Back to Journals » Infection and Drug Resistance » Volume 17
Hepatitis E Virus (HEV) Infection Among Immunocompromised Individuals: A Brief Narrative Review
Authors Alexandrova R, Tsachev I, Kirov P, Abudalleh A , Hristov H, Zhivkova T , Dyakova L , Baymakova M
Received 10 November 2023
Accepted for publication 21 February 2024
Published 14 March 2024 Volume 2024:17 Pages 1021—1040
DOI https://doi.org/10.2147/IDR.S449221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Radostina Alexandrova,1 Ilia Tsachev,2 Plamen Kirov,1 Abedulkadir Abudalleh,1 Hristo Hristov,1 Tanya Zhivkova,1 Lora Dyakova,3 Magdalena Baymakova4
1Department of Pathology, Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Sofia, Bulgaria; 2Department of Microbiology, Infectious and Parasitic Diseases, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria; 3Department of Synaptic Signaling and Communication, Institute of Neurobiology, Bulgarian Academy of Sciences, Sofia, Bulgaria; 4Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria
Correspondence: Magdalena Baymakova, Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria, Tel +359-882-28-50-87, Email [email protected]
Abstract: Hepatitis E virus (HEV) is a single-stranded positive-sense RNA virus that belongs to Hepeviridae family. HEV is the most common cause of acute viral hepatitis worldwide. According to the World Health Organization (WHO), there are estimated 20 million HEV infections worldwide every year, leading to estimated 3.3 million symptomatic cases of HEV infection. The WHO estimates that HEV infection caused approximately 44,000 deaths in 2015, which represents 3.3% of mortality rates due to viral hepatitis. In low-income (LI) countries and lower-middle-income (LMI) countries, HEV is a waterborne infection induced by HEV genotype (gt) 1 and HEV gt 2 that cause large outbreaks and affect young individuals with a high mortality rate in pregnant women from South Asian countries and patients with liver diseases. HEV gt 3, HEV gt 4, and HEV gt 7 are responsible for sporadic infections with zoonotic transmission mainly through the consumption of raw or undercooked meat from different animals. Acute HEV infection is relatively asymptomatic or mild clinical form, in rare cases the disease can be moderate/severe clinical forms and result in fulminant hepatitis or acute liver failure (ALF). Furthermore, HEV infection is associated with extrahepatic manifestations, including renal and neurological clinical signs and symptoms. Pregnant women, infants, older people, immunocompromised individuals, patients with comorbidities, and workers who come into close contact with HEV-infected animals are recognized as major risk groups for severe clinical form of HEV infection and fatal outcome. Chronic HEV infection can occur in immunocompromised individuals with the possibility of progression to cirrhosis.
Keywords: acute and chronic infection, cancer, cirrhosis, hepatitis E virus, HEV, HIV, solid organ transplants
Introduction
The hepatitis E virus (HEV) is the fifth known viral hepatitis (after A, B, C, and D) and the most common cause of acute viral hepatitis worldwide.1–5 It attracts the attention of the health community during an epidemic of the so-called non-A, non-B hepatitis in Kashmir, India, in 1978.1–5 HEV was discovered by immune electron microscopy in 1983 by Balayan et al, who were investigating an outbreak of unexplained hepatitis among Soviet (Russian) soldiers situated in Afghanistan.6 The genome of the virus was sequenced in 1991.1,4 In the same year, a diagnostic test for its detection was developed.5
Nowadays about twenty million HEV infections occur annually worldwide, resulting in approximately 3.3 million symptomatic cases with more than 70 thousand associated deaths and 3000 stillbirths mainly in Asia and Africa.1,7 One-third of the world’s population has been exposed to HEV.1,7
