101339
43. Gahlawat G, Choudhury AR. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9:12944–12967. doi:10.1039/C8RA10483B
44. Shantkriti S, Pradeep M, Unish KK, et al. Biosynthesis of silver nanoparticles using Dunaliella salina and its antibacterial applications. Appl Surf Sci Adv. 2023;13:100377. doi:10.1016/j.apsadv.2023.100377
45. Boukarma L, Aziam R, Abali M, et al. Algal biomass valorization for the removal of heavy metal ions. In: Lichtfouse E, Muthu SS, Khadir A, editors. Inorganic-Organic Composites for Water and Wastewater Treatment. Environmental Footprints and Eco-Design of Products and Processes. Singapore: Springer; 2022:267–302. doi:10.1007/978-981-16-5928-7_8
46. Makarov VV, Love AJ, Sinitsyna OV, et al. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:35–44. doi:10.32607/20758251-2014-6-1-35-44
47. Gowramma B, Keerthi U, Rafi M, Muralidhara Rao D. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech. 2015;5:195–201. doi:10.1007/s13205-014-0210-4
48. Palaniyandi T, Baskar G, Bhagyalakshmi V, et al. Biosynthesis of iron nanoparticles using brown algae Spatoglossum asperum and its antioxidant and anticancer activities through in vitro and in silico studies. Particulate Sci Technol. 2023;41:916–929. doi:10.1080/02726351.2022.2159900
49. El-Saadony MT, Sitohy MZ, Ramadan MF, Saad AM. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II). Innov Food Sci Emerg Technol. 2021;69:102645. doi:10.1016/j.ifset.2021.102645
50. Saad AM, Sitohy MZ, Sultan-Alolama MI, El-Tarabily KA, El-Saadony MT. Green nanotechnology for controlling bacterial load and heavy metal accumulation in Nile tilapia fish using biological selenium nanoparticles biosynthesized by Bacillus subtilis AS12. Front Microbiol. 2022;13:
51. Baker S, Harini B, Rakshith D, Satish S. Marine microbes: invisible nanofactories. J Pharm Res. 2013;6:83–388. doi:10.1016/j.jopr.2013.03.001
52. Bhattacharya D, Gupt R. Nanotechnology and potential of microorganisms. Crit Rev Biotechnol. 2005;25:1199–1204. doi:10.1080/07388550500361994
53. Singh A, Jain D, Upadhyay M, Khandelwal N, Verma H. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Bios. 2010;5:483–489.
54. Sathishkumar M, Sneha K, Yun Y. Palladium nanocrystal synthesis using Curcuma longa tuber extract. Int J Mater Sci. 2009;4:11–17.
55. Sriramulu M, Shanmugam S, Ponnusamy VK. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: an in vitro study. Colloids Interface Sci Commun. 2020;35:100254. doi:10.1016/j.colcom.2020.100254
56. Beveridge T, Murray R. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–887. doi:10.1128/Fjb.141.2.876-887.1980
57. Mehra RK, Winge DR. Metal ion resistance in fungi: molecular mechanisms and their regulated expression. J Cell Biochem. 1991;45:30–40. doi:10.1002/jcb.240450109
58. Southam G, Beveridge TJ. The in vitro formation of placer gold by bacteria. Geochim Cosmochim Acta. 1994;58:4527–4530. doi:10.1016/0016-7037(94)90355-7
59. Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. doi:10.1039/C1GC15386B
60. Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett. 2008;62:4588–4590. doi:10.1016/j.matlet.2008.08.044
61. Kuppusamy P, Yusoff MM, Maniam GP, Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–an updated report. Saudi Pharm J. 2016;24:473–484. doi:10.1016/j.jsps.2014.11.013
62. Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)− thiosulfate and gold (III)− chloride complexes. Langmuir. 2006;22:2780–2787. doi:10.1021/la052652c
63. Abdeen S, Geo S, Praseetha P, Dhanya R. Biosynthesis of silver nanoparticles from actinomycetes for therapeutic applications. Int J Nanodimension. 2014;5:155–162. doi:10.7508/ijnd.2014.02.008
64. Golinska P, Wypij M, Ingle AP, Gupta I, Dahm H, Rai M. Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicity. Applied Microbiol Biotechnol. 2014;98:8083–8097. doi:10.1007/s00253-014-5953-7
65. Karthik L, Kumar G, Kirthi AV, Rahuman A, Bhaskara Rao K. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst Eng. 2014;37:261–267. doi:10.1007/s00449-013-0994-3
66. Abd-Elhady HM, Ashor MA, Hazem A, et al. Biosynthesis and characterization of extracellular silver nanoparticles from Streptomyces aizuneusis: antimicrobial, anti-larval, and anticancer activities. Molecules. 2022;27:212. doi:10.3390/molecules27010212
67. Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotechnol. 2009;29:279–306. doi:10.3109/07388550903062462
68. Shah R, Oza G, Pandey S, Sharon M. Biogenic fabrication of gold nanoparticles using Halomonas salina. J Microbiol Biotechnol Res. 2012;2:485–492.
69. Rai A, Singh A, Ahmad A, Sastry M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanoparticles. Langmuir. 2006;22:736–741. doi:10.1021/la052055q
70. He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett. 2007;61:3984–3987. doi:10.1016/j.matlet.2007.01.018
71. Kumar A, Kaur K, Sharma S. Synthesis, characterization, and antibacterial potential of silver nanoparticles by Morus nigra leaf extract. Indian J Pharm Biol Res. 2013a;1:16–24. doi:10.30750/ijpbr.1.4.4
72. Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517. doi:10.1007/s11051-007-9275-x
73. Prasad TNV, Subba Rao Kambala V, Naidu R. A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr Nanosci. 2011;7:531–544. doi:10.2174/157341311796196736
74. Sunkar S, Nachiyar CV. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed. 2012;2:953–959. doi:10.1016/S2221-1691(13)60006-4
75. Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. Int Sch Res Notices. 2014;18:359316. doi:10.1155/2014/359316
76. Ahmad A, Senapati S, Khan MI, et al. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology. 2003;14:824. doi:10.1088/0957-4484/14/7/323
77. Constantin M, Spiridon M, VIchim DL, et al. Synthesis, biological and catalytic activity of silver nanoparticles generated and covered by oxidized pullulan. Mater Chem Phys. 2023;295:127141. doi:10.1016/j.matchemphys.2022.127141
78. Husseiny M, Abdel-Aziz M, Badr Y, Mahmoud M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67:1003–1006. doi:10.1016/j.saa.2006.09.028
79. Lin Z, Fu J, Wu J, Liu Y, Cheng H. Preliminary study on the mechanism of non-enzymatic bioreduction of precious metal ions. Acta Phys -Chim Sin. 2001;17:477–480. doi:10.3866/PKU.WHXB20010520
80. Leong YK, Show PL, Ooi CW, Ling TC, Lan JC-W. Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: insights from the recombinant Escherichia coli. J Biotechnol. 2014;180:52–65. doi:10.1016/j.jbiotec.2014.03.020
81. Pakalapati H, Chang CK, Show PL, Arumugasamy SK, Lan JCW. Development of polyhydroxyalkanoates production from waste feedstocks and applications. J Biosci Bioeng. 2018;126:282–292. doi:10.1016/j.jbiosc.2018.03.016
82. Pesante G, Frison N. Recovery of bio-based products from PHA-rich biomass obtained from biowaste: a review. Bioresour Technol Rep. 2023;21:101345. doi:10.1016/j.biteb.2023.101345