The incubation period of HEV is 2 to 6 weeks.8 The main sites of HEV replication are the liver as well as extrahepatic organs, including the small intestine, colon, spleen, stomach, kidney, and placenta.9,10 In general, acute HEV infection is a relatively asymptomatic or mild clinical form presenting with elevated liver enzymes (alanine aminotransferase, ALT, and aspartate aminotransferase, AST), fatigue, dark urine, abdominal pain, jaundice, malaise, nausea/vomiting, muscle pain, and pale stools.4,11 Acute icteric hepatitis is noted in around 5–30% of patients infected by HEV.9 These clinical signs usually last 1–6 weeks and are frequently similar to those observed in other liver diseases.4 In low-income (LI) countries and lower middle-income (LMI) countries, the virus affects especially badly pregnant women (especially those in the second or third trimester) and neonates; case fatality rates (CFR) of 20–30%.9,12 It is not clear why pregnant women are at greater risk as no suitable animal model exists, but it is thought to be related to changes in hormone levels during pregnancy and their effect on the immune system.9
Although HEV infection is usually a self-limited illness, a chronic presentation could be observed in immunocompromised persons such as solid organ and stem cell transplant recipients, people living with HIV, patients with cancer and rheumatic diseases.1,5
Additionally, HEV infection is associated with extrahepatic manifestations, including mainly renal and neurological symptoms and complications.10,13 Pregnant women, infants, adults/elderly, male sex, immunocompromised individuals, patients with comorbidities (such as hypertension, diabetes mellitus, cardiovascular diseases, malignant diseases, chronic liver diseases, immunodeficiency disorders, etc.) as well as workers who have close contact with HEV-infected animals have been recognized as major risk groups for severe clinical form, chronic infection, or fatal outcome.4,5,14 Serological and nucleic acid tests for HEV detection have been developed for both epidemiologic and diagnostic purposes.4
The present review summarizes the current knowledge of HEV infection and its presentation among solid organ transplant recipients, people living with HIV, cancer patients, persons with inflammatory bowel diseases, rheumatic disease patients, and individuals with chronic liver disease. All steps of manuscript preparation – from the collection of scientific information in the accessible databases before the critical analysis to the final design of the manuscript – were carried out according to the SANRA Guidelines (Scale for the Assessment of Narrative Review Articles).15
Etiology of HEV
HEV is a small virus with a spherical shape, icosahedral symmetry, and a single-stranded, positive-sense RNA genome. Its diameter is 27–30 nm as determined by immunoelectron microscopy, 32–34 nm after sucrose density gradient centrifugation, and 38.5–42 nm when analyzed by freezing electron microscopy.2,16
Today, HEV is a part of the Hepeviridae family.2,3 The current classification divided the Hepeviridae family into two subfamilies: Orthohepevirinae (contains all mammalian and avian HEV isolates) and Parahepevirinae (includes fish HEVs) based on the analysis of the existing sequence information.2,17 More information about them is summarized in Table 1.
 |
Table 1 Classification of Hepeviruses |
The Orthohepevirinae subfamily contains four genera: Avihepevirus, Chirohepevirus, Paslahepevirus, and Rocahepevirus. Members of Avihepevirus genus infect chickens and other avian species.2,4 As the virus spreads through the fecal-oral route, a vertical transmission is also assumed. Among leghorn hens, broiler breeders, and dual-purpose hens, the HEV infection was related to increased mortality, reduced egg production, abdominal bleeding, splenomegaly, enlarged not fatty livers, and pale combs and wattles.2,18 Coinfections with avian HEV and other pathogens (eg, avian leukosis virus subgroup J and Marek’s disease virus) have been reported.2,4 Avian HEVs share an antigenic and genetic resemblance to human and swine HEV strains.2,19 The possibility of an avian HEV transmission to humans should be taken into consideration.10
Members of Chirohepevirus genus are isolated from bats.2 So far, there is no evidence of human infection with these viruses.2,7 There is great attention on these members of the Hepeviridae family because of the likely association of bats (order Chiroptera) with the emergence of new human pathogenic viruses, including Ebola disease virus, Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1), and SARS- CoV-2.2,14
Paslahepevirus genus includes eight genotypes. They infect humans (HEV gt 1 – HEV gt 4, HEV gt 7), pigs (HEV gt 3 and HEV gt 4), wild boar (HEV gt 3 – HEV gt 6), mongoose (HEV gt 3), deer (HEV gt 3), yak (HEV gt 4), camels (HEV gt 7 and HEV gt 8), and other animal species.2,4,14 HEV gt 3 and HEV gt 4 are restricted to higher primates and adapted specifically to humans.
Members of Rocahepevirus genus have been isolated from rodents, insectivore and carnivore species. The ability of C1 genotype viruses to infect humans has been reported.2
Parahepevirinae subfamily includes only one genus – Piscihepevirus, the only representative of which is Piscihepevirus A (also known by its historic name of Cutthroat Trout Virus – CTV).2 In 1988, in California CTV was first found in obviously healthy cutthroat trout (Oncorhynchus clarkii) and since then has been established in several different species of trout and Atlantic salmon (Salmo salar) in New Brunswick, Canada, and North America.2,7,12 Two genotypes (CTV-1 and CTV-2) Canadian CTV isolates have been described.2,7,12 Infected Atlantic salmon (Salmo salar) and chinook salmon (Oncorhynchus tshawytscha) served as hosts and potential reservoirs of CTV-2.2,7,12 Both genotypes CTV-1 and CTV-2 have not been linked with any disease in the fish and do not seem to affect people.2,7,12
Epidemiology and Animal Reservoirs
Two distinct epidemiological patterns of HEV infection have been described. HEV gt 1 and HEV gt 2 are prevalent in LI/LMI countries and can result in huge outbreaks originating from contaminated water.1,4 Acute hepatitis in pregnant women and infants exists and could be fatal.9 In contrast, HEV gt 3 and HEV gt 4 are zoonotic, and the transmission of these genotypes to humans occurs mainly through the consumption of raw or undercooked meat from infected animals.1,14
Infections by HEV gt 3 and HEV gt 4 have become the most common cause of acute viral hepatitis in several upper middle-income (UMI) countries and high-income (HI) countries. The situation in China is a good example. In that country HEV gt 1 was previously the most common genotype but was subsequently overtaken by HEV gt 4 as a result of improved sanitary and hygienic conditions.4,10
Several routes for transmission of HEV infection are known (Figure 1). One of them is through contamination with human feces water (refers to HEV gt 1 and HEV gt 2) and occurs in poor sanitary conditions (mainly in LI/LMI countries).1 The presence of HEV in water supply systems with safe sanitary practices has also been demonstrated even in UMI/HI countries.13
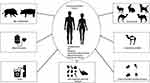 |
Figure 1 The Main Known Routes of HЕV Transmission. |
HEV gt 3 and HEV gt 4 are spread zoonotically (mainly through infected meat, but also through contact), and the sources are infected animals, mainly domestic pigs and wild boars, sika deer in Japan, but also rabbits, sheep, cattle, dogs, camels.2,5,17,20 HEV gt 3 and HEV gt 4 spread mainly through the consumption of undercooked animal meat (including pork, game meat, pig liver, pork sausages, pates, salami, dry sausages, and others).1,4,14 Inactivation of the virus in meat can be achieved at a temperature of 70.0°C for 30 min and by heating to 100.0°C in milk.20 Domestic pigs are considered for the main reservoir of zoonotic HEV gt 3 and HEV gt 4 in UMI/HI countries. HEV is endemic in domestic swine farms worldwide and can infect pigs of all ages. Consequently, pig liver and pig liver containing products have been identified as the source of many of the foodborne HEV outbreaks in Europe.5,21 Fruits and vegetables that are washed with contaminated water can be a putative source of HEV.2,4 Bivalve mollusks (shellfish) such as mussels and oysters who are filter feeders can concentrate HEV particles and are also recognized as a potential source of transmission.7,14
HEV infection can also occur through direct contact with infected animals.9 High seroprevalence rates were observed among swine workers, veterinarians, farmers, people working at a slaughterhouse and hunters.10 Considering these data and the routes of transmission, it could be suggested that individuals who are exposed to contact with infected animal reservoirs are at an increased risk of obtaining the virus than the general population.13 New HEV strains have continuously been discovered in a wide range of hosts. So the number of animal reservoirs is expanding.
HEV can be transmitted by blood transfusion.19 Blood or blood products are frequently required for different clinical conditions. HEV infection that occurs by blood transfusion could be more severe or lead to chronic hepatitis and cirrhosis.4,10 Individuals at risk are pregnant women, people with liver diseases, transplanted organs and tissues, HIV infections, and leukemias.9 Screening for HEV RNA among blood donors is currently recognized as the only effective method of preventing transfusion-transmitted HEV infection.