83. Dhillon GS, Brar SK, Kaur S, Verma M. Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit Rev Biotechnol. 2012;32:449–473. doi:10.3109/07388551.2010.550568
84. Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mat Chem. 2003;13:1822–1826. doi:10.1039/B303808B
85. Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418. doi:10.1016/j.matlet.2006.07.042
86. Kathiresan K, Manivannan S, Nabeel M, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B. 2009;71:133–137. doi:10.1016/j.colsurfb.2009.01.016
87. Philip D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim Acta A Mol Biomol Spectrosc. 2009;73:374–381. doi:10.1016/j.saa.2009.02.037
88. Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi:10.1016/j.cis.2010.02.001
89. Volesky B, Holan Z. Biosorption of heavy metals. Biotech Prog. 1995;11:235–250. doi:10.1021/bp00033a001
90. Mukherjee P, Senapati S, Mandal D, et al. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chem BioChem. 2002;3:461–463. doi:10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X
91. Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J Biomed Nanotechnol. 2005;1:47–53. doi:10.1166/jbn.2005.012
92. Kale A, Bao Y, Zhou Z, Prevelige PE, Gupta A. Directed self-assembly of CdS quantum dots on bacteriophage P22 coat protein templates. Nanotechnology. 2013;24:045603. doi:10.1088/0957-4484/24/4/045603
93. Kulkarni N, Muddapur UJ. Biosynthesis of metal nanoparticles: a review. J Nanotechnol. 2014;510246. doi:10.1155/2014/510246
94. Khan AA, Fox EK, GóRzny MŁ, et al. pH control of the electrostatic binding of gold and iron oxide nanoparticles to tobacco mosaic virus. Langmuir. 2013;29:2094–2098. doi:10.1021/la3044126
95. Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014;302429. doi:10.1155/2014/302429
96. Akhtar MS, Panwar J, Yun Y. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustainable Chem Eng. 2013;1:591–602. doi:10.1021/sc300118u
97. Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A Physicochem Eng Asp. 2010;369:27–33. doi:10.1016/j.colsurfa.2010.07.020
98. Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–356. doi:10.1016/j.biotechadv.2013.01.003
99. Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:
100. Luangpipat T, Beattie IR, Chisti Y, Haverkamp RG. Gold nanoparticles are produced in a microalga. J Nanopart Res. 2011;13:6439–6445. doi:10.1007/s11051-011-0397-9
101. Xie J, Lee JY, Wang DI, Ting YP. Silver nanoplates: from biological to biomimetic synthesis. ACS Nano. 2007b;1:429–439. doi:10.1021/nn7000883
102. Govindaraju K, Basha SK, Kumar VG, Singaravelu G. Silver, gold, and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci. 2008;43:5115–5122. doi:10.1007/s10853-008-2745-4
103. Singaravelu G, Arockiamary J, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B. 2007;57:97–101. doi:10.1016/j.colsurfb.2007.01.010
104. Rajasulochana P, Krishnamoorthy P, Dhamotharan R. Potential application of Kappaphycus alvarezii in agricultural and pharmaceutical industry. J Chem Pharm Res. 2012;4:33–37.
105. Mata YN, Torres E, Blazquez ML, Ballester A, González FMJA, Munoz JA. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J Hazard Mater. 2009;166:612–618. doi:10.1016/j.jhazmat.2008.11.064
106. Senapati S, Syed A, Moeez S, Kumar A, Ahmad A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett. 2012;79:116–118. doi:10.1016/j.matlet.2012.04.009
107. Castro L, Blázquez ML, Muñoz JA, González F, Ballester A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013;7:109–116. doi:10.1049/iet-nbt.2012.0041
108. El-Sheekh MM, Deyab MA, Hassan NI, Abu Ahmed SE. Bioremediation of malachite green dye using sodium alginate, Sargassum latifolium extract, and their silver nanoparticles. BMC Chem. 2023;17:108. doi:10.1186/s13065-023-01022-0
109. Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8:7278–7308. doi:10.3390/ma8115377
110. Hossain M, Su M. Nanoparticle location and material-dependent dose enhancement in X-ray radiation therapy. J Phys Chem. 2012;116:23047–23052. doi:10.1021/jp306543q
111. Mcnamara A, Kam W, Scales N, et al. Dose enhancement effects to the nucleus and mitochondria from gold nanoparticles in the cytosol. Phys Med Biol. 2016;61:5993. doi:10.1088/0031-9155/61/16/5993
112. Kwatra D, Venugopal A, Anant S. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl Cancer Res. 2013;2:330–342. doi:10.3978/j.issn.2218-676X.2013.08.06
113. Hashemi S, Aghamiri M, Kahani M, Jaberi R. Investigation of gold nanoparticle effects in brachytherapy by an electron emitter ophthalmic plaque. Int J Nanomed. 2019;14:4157–4165. doi:10.2147/IJN.S205814
114. Altundal Y, Cifter G, Detappe A, et al. New potential for enhancing concomitant chemoradiotherapy with FDA approved concentrations of cisplatin via the photoelectric effect. Physica Medica. 2015;31:25–30. doi:10.1016/j.ejmp.2014.11.004
115. Gao Y, Chen K, Ma -L-L, Gao F. Cerium oxide nanoparticles in cancer. OncoTargets Ther. 2014;7:835–840. doi:10.2147/OTT.S62057
116. Vodyashkin AA, Kezimana P, Prokonov FY, Vasilenko IA, Stanishevskiy YM. Current methods for synthesis and potential applications of cobalt nanoparticles: a review. Crystals. 2022;12:272. doi:10.3390/cryst12020272
117. Bilal M, Mehmood S, Rasheed T, Iqbal HMN. Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry. 2019;5:42. doi:10.3390/magnetochemistry5030042
118. Abbasi BA, Iqbal J, Khan Z, et al. Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc Res Tech. 2021;84:192–201. doi:10.1002/jemt.23577
119. Chattopadhyay S, Dash SK, Tripathy S, et al. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem Biol Interact. 2015;226:58–71. doi:10.1016/j.cbi.2014.11.016
120. Papis E, Gornati R, Prati M, Ponti J, Sabbioni E, Bernardini G. Gene expression in nanotoxicology research: analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxico Letters. 2007;170:185–192. doi:10.1016/j.toxlet.2007.03.005
121. Colognato R, Bonelli A, Ponti J, et al. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23:377–382. doi:10.1093/mutage/gen024
122. Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi:10.1021/tx800064j
123. Hu W, Culloty S, Darmody G, et al. Toxicity of copper oxide nanoparticles in the blue mussel, Mytilus edulis: a redox proteomic investigation. Chemosphere. 2014;108:289–299. doi:10.1016/j.chemosphere.2014.01.054
124. Raj DP, Shairam M, Suganya N, et al. Green synthesis of copper oxide nanoparticles using sinapic acid: an underpinning step towards antiangiogenic therapy for breast cancer. J Biol Inorg Chem. 2019;24:633–645. doi:10.1007/s00775-019-01676-z
125. Gnanavel V, Palanichamy V, Roopan SM. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J Photochem Photobiol B Biol. 2017;171:133–138. doi:10.1016/j.jphotobiol.2017.05.001