The ability of HEV to be transmitted vertically to the fetus and infant from an infected mother deserves special attention. Cases of maternal death, abortions, birth of premature babies and cases of neonatal death due to liver failure have been described.9,10 Fortunately, in some cases, the pregnancy ended with a normal birth.12 A relationship has been found between fetal and maternal disease severity and outcome.9 Although all viral liver pathogens can harm the mother and the child, the greatest risk for the woman and subsequently for the fetus is observed in case of acute infection with hepatitis A virus (HAV) or HEV during the period of pregnancy.9 While HAV infection is self-limiting during pregnancy, HEV has a higher prevalence and is related to significant morbidity and very high maternal mortality (approximately 20%).9,10 Therefore, HEV infection in pregnancy in endemic areas requires special attention.
HEV in Solid Organ Transplant (SOT) Recipients
In clinical practice, differences in HEV infection between groups of healthy donors and immunocompromised individuals are noted.22,23 One explanation could be the longer durations of HEV viremia and the less effective HEV clearance in immunocompromised patients.5,24
Chronic HEV infection in SOT recipients was defined as HEV replication (viremia) – HEV ribonucleic acid detectable in plasma, for more than 3 months after the onset of infection.5,25 Furthermore, spontaneous clearance of HEV infection in transplant recipients could occur 3 months after the first detection of HEV viremia.5,26 However, it was reported that some healthy individuals were HEV positive for more than 6 months.5,27
In 2008, Kamar et al reported chronic HEV infection in SOT. They presented patients after a kidney or liver transplantation with acute HEV infection, persistent elevation of liver enzymes, liver fibrosis, and histological activity on follow-up.28 A chronic HEV gt 7 post-transplantation infection has been recorded in patients who had eaten camel-derived food products.29
About 20–50% up to 66% of SOT recipients developed chronic HEV infection. The infection could be asymptomatic, but sometimes fatigue, diarrhea, arthralgia, and liver enzyme abnormality could be found.5
It is known that HEV gt 3 and gt 4 have been associated with chronic infection, whereas HEV gt 1 and gt 2 have not been documented to cause chronification.30
HEV prevalence in non-endemic areas was 1–2% after liver transplantation.10,31 Histopathological findings in chronic infection were fibrotic damage, nodules, fibrous septa, and cirrhosis.5 Almost 10% of people with chronic presentation could develop cirrhosis within 2–5 years.32
Liver inflammation after HEV clearance has been observed.32 Chronic HEV may induce post-transplant hepatitis, cirrhosis, liver failure, and even retransplantation, which may cause relapse of HEV infection in the newly transplanted liver.10
Lung transplant recipients are less frequently infected with HEV compared to liver transplant recipients.32 One explanation could be the small number of lung transplantation per year.31 Other reasons could be ribavirin therapy for respiratory syncytial virus (RSV) or hepatitis C virus (HCV) infections, which lung recipients received.
A meta-analysis for HEV seroprevalence in 14,626 SOT people found a range of 6.0% to 29.6% depending on different diagnostic assays.30 In this study, HEV-RNA was positive in 1.2%.30 Another report found 0.66% HEV-RNA positivity in 2419 SOT recipients.31 A study published in 2021 presented a high HEV prevalence in SOT recipients (20.2%), whereas the highest was in a group of liver recipients (27.2%) and the lowest in lung recipients (5.6%).32 A significantly higher HEV prevalence was estimated in LI/LMI countries compared to UMI/HI countries (41.8% vs 18.9%).32
Unlike most immunocompetent individuals who do not require specific treatment for acute HEV infection, chronic infection in immunocompromised hosts (ie, organ transplant recipients) should be treated to avoid a rapid progression to cirrhosis or even death.33
The first-line therapeutic option is to reduce immunosuppressive therapy. In one-third of patients, this pattern leads to viral clearance. However, this stage increased the risk of organ rejection and graft-vs-host reaction; consequently, these patients should be followed up closely.33 It is documented that drugs such as cyclosporine and tacrolimus may increase HEV replication in vitro, whereas mycophenolate mofetil demonstrated an antiviral effect.34 This information is useful for physicians in selecting immunosuppressive therapies for transplant patients who are infected with HEV.
Ribavirin has been used off-label as a second step in the treatment of chronic HEV infection. Ribavirin is a guanosine analog that expresses an antiviral action by inducing lethal mutations and inhibiting viral RNA-dependent RNA polymerase (RdRp).35
The achieved sustained virological response (SVR) rate was 91%, 76%, 67%, and 63% in liver, kidney, lung, and heart transplant recipients, respectively.33 Six percent of patients were non‐responders, and relapse has been noted in 18%.33
A large European retrospective multicenter study estimated that ribavirin was highly efficient for treating chronic HEV infection in SOT recipients.36 There are probably at least two reasons that could explain the satisfactory SVR: (i) better awareness among physicians, resulting in earlier diagnosis and initiation of treatment for chronic HEV infection; (ii) published guidelines and improved knowledge for treatment strategies (optimal timing, dosage, and duration of therapy).5,33 Some aspects of the ribavirin administration schedule (eg dose) in SOT recipients needed further optimization.33,35 Clinical guidelines recommend at least 3 months of therapy in case of chronic infection.5 However, the optimal duration and dose of ribavirin for treating HEV infection are still undetermined.35 According to the EASL guideline, HEV RNA should be assessed in the serum and the stool before treatment discontinuation.5 The monitoring of HEV RNA in stools could be useful to determine the optimal regimen of ribavirin therapy.33 Unfortunately, treatment failure may occur and the only therapeutic strategy in such cases is interferon.5
Some single nucleotide variants (SNVs) as well as in-frame insertions in the hypervariable region of ORF1 and other mutations in viral polymerase (Y1320H, G1634R, and K1383N) have been connected with ribavirin failure.37 Ribavirin treatment failure-associated mutation Y1320H in the RNA-dependent RNA polymerase of HEV gt 3 has been found to enhance virus replication in a rabbit HEV infection model.38 The ability of ribavirin to induce HEV mutagenesis in treated patients has been reported.37 The analysis of plasma HEV kinetic patterns in 41 SOT patients during ribavirin therapy demonstrated no association with response to therapy, with the exception of a flat-partial response.39
A second treatment attempt with ribavirin can be performed for 6 months in cases of ribavirin failure, and the data showed sustained virological response in 76% of these patients.33 As another therapeutic option, pegylated-interferon alpha (PEG-IFNα) could be applied to liver transplant recipients, but it is contraindicated after other SOTs, because of the increased risk of allograft rejection.5,33
Sofosbuvir is a nucleotide analog approved by the US Food and Drug Administration (FDA) for the treatment of chronic HCV infection, which has been reported to inhibit HEV-3 replication in vitro and possess an additive effect when combined with ribavirin.40,41 However, in clinical studies, only modest antiviral activity was observed and SVR was not achieved.33,42
HEV in HIV-Positive Individuals
According to UN AIDS data up to 2022, 39 million people are living with HIV (PLWH). The distribution is the following: 37.5 million adults and 1.5 million children <15 years old; 53% of them were women and girls, and 25 million live in sub-Saharan Africa.43,44 The number of newly diagnosed HIV infections in 2022 is 1.3 million.44 Despite the increased coverage of antiretroviral therapy, about 15% of HIV individuals are not included.45
HIV-infected patients possess many specific immunological, epidemiological, and clinical characteristics, which could influence the pathogenesis of HEV and the outcome of the infection.46 Some of them will be discussed in the following lines.
HIV-positive patients are highly sensitive to infectious diseases and cancer due to the alteration of their immune status.43,44 HEV seropositivity in HIV-infected population varies among different continents. The prevalence of HEV infection is presented in Table 2.47–115 We applied a search strategy to the following scientific databases: PubMed, Scopus, and Web of Science. We used free-text terms: “Hepatitis E virus (HEV) in HIV individuals” AND “Hepatitis E virus (HEV) in AIDS patients” AND “Hepatitis E virus (HEV) in HIV/AIDS persons”. The present analysis included original articles, short communications, and case series (more than 20 participants). The current analysis excluded reviews and articles with missing data for the number of investigated persons and case reports. Scientific papers for the period from January 1983 to August 2023 were studied.