126. Taherzadeh‑Soureshjani P, Chehelgerdi M. Algae‑meditated route to cuprous oxide (Cu2O) nanoparticle: differential expression profile of MALAT1 and GAS5 LncRNAs and cytotoxic effect in human breast cancer. Cancer Nanotechnol. 2020;11:11. doi:10.1186/s12645-020-00066-4
127. Bensy ADV, Christobel GJ, Muthusamy K, Alfarhan A, Anantharaman P. Green synthesis of iron nanoparticles from Ulva lactuca and bactericidal activity against enteropathogens. J King Saud Univ Sci. 2022;34:101888. doi:10.1016/j.jksus.2022.101888
128. Alphandéry E. Bio-synthesized iron oxide nanoparticles for cancer treatment. Intl J Pharm. 2020;586:119472. doi:10.1016/j.ijpharm.2020.119472
129. Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2:282–289. doi:10.4103/0975-7406.72127
130. Wu H, Yin -J-J, Wamer WG, Zeng M, Lo YM. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J Food Drug Anal. 2014;22:86–94. doi:10.1016/j.jfda.2014.01.007
131. Seabra AB, Haddad P, Duran N. Biogenic synthesis of nanostructured iron compounds: applications and perspectives. IET Nanobiotech. 2013;7:90–99. doi:10.1049/iet-nbt.2012.0047
132. Haris M, Fatima N, Iqbal J, et al. Oscillatoria limnetica mediated green synthesis of iron oxide (Fe2O3) nanoparticles and their diverse in vitro bioactivities. Molecules. 2023;28:2091. doi:10.3390/molecules28052091
133. Alangari A, Alqahtani MS, Mateen A, et al. Iron oxide nanoparticles: preparation, characterization, and assessment of antimicrobial and anticancer activity. Adsorp Sci Technol. 2022;2022:1–9. doi:10.1155/2022/1562051
134. Zarzzeka C, Goldoni J, Marafon F, et al. Use of titanium dioxide nanoparticles for cancer treatment: a comprehensive review and bibliometric analysis. Biocatal Agric Biotechnol. 2023;50:102710. doi:10.1016/j.bcab.2023.102710
135. Mccarthy J, Inkielewicz-StęPniak I, Corbalan JJ, Radomski MW. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: protective effects of fisetin. Chem Res Toxicol. 2012;25:2227–2235. doi:10.1021/tx3002884
136. Zheng Y, Wang Z, Peng F, Fu L. Application of biosynthesized ZnO nanoparticles on an electrochemical H2O2 biosensor. Braz J Pharm Sci. 2016;52:781–786. doi:10.1590/S1984-82502016000400023
137. Shukla AK, Iravani S. Metallic nanoparticles: green synthesis and spectroscopic characterization. Environ Chem Lett. 2017;15:223–231. doi:10.1007/s10311-017-0618-2
138. Sharma G, Jasuja ND, Kumar M, Ali MI. Biological synthesis of silver nanoparticles by cell-free extract of Spirulina platensis. J Nanotechnol. 2015;2015:132675. doi:10.1155/2015/132675
139. Sharma D, Kanchi S, Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab J Chem. 2019;12:3576–3600. doi:10.1016/j.arabjc.2015.11.002
140. Poinern GEJ. A Laboratory Course in Nanoscience and Nanotechnology. Boca Raton, Florida: CRC Press; 2014:261.
141. Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5:33–40. doi:10.2217/nnm.09.77
142. Aboelfetoh EF, El-Shenody RA, Ghobara MM. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess. 2017;189:349. doi:10.1007/s10661-017-6033-0
143. Jena J, Pradhan N, Dash BP, Sukla LB, Panda PK. Biosynthesis, and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int J Nanomater Biostruct. 2013;3:1–8.
144. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017;12:908. doi:10.1016/j.arabjc.2017.05.011
145. Dahoumane SA, Wujcik EK, Jeffryes C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzyme Microb Technol. 2016;95:13–27. doi:10.1016/j.enzmictec.2016.06.008
146. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:
147. Rajathi FAA, Parthiban C, Kumar VG, Anantharaman P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim Acta A Mol Biomol Spectrosc. 2012;99:166–173. doi:10.1016/j.saa.2012.08.081
148. Tomaszewska E, Soliwoda K, Kadziola K, et al. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater. 2013;2013:313081. doi:10.1155/2013/313081
149. Shard AG, Schofield RC, Minelli C. Ultraviolet–visible spectrophotometry. In: Hodoroaba V, Unger WES, Shard AG, editors. Micro and Nano Technologies, Characterization of Nanoparticles. Amsterdam, Netherlands: Elsevier; 2020:185–196. doi:10.1016/B978-0-12-814182-3.00012-2
150. Hall BD, Zanchet D, Ugarte D. Estimating nanoparticle size from diffraction measurements. J Appl Crystallogr. 2000;33:1335–1341. doi:10.1107/S0021889800010888
151. Deloncle R, Coppel Y, Rebout C, Majoral JP, Caminade AM. Characterization of two series of nitrogen‐containing dendrimers by natural abundance 15N NMR. Magn Reson Chem. 2008;46:493–496. doi:10.1002/mrc.2203
152. Sapsford KE, Tyner KM, Dair BJ, Deschamps JR, Medintz IL. Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem. 2011;83:4453–4488. doi:10.1021/ac200853a
153. Lin PC, Lin S, Wang PC, Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32:711–726. doi:10.1016/j.biotechadv.2013.11.006
154. Sharma G, Pandey S, Ghatak S, Watal G, Rai PK. Potential of spectroscopic techniques in the characterization of “green nanomaterials”. In: Tripathi DK, Ahmad P, Sharma S, Chauhan DK, Dubey NK, editors. Nanomaterials in Plants, Algae and Microorganisms: Concepts and Controversies. Amsterdam, The Netherland: Elsevier; 2018:59–77. doi:10.1016/B978-0-12-811487-2.00003-7
155. Lange H. Comparative test of methods to determine particle size and particle size distribution in the submicron range. Part Part Syst Charact. 1995;12:148–157. doi:10.1002/ppsc.19950120307
156. Bootz A, Vogel V, Schubert D, Kreuter J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly (butyl cyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 2004;57:369–375. doi:10.1016/S0939-6411(03)00193-0
157. Stephan TS, Scott EM, Anil KP, Marina AD. Preclinical characterization of engineered nanoparticles intended for cancer therapeutics. In: Amiji MM, editor. Nanotechnology for Cancer Therapy. Boca Raton, FL, USA: CRC Press; 2006:105–137.
158. Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008;101:239–253. doi:10.1093/toxsci/kfm240
159. Uskoković V. Dynamic light scattering based microelectrophoresis: main prospects and limitations. J Dispers Sci Technol. 2012;33:1762–1786. doi:10.1080/01932691.2011.625523
160. Shukla AK, Iravani S. Green Synthesis, Characterization and Applications of Nanoparticles. Amsterdam, The Netherland: Elsevier; 2019:523. doi:10.1016/C2017-0-02526-0
161. Zscherp C, Barth A. Reaction-induced infrared difference spectroscopy for the study of protein reaction mechanisms. Biochemistry. 2001;40:1875–1883. doi:10.1021/bi002567y
162. Faghihzadeh F, Anaya NM, Schifman LA, Oyanedel-Craver V. Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnol Environ Eng. 2016;1:1. doi:10.1007/s41204-016-0001-8
163. Pathak Y, Thassu D. Drug Delivery Nanoparticles Formulation and Characterization. New York, USA: Informa Healthcare; 2009:416.