 |
Table 2 Distribution of HEV Infection Among HIV-Positive Individuals |
There is probably no connection between HIV viremia and a higher risk for HEV infection.46 No significant association between HIV viral load and HEV seroprevalence in HIV-infected patients has been documented.46,91,111 Probably HIV infection itself is not a risk factor for HEV infection.46 Differences in HEV seropositivity between HIV-1- and HIV-2-infected patients have not been noted.56
Both acute and chronic HEV infections have been described in PLWH, especially among MSM and in patients with severe immunodeficiency.46 Chronic HEV infection can persist despite a CD4+ T-cell count >200 cells/mm3,56 The rate of chronic HEV infection among PLWH is low (0.0–0.5%) and mainly for individuals receiving appropriate antiretroviral therapy.46,81
Men who have sex with men are at higher risk for HIV infection, and also for pathogens transmitted by the oro-fecal route, for example HAV and HEV.116 In contrast to HAV, the available data do not indicate that sexual transmission is among the major routes of HEV infection in MSM.85,117 MSM and PWUD are not at higher risk for being HEV IgG seropositive than blood donors.85,117
Over the course of evolution, HEV has developed strategies to counteract and escape the host immune response and the development of chronic HEV infection is associated with immunosuppression.46 Effective immune control by both CD4+ and CD8+ T cells is necessary to prevent chronic viral replication.118 It has been reported that HEV-specific CD8+ T cells do not function properly in immunocompromised individuals and this contributes to the establishment of chronic infection with this virus.119 So, the measures to improve the immune response could have a good influence on HEV clearance. In agreement with this hypothesis was a manuscript that presented a self-limiting HEV infection in people living with HIV as a result of initiation of antiretroviral therapy and decreasing HIV viral load.55 Serologic screening alone may be insufficient to diagnose HEV infection in HIV-infected patients with very low CD4 counts because seroconversion (IgG) may be delayed or may not occur.55
While abundant information is available on therapeutic options for chronic HEV infection in liver transplant patients, data for PLWH are extremely limited.5 There were reports for ribavirin (RBV) monotherapy; a combination of pegylated interferon and RBV for 12 and 24 weeks of treatment; and a combination of sofosbuvir and RBV for 12 weeks.5,120–122
HEV in Patients with Neoplasm
The research on the relationship “HEV–cancer” is important for at least three reasons. First, patients with neoplastic diseases often have a suppressed immune system due to the cancer itself or the applied therapy (cancer chemotherapy or immunosuppressive drugs because of stem cell transplantation), which makes them more susceptible to further bacterial and viral infections or reactivation of latent pathogens such as Toxoplasma gondii, HIV, HBV, HCV, herpes simplex virus, and so on. This may increase morbidity and cancer death rates.123–125 Second, oncology patients are commonly transfused with blood products, which pose a possible route of HEV transmission.126,127 Third, 15.4% of all cancers are caused by infectious agents of various categories, and more than 10% of them are attributed to viruses, including HBV and HCV.126,127
It seems that the incidence of HEV in patients with neoplasms is similar in the general population.128 In the scientific literature, we can find publications that presented a case, cases, or series of cases on HEV infection and neoplastic patients. Chronic HEV infection has been registered in a person with non-Hodgkin’s lymphoma undergoing treatment.129 A case has been published of acute lymphoblastic leukaemia after allogeneic stem cell transplantation who developed reactivation of HEV infection.130 The results of a multicentre cohort study across 11 European centres were published in 2019 and showed liver morbidity and mortality related to HEV among patients with hematologic cancers.131
A case series of women with underlying gynecological cancers who developed HEV infection has been reported.132
A study (950 cancer patients and 950 control volunteers) from Shandong province, Eastern China revealed a higher seroprevalence of IgG and IgM antibodies in cancer patients.133 The highest incidence of HEV infection has been found in leukemia patients (32.3%), followed by liver cancer patients (31.1%), the lowest HEV seropositivity was noted in individuals with gastric cancer (18.9%).133 The highest seroprevalence in a group of leukemia patients can be attributed to severe immune dysfunctions observed in acute and chronic leukemia.134,135 Also, the treatment of acute lymphoblastic leukemia often included stem cell transplantation, radiation therapy, and application of immunosuppressive drugs or blood transfusions – so there were predispositions for immune disturbances, on one hand, and transfusion-transmitted ability, on the other hand.126,127