164. Gaba F, Tipping WJ, Salji M, Faulds K, Graham D, Leung HY. Raman spectroscopy in prostate cancer: techniques, applications, and advancements. Cancers. 2022;14:1535. doi:10.3390/cancers14061535
165. Kämpfe B, Luczak F, Michel B. Energy Dispersive X-Ray Diffraction. Part Syst Charact. 2005;22:391–396. doi:10.1002/ppsc.200501007
166. Scimeca M, Bischetti S, Lamsira HK, Bonfiglio R, Bonanno E. Energy Dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur J Histochem. 2018;62:
167. Khanna P, Kaur A, Goyal DJ. Algae-based metallic nanoparticles: synthesis, characterization and applications. J Microbiol Methods. 2019;163:105656. doi:10.1016/j.mimet.2019.105656
168. Ingham B. X-ray scattering characterization of nanoparticles. Crystallogr Rev. 2015;21:229–303. doi:10.1080/0889311X.2015.1024114
169. Khan I, Yamani ZH, Qurashi A. Sonochemical-driven ultrafast facile synthesis of SnO2 nanoparticles: growth mechanism structural electrical and hydrogen gas sensing properties. Ultrason Sonochem. 2017;34:484–490. doi:10.1016/j.ultsonch.2016.06.025
170. Kaplan D. Absorption and adsorption of heavy metals by microalgae. In: Richmond A, Hu Q, editors. Handbook of Microalgal Culture: Applied Phycology and Biotechnology.
171. Bwapwa J, Jaiyeola A, Chetty R. Bioremediation of acid mine drainage using algae strains: a review. S Afr J Chem Eng. 2017;24:62–70. doi:10.1016/j.sajce.2017.06.005
172. Adityosulindro S, Wulandari D. Utilization of wild algae biomass as biosorbent for removal of heavy metal Zinc (Zn2+) from aqueous solution. Earth Environ Sci. 2021;824:012017. doi:10.1088/1755-1315/824/1/012017
173. Fawcett D, Verduin JJ, Shah M, Sharma SB, Poinern GEJ. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J Nanosci Nanotechnol. 2017;2017:8013850. doi:10.1155/2017/8013850
174. Morowvat MH, Kazemi K, Jaberi MA, Amini A, Gholami A. Biosynthesis and antimicrobial evaluation of zinc oxide nanoparticles using Chlorella vulgaris biomass against multidrug-resistant pathogens. Materials. 2023;16:842. doi:10.3390/ma16020842
175. Prasad R, Pandey R, Barman I. Engineering tailored nanoparticles with microbes: quo vadis?. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:316–330. doi:10.1002/wnan.1363
176. Vijayan SR, Santhiyagu P, Singamuthu M, Kumari AN, Jayaraman R, Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci World J. 2014;2014:10. doi:10.1155/2014/938272
177. Chakraborty N, Pal R, Ramaswami A, Nayak D, Lahiri S. Diatom: a potential bio-accumulator of gold. J Radioanal Nucl Chem. 2006;270:645–649. doi:10.1007/s10967-006-0475-0
178. Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation–a novel phenomenon. J Appl Phycol. 2009;21:145–152. doi:10.1007/s10811-008-9343-3
179. Shukla AK, Upadhyay AK, Singh L. Algae-mediated biological synthesis of nanoparticles: applications and prospects. In: Mandotra SK, Upadhyay AK, Ahluwalia AS, editors. Algae. Singapore: Springer; 2021:325–338. doi:10.1007/978-981-15-7518-1_14
180. Dahoumane SA, Yéprémian C, Djédiat C, et al. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J Nanopart Res. 2014;16:2607. doi:10.1007/s11051-014-2607-8
181. Namvar F, Azizi S, Ahmad MB, et al. Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res Chem Intermed. 2015;41:5723–5730. doi:10.1007/s11164-014-1696-4
182. Ismail MM, Alotaibi BS, El-Sheekh MM. Therapeutic uses of red macroalgae. Molecules. 2020;25:4411. doi:10.3390/molecules25194411
183. Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:87–96. doi:10.1263/jbb.101.87
184. Stablein MJ, Baracho DH, Watson JT, Silva JC, Zhang Y, Lombardi AT. Microalgal photosynthetic inhibition and mixotrophic growth in post hydrothermal liquefaction wastewater (PHW). Algal Res. 2021;60:102548. doi:10.1016/j.algal.2021.102548
185. Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B Biol. 2019;190:86–97. doi:10.1016/j.jphotobiol.2018.11.014
186. Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem. 2017;10:S3029. doi:10.1016/j.arabjc.2013.11.044
187. Castro L, Blázquez ML, González F, Muñoz JÁ, Ballester A. Exploring the possibilities of biological fabrication of gold nanostructures using Orange peel extract. Metals. 2015;5:1609–1619. doi:10.3390/met5031609
188. Sharma B, Purkayastha DD, Hazra S, et al. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst Eng. 2014;37:2559–2565. doi:10.1007/s00449-014-1233-2
189. Kumar P, Senthamilselvi S, Lakshmipraba A, et al. Efficacy of bio-synthesized silver nanoparticles using Acanthophora spicifera to encumber biofilm formation. Dig J Nanomater Biostruct. 2012;7:511–522.
190. Devi JS, Bhimba BV, Ratnam K. In vitro anticancer activity of silver nanoparticles synthesized using the extract of Gelidiella sp. Int J Pharm Sci. 2012;4:710–715.
191. Venkatesan J, Manivasagan P, Kim SK, Kirthi AV, Marimuthu S, Rahuman AA. Marine algae-mediated synthesis of gold nanoparticles using a novel Ecklonia cava. Bioprocess Biosyst Eng. 2014;37:1591–1597. doi:10.1007/s00449-014-1131-7
192. Prasad TN, Kambala VSR, Naidu R. Phyconanotechnology: synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterization. J Appl Phycol. 2013;25:177–182. doi:10.1007/s10811-012-9851-z
193. Liu B, Xie J, Lee J, Ting Y, Chen JP. Optimization of high-yield biological synthesis of single-crystalline gold nanoplates. J Phys Chem B. 2005;109:15256–15263. doi:10.1021/jp051449n
194. Madhiyazhagan P, Murugan K, Kumar AN, et al. Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitol Res. 2015;114:4305–4317. doi:10.1007/s00436-015-4671-0
195. Rajeshkumar S, Malarkodi C, Gnanajobitha G, et al. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J Nanostructure Chem. 2013;3:44. doi:10.1186/2193-8865-3-44
196. Singh M, Kalaivani R, Manikandan S, Sangeetha N, Kumaraguru AK. Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl Nanosci. 2013;3:145–151. doi:10.1007/s13204-012-0115-7
197. El-Rafie H, El-Rafie M, Zahran M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohyd Polym. 2013;96:403–410. doi:10.1016/j.carbpol.2013.03.071
198. Parial D, Gopal PK, Paul S, Pal R. Gold (III) bioreduction by cyanobacteria with special reference to in vitro biosafety assay of gold nanoparticles. J Appl Phycol. 2016;28:3395–3406. doi:10.1007/s10811-016-0880-x
199. Focsan M, Ardelean I, Craciun C, Astilean S. Interplay between gold nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803. Nanotechnology. 2011;22:485101. doi:10.1088/0957-4484/22/48/485101
200. Mosulishvili L, Kirkesali E, Belokobylsky A, et al. Experimental substantiation of the possibility of developing selenium-and iodine-containing pharmaceuticals based on blue–green algae Spirulina platensis. J Pharm Biomed. 2002;30:87–97. doi:10.1016/S0731-7085(02)00199-1
201. Ali A, Ali MA, Ali MU, Mohammad S. Hospital outcomes of obstetrical-related acute renal failure in a tertiary care teaching hospital. Ren Fail. 2011;33:285–290. doi:10.3109/0886022X.2011.560400
202. Doshi H, Ray A, Kothari I. Bioremediation potential of live and dead Spirulina: spectroscopic, kinetics and SEM studies. Biotechnol Bioeng. 2007;96:1051–1063. doi:10.1002/bit.21190
203. Wei D, Qian W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf B. 2008;62:136–142. doi:10.1016/j.colsurfb.2007.09.030
204. Sinha SN, Paul D, Halder N, Sengupta D, Patra SK. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl Nanosci. 2015;5:703–709. doi:10.1007/s13204-014-0366-6
205. Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci. 2015;5:499–504. doi:10.1007/s13204-014-0341-2
206. Xie J, Lee JY, Wang DI, Ting YP. Identification of active biomolecules in the high‐yield synthesis of single‐crystalline gold nanoplates in algal solutions. Small. 2007;3:672–682. doi:10.1002/smll.200600612
207. Barwal I, Ranjan P, Kateriya S, Yadav SC. Cellular oxido-reductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles. J Nanobiotechnol. 2011;9:56. doi:10.1186/1477-3155-9-56
208. Shakibaie M, Forootanfar H, Mollazadeh‐Moghaddam K, et al. Green synthesis of gold nanoparticles by the marine microalga Tetraselmis suecica. Biotechnol Appl Biochem. 2010;57:71–75. doi:10.1042/BA20100196
209. Nurmi JT, Tratnyek PG, Sarathy V, et al. Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environ Sci Technol. 2005;39:1221–1230. doi:10.1021/es049190u
210. Priyadharshini RI, Prasannaraj G, Geetha N, Venkatachalam P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl Biochem Biotechnol. 2014;174:2777–2790. doi:10.1007/s12010-014-1225-3
211. Dhanalakshmi PK, Azeez R, Rekha R, Poonkodi S, Nallamuthu T. Synthesis of silver nanoparticles using green and brown seaweeds. Phykos. 2012;42:39–45.
212. Parial D, Patra HK, Roychoudhury P, Dasgupta AK, Pal R. Gold nanorod production by cyanobacteria—a green chemistry approach. J Appl Phycol. 2012b;24:55–60. doi:10.1007/s10811-010-9645-0
213. Kannan RRR, Stirk W, Van Staden J. Synthesis of silver nanoparticles using the seaweed Codium capitatum PC Silva (Chlorophyceae). S Afr J Bot. 2013b;86:1–4. doi:10.1016/j.sajb.2013.01.003
214. Kannan R, Arumugam R, Ramya D, Manivannan K, Anantharaman P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl Nanosci. 2013a;3:229–233. doi:10.1007/s13204-012-0125-5
215. Marslin G, Siram K, Maqbool Q, et al. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials. 2018;11:940. doi:10.3390/ma11060940
216. Halder A, Das S, Bera T, Mukherjee A. Rapid synthesis for monodispersed gold nanoparticles in kaempferol and anti-leishmanial efficacy against wild and drug resistant strains. RSC Adv. 2017;7:14159–14167. doi:10.1039/C6RA28632A
217. Oza G, Pandey S, Mewada A, et al. Facile biosynthesis of gold nanoparticles exploiting optimum pH and temperature of freshwater algae Chlorella pyrenoidusa. Adv Appl Sci Res. 2012;3:1405–1412.
218. Parial D, Pal R. Biosynthesis of monodisperse gold nanoparticles by green alga Rhizoclonium and associated biochemical changes. J Appl Phycol. 2015;27:975–984. doi:10.1007/s10811-014-0355-x
219. Gardea-Torresdey J, Tiemann K, Dokken K, Pingitore N. Recovery of gold (III) by alfalfa biomass and binding characterization using X-ray microfluoresence. Adv Environ Res. 1999;U7–93.
220. Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B. 2009;74:328–335. doi:10.1016/j.colsurfb.2009.07.048
221. Sathishkumar M, Sneha K, Yun YS. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour Technol. 2010;101:7958–7965. doi:10.1016/j.biortech.2010.05.051
222. Singh AK, Pal P, Gupta V, Yadav TP, Gupta V, Singh SP. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater Chem Phys. 2018;203:40–48. doi:10.1016/j.matchemphys.2017.09.049
223. Darroudi M, Ahmad MB, Zamiri R, Zak AK, Abdullah AH, Ibrahim NA. Time-dependent effect in green synthesis of silver nanoparticles. Int J Nanomed. 2011;26:677. doi:10.2147/IJN.S17669
224. Kuchibhatla SV, Karakoti AS, Baer DR, et al. Influence of aging and environment on nanoparticle chemistry: implication to confinement effects in nanoceria. J Phys Chem. 2012;116:14108–14114. doi:10.1021/jp300725s
225. Mudunkotuwa IA, Pettibone JM, Grassian VH. Environmental implications of nanoparticle aging in the processing and fate of copper-based nanomaterials. Environ Sci Technol. 2012;46:7001–7010. doi:10.1021/es203851d
226. Baer DR. Surface characterization of nanoparticles: critical needs and significant challenges. J Surf Anal. 2011;17:163–169. doi:10.1384/jsa.17.163
227. Sahayaraj K, Rajesh S, Rathi JM. Silver nanoparticles biosynthesis using marine alga Padina pavonica (LINN.) and its microbicidal activity. Dig J Nanomater Bios. 2012;7:1557–1567.
228. Chia SR, Ong HC, Chew KW, et al. Sustainable approaches for algae utilization in bioenergy production. Renew Energy. 2018;129:838–852. doi:10.1016/j.renene.2017.04.00
229. Wang K, Khoo KS, Chew KW, et al. Microalgae: the future supply house of biohydrogen and biogas. Front Energy Res. 2021;9:660399. doi:10.3389/fenrg.2021.660399
230. Xie Y, Khoo KS, Chew KW, et al. Advancement of renewable energy technologies via artificial and microalgae photosynthesis. Bioresour Technol. 2022;363:
231. Kumar SS, Kumar Y, Khan MSY, Anbu J, De Clercq E. Antihistaminic and antiviral activities of steroids of Turbinaria conoides. Nat Prod Res. 2011;25:723–729. doi:10.1080/14786411003781515
232. Ghodake G, Lee DS. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J Nanoelectron Optoelectron. 2011;6:268–271. doi:10.1166/jno.2011.1166
233. Rajeshkumar S, Kannan C, Annadurai G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int J Pharma Bio Sci. 2012;3:502–510.