Nevertheless, the influence of HEV infection on cancer development (in particular liver carcinogenesis) has been poorly studied for a variety of reasons:
- Chronic HEV infection mainly presents in immunocompromised individuals, and the overall number of cases is low.5,136
- The onset of liver cirrhosis occurs relatively soon after chronic HEV infection, whereas the development of HCC takes longer – at least 8 years.28,137 Other hepatotropic oncoviruses such as HBV or HCV usually lead to HCC 20–30 years after the initial infection.28,137
- An extremely limited number of clinical cases of HEV-related HCC has been reported. Atsama et al described the association between anti-HEV IgG prevalence and HCC in a study performed in Cameroon.138 HEV positivity has been detected in 31.1% of liver cancer patients.133 A case of HCC complicating chronic HEV infection has been reported in 2018 by French researchers.137 The possibility of cancer cells being more sensitive to HEV infection is discussable.139
- Globally, HEV infection is not routinely tested.1,5
- Basically, cancer develops in a small fraction of people infected with the respective oncovirus.5,10
- Studies on biology and oncogenic potential of HEV are limited because of the difficulties to propagate at conventional cell lines and the available appropriate experimental systems (cell cultures and animal models).139,140
HEV Among Individuals with Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease (IBD) is characterized by repetitive episodes of inflammation of the gastrointestinal tract caused by an abnormal immune response to gut microflora.141,142 Two main diseases are ulcerative colitis and Crohn’s disease.141,142 There were a few articles on HEV and IBD. Senosiain et al presented 87 patients with IBD from Madrid, Spain. The estimated prevalence of anti-HEV IgG antibodies was 1.3%, HEV IgM – 2.7%, HEV RNA – 0.0%, chronic HEV infection – 0.0%.143 In 2019, German scientists performed a study on HEV among 328 patients with Crohn’s disease and 150 patients with ulcerative colitis.144 They reported HEV IgG seropositivity of 17.4% in Crohn’s disease individuals, 24.7% in ulcerative colitis patients, and no positive results for HEV by RT-PCR.144 Grigas et al presented 203 patients with inflammatory bowel disease (Crohn’s disease – 47 persons and 156 with ulcerative colitis).145 These Lithuanian scientists announced higher levels of anti-HEV IgG and IgM antibodies among Crohn’s disease patients – 12.8% (95% CI: 3.2–22.3) and 8.5% (95% CI: 0.5–16.5), versus lower level of anti-HEV IgG and IgM antibodies in ulcerative colitis individuals – 12.2% (95% CI: 7.1–17.3) and 5.8% (95% CI: 2.1–9.4).145 Furthermore, Grigas et al reported 22-year-old woman with ulcerative colitis and HEV gt 3i (GenBank accession number: MT585816), which was genetically closer to wild boar and domestic pig isolates from Lithuania.145 In retrospective, multicenter, observational research of 327 patients with Crohn’s disease and 161 ulcerative colitis individuals from 16 French general hospitals was presented.146 These French authors established a HEV IgG seroprevalence rate of 14.2% among all patients, and HEV IgM was detected in 0.9%, and HEV RNA was undetectable in all participants.146 At this stage of knowledge, the number of studies on HEV infection among IBD individuals with IBD is small and are not enough to draw up conclusions and recommendations for this specific patient group.
HEV Among Rheumatic Diseases Patients
Rheumatic diseases include different autoimmune and autoinflammatory illnesses that are characterised by musculoskeletal disorders and systemic presentations.147,148 Furthermore, the adaptive and innate immunity can support to the inflammatory processes that take part in the pathogenesis of these debilitating diseases.147,148 There are still few studies on HEV infection in individuals with rheumatic diseases. Bauer et al did a retrospective multicenter questionnaire-based survey (initiated by the Society of Rheumatology in France) among nearly 2400 French physicians.149 For the period of 44 months (between January 2010 and August 2013) the surveyed physicians announced 23 clinical cases of HEV infection among patients with rheumatic diseases (axial spondyloarthritis, n = 5; other types of arthritides, n = 3; psoriatic arthritis, n = 4; and rheumatoid arthritis, n = 11).149 All 23 patients had an acute infection, and the diagnosis was made by positive results from the RT-PCR test (n = 14) and ELISA HEV IgM test (n = 9). Due to the initial phase of investigations on HEV infection in rheumatic patients, it is hard to make in-depth conclusions and recommendations.