234. Azizi S, Ahmad MB, Namvar F, Mohamad R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett. 2014;116:275–277. doi:10.1016/j.matlet.2013.11.038
235. Maneerung T, Tokura S, Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym. 2008;72:43–51. doi:10.1016/j.carbpol.2007.07.025
236. Vidyanathan R, Llishwaralal K, Gopalram S, Gurunathan S. Nanosilver-the burgeoning therapeutic molecule and its green synthesis. Biotechnol Adv. 2009;27:924–937. doi:10.1016/j.biotechadv.2009.08.001
237. Abo-Neima SE, Ahmed AA, El-Sheekh M, Makhlof MEM. Polycladia myrica-based delivery of selenium nanoparticles in combination with radiotherapy induces potent in vitro antiviral and in vivo anticancer activities against Ehrlich ascites tumor. Front Mol Biosci. 2023;10:1120422. doi:10.3389/fmolb.2023.1120422
238. Abboud Y, Saffaj T, Chagraoui A, et al. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl Nanosci. 2014;4:571–576. doi:10.1007/s13204-013-0233-x
239. El-Sheekh MM, El-Kassas HY, Shams El-Din NG, Eissa DI, El-Sherbiny BA. Green synthesis, characterization applications of iron oxide nanoparticles for antialgal and wastewater bioremediation using three brown algae. Int J Phytoremediation. 2021;23:1538–1552. doi:10.1080/15226514.2021.1915957
240. Elrefaey AAK, El-Gamal AD, Hamed SM, El-belely EF. Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it’s antimicrobial and antioxidant activities. Egypt J Chem. 2022;65:231–240. doi:10.21608/EJCHEM.2021.87722.4231
241. Touliabah H, EL-Sheekh MM, Makhlof MEM. Evaluation of Polycladia myrica mediated selenium nanoparticles (CsSeNPs) cytotoxicity against PC-3 cells and antiviral activity against HAV HM175 (Hepatitis A), HSV-2 (Herpes simplex II) and Adenovirus strain 2. Front Mar Sci. 2022;9:1092343. doi:10.3389/fmars.2022.1092343
242. Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem. 2014;7:1131–1139. doi:10.1016/j.arabjc.2013.04.007
243. Kumar-Krishnan S, Prokhorov E, Hernández-Iturriaga M, et al. Chitosan/silver nanocomposites: synergistic antibacterial action of silver nanoparticles and silver ions. Eur Polym J. 2015;6:242–251. doi:10.1016/j.eurpolymj.2015.03.066
244. Kushnerova NF, Fomenko SE, Sprygin VG, et al. An extract from the brown alga Laminaria japonica: a promising stress-protective preparation. Russ J Mar Biol. 2010;36:209–214. doi:10.1134/S1063074010030077
245. Khodashenas B, Ghorbani HR. Synthesis of silver nanoparticles with different shapes. Arab J Chem. 2019;12:1823–1838. doi:10.1016/j.arabjc.2014.12.014
246. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7:219–242. doi:10.1007/s40820-015-0040-x
247. Azizi S, Shahri MM, Mohamad R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Molecules. 2017;22:831. doi:10.3390/molecules22060831
248. Yoon HS, Müller KM, Sheath RG, Ott FD, Bhattacharya D. Defining the major lineages of red algae (Rhodophyta). J Phycol. 2006;42:482–492. doi:10.1111/j.1529-8817.2006.00210.x
249. Rao PS, Mantri VA, Ganesan K. Mineral composition of edible seaweed Porphyra vietnamensis. Food Chem. 2007;102:215–218. doi:10.1016/j.foodchem.2006.05.009
250. Venkatpurwar V, Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in vitro antibacterial activity. Mater Lett. 2011;65:999–1002. doi:10.1016/j.matlet.2010.12.057
251. Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi:10.1016/j.micpath.2017.11.013
252. Vadlapudi V, Amanchy R. Synthesis, characterization, and antibacterial activity of silver nanoparticles from red algae, Hypnea musciformis. Adv Biol Res. 2017;11:242–249. doi:10.5829/idosi.abr.2017.242.249
253. Kumar P, Govindaraju M, Senthamilselvi S, Premkumar K. Photocatalytic degradation of methyl Orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf B. 2013b;103:658–661. doi:10.1016/j.colsurfb.2012.11.022
254. Barciela P, Carpena M, Li N-Y, et al. Macroalgae as biofactories of metal nanoparticles; biosynthesis and food applications. Adv Colloid Interface Sci. 2023;311:102829. doi:10.1016/j.cis.2022.102829
255. Singh CR, Kathiresan K, Anandhan S. A review on marine based nanoparticles and their potential applications. Afr J Biotechnol. 2015;14:1525–1532. doi:10.5897/AJB2015.14527
256. Murugesan S, Bhuvaneswari S, Sivamurugan V. Green synthesis, characterization of silver nanoparticles of a marine red alga Spyridia fusiformis and their antibacterial activity. Int J Pharm Pharm Sci. 2017;9:192–197. doi:10.22159/ijpps.2017v9i5.17105
257. Al-Naamani L, Dobretsov S, Dutta J, Burgess JG. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere. 2017;168:408–417. doi:10.1016/j.chemosphere.2016.10.033
258. Mosulishvili LM, Belokobylsky AI, Kirkesali EI, Frontasyeva MV, Pavlov SS, Aksenova NG. Neutron activation analysis for studying Cr uptake in the blue-green microalga Spirulina platensis. J Neutron Res. 2007;5:49–54. doi:10.1080/10238160601025138
259. Khan AU, Khan M, Malik N, Cho MH, Khan MM. Recent progress of algae and blue–green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess Biosyst Eng. 2019;42:1–15. doi:10.1007/s00449-018-2012-2
260. Suganya KU, Govindaraju K, Kumar VG, et al. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater Sci Eng C. 2015;47:351–356. doi:10.1016/j.msec.2014.11.043
261. Parial D, Patra HK, Dasgupta AK, Pal R. Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol. 2012a;47:22–29. doi:10.1080/09670262.2011.653406
262. Rösken LM, Cappel F, Körsten S, et al. Time-dependent growth of crystalline Au(0)-nanoparticles in cyanobacteria as self-reproducing bioreactors: 2. Anabaena cylindrica. Beilstein J Nanotechnol. 2016;7:312–327. doi:10.3762/bjnano.7.30
263. Hanna AL, Hamouda HM, Goda HA, et al. Biosynthesis and characterization of silver nanoparticles produced by Phormidium ambiguum and Desertifilum tharense cyanobacteria. Bioinorg Chem Appl. 2022;2022:9072508. doi:10.1155/2022/9072508
264. Sudha SS, Rajamanickam K, Rengaramanujam J. Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J Exp Biol. 2013;51:393–399.
265. Husain S, Afreen S, Yasin D, Afzal B, Fatma T. Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J Microbiol Methods. 2019;162:77–82. doi:10.1016/j.mimet.2019.05.011
266. Husain S, Sardar M, Fatma T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J Microbiol Biotechnol. 2015;31:1279–1283. doi:10.1007/s11274-015-1869-3
267. Mahdieh M, Zolanvari A, Azimee AJS. Green biosynthesis of silver nanoparticles by Spirulina platensis. Scientia Iranica. 2012;19:926–929. doi:10.1016/j.scient.2012.01.010
268. Banerjee S, Bhattacharya A, Roychoudhury P, Dasgupta AK, Dutta M, Pal R. Arthrospira platensis (Cyanobacteria) – a potential biofactory for fluoromagnetic nanoiron production. Phycologia. 2021;60:62–72. doi:10.1080/00318884.2020.1851010
269. MubarakAli D, Gopinath V, Rameshbabu N, Thajuddin N. Synthesis and characterization of CdS nanoparticles using C-phycoerythrin from the marine cyanobacteria. Mater Lett. 2012;74:8–11. doi:10.1016/j.matlet.2012.01.026
270. Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir. 2007;23:2694–2699. doi:10.1021/la0613124
271. Bishoyi AK, Sahoo CR, Sahoo AP, Padhy RN. Bio-synthesis of silver nanoparticles with the brackish water blue-green alga Oscillatoria princeps and antibacterial assessment. Appl Nanosci. 2021;11:389–398. doi:10.1007/s13204-020-01593-7
272. Che X, Ding R, Zhang Q, et al. The severe toxicity of CuO nanoparticles to the photosynthesis of the prokaryotic algae Arthrospira sp. Environ Sci Pollut Res. 2021;28:54105–54116. doi:10.1007/s11356-021-14341-3
273. Thota S, Crans DC. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences. John Wiley & Sons New Jersey. USA; 2018:261.
274. Sosa IO, Noguez C, Barrera RG. Optical properties of metal nanoparticles with arbitrary shapes. J Phys Chem B. 2003;107:6269–6275. doi:10.1021/jp0274076
275. Yousefzadi M, Rahimi Z, Ghafori V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen). J Agardh Mate Lett. 2014;137:1–4. doi:10.1016/j.matlet.2014.08.110
276. Jena J, Pradhan N, Nayak RR, et al. Microalga Scenedesmus sp. A potential low-cost green machine for silver nanoparticle synthesis. J Microbiol Biotechnol. 2014;24:522–533. doi:10.4014/jmb.1306.06014
277. Beganskienė A, Sirutkaitis V, Kurtinaitienė M, Juškėnas R, Kareiva A. FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater Sci. 2004;10:287–290. doi:10.5755/j01.ms.10.4.26643
278. Arya AK, Gupta K, Chundawat TS, Vaya D. Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg Chem Appl. 2018;7879403. doi:10.1155/2018/7879403
279. Mishra V, Arya A, Chundawat TS. High catalytic activity of Pd nanoparticles synthesized from green alga Chlorella vulgaris in Buchwald-Hartwig synthesis of N-aryl piperazines. Curr Organo. 2020;7:23–33. doi:10.2174/2213337206666190515091945
280. Rao MD, Pennathur G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater Res Bull. 2017;85:64–73. doi:10.1016/j.materresbull.2016.08.049
281. Roychoudhury P, Pal R. Spirogyra submaxima—a green alga for nanogold production. J Algal Biomass Utln. 2014;5:15–19.
282. Xu L, Zhao Z, Yan Z, et al. Defense pathways of Chlamydomonas reinhardtii under silver nanoparticle stress: extracellular biosorption, internalization and antioxidant genes. Chemosphere. 2022;291:132764. doi:10.1016/j.chemosphere.2021.132764
283. Mahana A, Mehta SK. Potential of Scenedesmus-fabricated ZnO nanorods in photocatalytic reduction of methylene blue under direct sunlight: kinetics and mechanism. Environ Sci Pollut Res. 2021;2822:28234–28250. doi:10.1007/s11356-021-12682-7
284. Lu H, Wang J, Wang T, Zhong J, Bao Y, Hao H. Recent progress on nanostructures for drug delivery applications. Nanomater. 2016;2016:5762431. doi:10.1155/2016/5762431
285. Zhang S, Qamar SA, Junaid M, Munir B, Badar Q, Bilal M. Algal polysaccharides‐based nanoparticles for targeted drug delivery applications. Starch‐Stärke. 2022;74:2200014. doi:10.1002/star.202200014
286. Choi YH, Han HK. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Invest. 2018;48:43–60. doi:10.1007/s40005-017-0370-4
287. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71. doi:10.1186/s12951-018-0392-8
288. Yan N, Fan C, Chen Y, Hu Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci. 2016;17:
289. Khavari F, Saidijam M, Taheri M, Nouri F. Microalgae: therapeutic potentials and applications. Mol Biol Rep. 2021;48:4757–4765. doi:10.1007/s11033-021-06422-w
290. Michael A, Singh A, Roy A, Islam MR. Fungal-and algal-derived synthesis of various nanoparticles and their applications. Bioinorg Chem Appl. 2022;2022:3142674. doi:10.1155/2022/3142674
291. Kalimuthu K, Lubin BC, Bazylevich A, et al. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J Nanobiotechnology. 2018;16:34. doi:10.1186/s12951-018-0362-1
292. El-Sheekh MM, Nassef M, Bases E, El Shafay S, El-shenody R. Antitumor immunity and therapeutic properties of marine seaweeds-derived extracts in the treatment of cancer. Cancer Cell Int. 2022;22:267. doi:10.1186/s12935-022-02683-y
293. Paciotti GF, Kingston DGI, Tamarkin L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev Res. 2006;67:47–54. doi:10.1002/ddr.20066
294. Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi:10.1080/10717540490433895
295. Gupta AK, Gupta M. Synthesis, and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi:10.1016/j.biomaterials.2004.10.012
296. Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deli Rev. 2011;63:24–46. doi:10.1016/j.addr.2010.05.006
297. Uzair B, Liaqat A, Iqbal H, et al. Green and cost-effective synthesis of metallic nanoparticles by algae: safe methods for translational medicine. Bioengineering. 2020;7:
298. Lo JH, von Maltzahn G, Douglass J, et al. Nanoparticle amplification via photothermal unveiling of cryptic collagen binding sites. J Mater Chem. 2013;20:1630–1635. doi:10.1039/C3TB20619J
299. Manivasagan P, Kim SK. Biosynthesis of nanoparticles using marine algae: a review. In: Marine Algae Extracts: Processes, Products, and Applications; 2015:295–304. doi:10.1002/9783527679577.ch17
300. Bhattacharya P, Swarnakar S, Ghosh S, Majumdar S, Banerjee S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J Environ Chem Eng. 2019;7:102867. doi:10.1016/j.jece.2018.102867
301. Cortese B, D’Amone S, Testini M, Ratano P, Palamà IE. Hybrid clustered nanoparticles for chemo-antibacterial combinatorial cancer therapy. Cancers. 2019;11:
302. Al-Dulimi AG, Al-Saffar AZ, Sulaiman GM, et al. Immobilization of L-asparaginase on gold nanoparticles for novel drug delivery approach as anti-cancer agent against human breast carcinoma cells. J Mater Res Technol. 2020;9:15394–15411. doi:10.1016/j.jmrt.2020.10.021
303. Ramaswamy SVP, Narendhran S, Sivaraj R. Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: antimicrobial and anticancer activities. Bull Mater Sci. 2016;39:361–364. doi:10.1007/s12034-016-1173-3
304. González-Ballesteros N, Prado-López S, Rodríguez-González J, Lastra M, Rodríguez-Argüelles M. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surf B. 2017;153:190–198. doi:10.1016/j.colsurfb.2017.02.020
305. Sonbol H, Ameen F, Alyahya S, Almansob A, Alwakeel S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci Rep. 2021;11:5444. doi:10.1038/s41598-021-84794-6
306. Babu B, Palanisamy S, Vinosha M, et al. Bioengineered gold nanoparticles from marine seaweed Acanthophora spicifera for pharmaceutical uses: antioxidant, antibacterial, and anticancer activities. Bioprocess Biosyst Eng. 2020;43:2231–2242. doi:10.1007/s00449-020-02408-3
307. Sathiyaraj G, Vinosha M, Sangeetha D, et al. Bio-directed synthesis of Pt-nanoparticles from aqueous extract of red algae Halymenia dilatata and their biomedical applications. Colloids Surf A Physicochem Eng Asp. 2021;618:126434. doi:10.1016/j.colsurfa.2021.126434
308. Gopu M, Kumar P, Selvankumar T, et al. Green biomimetic silver nanoparticles utilizing the red algae Amphiroa rigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioprocess Biosyst Eng. 2021;44:217–223. doi:10.1007/s00449-020-02426-1
309. Sathishkumar R, Sundaramanickam A, Srinath R, et al. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J Saudi Chem Soc. 2019;23:1180–1191. doi:10.1016/j.jscs.2019.07.008
310. Elgamouz A, Idriss H, Nassab C, et al. Green synthesis, characterization, antimicrobial, anti-cancer, and optimization of colorimetric sensing of hydrogen peroxide of algae extract capped silver nanoparticles. Nanomaterials. 2020;10:1861. doi:10.3390/nano10091861
311. Singh AK, Tiwari R, Singh V, Singh P, Khadim S, Singh U. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J Drug Deliv Sci Technol. 2019;51:164–176. doi:10.1016/j.jddst.2019.02.023
312. Amina SJ, Iqbal M, Faisal A, et al. Synthesis of diosgenin conjugated gold nanoparticles using algal extract of Dictyosphaerium sp. and in vitro application of their antiproliferative activities. Mater Today Commun. 2021;27:102360. doi:10.1016/j.mtcomm.2021.102360
313. Ebrahiminezhad A, Bagheri M, Taghizadeh S-M, Berenjian A, Ghasemi Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv Nat Sci. 2016;7:015018. doi:10.1088/2043-6262/7/1/015018
314. Boca SC, Potara M, Gabudean A-M, Juhem A, Baldeck PL, Astilean S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011;311:131–140. doi:10.1016/j.canlet.2011.06.022
315. Khatik N. Green synthesis of nanomaterials and their utilization as potential vehicles for targeted cancer drug delivery. Nanomedicine. 2022;19:21. doi:10.13189/app.2022.100205
316. Sun B, Hu N, Han L, Pi Y, Gao Y, Chen K. Anticancer activity of green synthesized gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif Cells Nanomed Biotechnol. 2019;47:4012–4019. doi:10.1080/21691401.2019.1575844
317. Geetha R, Ashokkumar T, Tamilselvan S, Govindaraju K, Sadiq M, Singaravelu G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. 2013;4:91–98. doi:10.1007/s12645-013-0040-9
318. Baskar G, Garrick B, Lalitha K, Chamundeeswari M. Gold nanoparticle mediated delivery of fungal asparaginase against cancer cells. J Drug Deliv Sci Technol. 2018;44:498–504. doi:10.1016/j.jddst.2018.02.007
319. Naveena BE, Prakash S. Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J Pharm Clin Res. 2013;6:179–182.
320. Ogi T, Saitoh N, Nomura T, Konishi Y. Room-temperature synthesis of gold nanoparticles and nanoplates using Shewanella algae cell extract. J Nanopart Res. 2010;12:2531–2539. doi:10.1007/s11051-009-9822-8
321. Santhosh PB, Genova J, Chamati H. Green synthesis of gold nanoparticles: an eco-friendly approach. Chemistry. 2022;4:345–369. doi:10.3390/chemistry4020026
322. Rezaeian A, Amini SM, Najafabadi MRH, Farsangi ZJ, Samadian H. Plasmonic hyperthermia or radiofrequency electric field hyperthermia of cancerous cells through green-synthesized curcumin-coated gold nanoparticles. Lasers Med Sci. 2022;37:1333–1341. doi:10.1007/s10103-021-03399-7
323. Fatima H, Charinpanitkul T, Kim KS. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials. 2021;11:
324. Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep. 2022;12:2421. doi:10.1038/s41598-022-06412-3
325. Viswanathan S, Palaniyandi T, Shanmugam R, et al. Synthesis, characterization, cytotoxicity, and antimicrobial studies of green synthesized silver nanoparticles using red seaweed Champia parvula. Biomass Conv Bioref. 2023. doi:10.1007/s13399-023-03775-z
326. Patil MP, Kim GD. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol. 2017;101:79–92. doi:10.1007/s00253-016-8012-8
327. Veeramani S, Ravindran E, Ramadoss P, Joseph C, Shanmugam K, Renganathan S. Silver nanoparticles green synthesis with Aq. extract of stems Ipomoea pes-caprae, characterization, antimicrobial and anti-cancer potential. Int J Med Nano Res. 2018;5:024. doi:10.23937/2378-3664.1410024
328. Ratan ZA, Haidere MF, Nurunnabi M, et al. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers. 2020;12:855. doi:10.3390/cancers12040855
329. Jain N, Jain P, Rajput D, Patil UK. Green synthesized plant-based silver nanoparticles: therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst Lett. 2021;9:5. doi:10.1186/s40486-021-00131-6
330. Orhan H, Aktaş Uygun D. Immobilization of L-asparaginase on magnetic nanoparticles for cancer treatment. Appl Biochem Biotechnol. 2020;191:1432–1443. doi:10.1007/s12010-020-03276-z
331. Kamaruzaman NH, Noor NNM, Mohamed R, et al. Applicability of bio-synthesized nanoparticles in fungal secondary metabolites products and plant extracts for eliminating antibiotic-resistant bacteria risks in non-clinical environments. Environ Res. 2022;209:112831. doi:10.1016/j.envres.2022.112831
332. Ismail AM, Menazea AA, Kabary HA, El-Sherbiny AE, Samy A. The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol–Gel method. J Mol Struct. 2019;1196:332–337. doi:10.1016/j.molstruc.2019.06.084
333. Govindasamy R, Gayathiri E, Sankar S, et al. Emerging trends of nanotechnology and genetic engineering in cyanobacteria to optimize production for future applications. Life. 2022;12:2013. doi:10.3390/life12122013
334. El-Sheekh MM, El-Kasas H. Algal production of nano-silver and gold: their antimicrobial and cytotoxic activities: a review. J Genet Eng Biotechnol. 2016;14:299–310. doi:10.1016/j.jgeb.2016.09.008
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.