HEV Among Individuals with Chronic Liver Disease
Chronic hepatitis B virus (HBV) is an immune-related chronic liver disease.150 Essentially, HBV does not directly damage the liver but through abnormal immune response.151 In chronic HBV infection, the virus continues to replicate and the host immune response is insufficient.152 The most common obstacles to eliminate HBV are the following: dysfunction of T cells; persistent presence of HBV cccDNA (covalently closed circular DNA); insufficient response of B cells; and integration of HBV DNA in hepatocytes.153 In 2007, Bayram et al reported 13.7% (26/190) anti-HEV IgG positive results among Turkish adult patients with chronic HBV.154 Chinese authors presented a study including 188 persons with chronic HBV, 136 with HEV superinfection and 52 with HAV superinfection.155 Most of the individuals in the group “chronic HBV + HEV” had complications (94.9%), hepatic failure (39.7%), and high mortality (33.8%).155 In 2019, McGivern et al announced 28.5%/1.7% anti-HEV IgG/IgM seroprevalence in 600 adult persons with chronic HBV living in the USA and Canada.156 Chinese scientists found 1.27% (10/790) anti-HEV-IgG positive results in patients with chronic HBV versus 4.21% (22/522) in persons with HBV-related cirrhosis (OR = 3.04; p < 0.05).157 In 2019, Kilonzo et al established 35.9% anti-HEV IgG seroprevalence among chronic HBV patients.158 American authors reported 19.86% HEV IgG seroprevalence in US adults with chronic HBV.159
Immune cell detection of HCV activates signaling pathways that produce interferons and trigger the innate immune response against this virus, ie preventing HCV spread and replication.160 Furthermore, cells in the innate immune system (including dendritic cells, Kupffer cells, and natural killer cells) interact with infected hepatocytes and present viral antigens to B and T cells where their effector responses contribute to HCV infection outcome.161 Despite all these immune mechanisms, HCV can evade the host immune response and establish chronic infection. Turkish authors reported 54.0% (94/174) anti-HEV IgG positive results in adult persons with chronic HCV.154 In 2017, Mellgren et al found 30.0% anti-HEV IgG prevalence among 204 Swedish patients with chronic HCV infection.162 Chinese scientists announced 3.23% anti-HEV-IgG positive results in individuals with chronic HCV versus 10.81% in persons with HCV-related cirrhosis.157 In 2019, Bricks et al noted 13.2% anti-HEV seroprevalence among chronic HCV patients with cirrhosis and 8.0% in chronic HCV persons without cirrhosis (OR = 1.74; p = 0.04).163 Brazilian authors found 12.0% (22/181) anti-HEV IgG seropositivity in individuals with chronic HCV.164 In 2021, Wong et al reported 8.66% HEV IgG seroprevalence among adults with chronic HCV.159 Korean scientists presented 33.3% anti-HEV IgG positivity prevalence in 502 patients with chronic HCV.165
Cirrhosis is a dynamic chronic liver disease. Furthermore, cirrhosis and acute-on-chronic liver failure (ACLF) are associated with severe systemic inflammation, respectively, with oxidative stress, increased inflammatory cytokines, and some markers of activated macrophages and neutrophils in the liver and in the blood.166 In liver cirrhosis, an immune deficiency or “immune paralysis” is often observed – cirrhosis-associated immune dysfunction (CAID) syndrome.167 The intensity of CAID syndrome correlates with the severity of acute/chronic liver failure and bacterial translocation.168 Kumar Acharya et al reported that HEV infection among 107 Indian patients with cirrhosis is associated with rapid decompensation and death.169 Spanish authors reported 17.5% anti-HEV IgG positive results in persons with chronic liver disease and cirrhosis versus 32.1% in individuals with liver transplant and cirrhosis.67 In 2019, Fantilli et al found 25.0% HEV seroprevalence among 140 Argentinian patients with cirrhosis.170 Chinese scientists noted 1.41% anti-HEV-IgG positive results in persons with chronic alcoholic hepatitis versus 9.40% in individuals with alcoholic cirrhosis.157 American researchers recorded 6.58% HEV IgG seroprevalence among adults with alcoholic liver disease.159 In 2022, Korean authors reported that acute HEV infection mortality rate was higher among individuals with cirrhosis, and especially high in those with ACLF.171
Non-alcoholic fatty liver disease (NAFLD) has been recently re-named as “metabolic dysfunction-associated fatty liver disease” (MAFLD).172 MAFLD is the main cause of chronic liver disease worldwide. MAFLD can range from steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocarcinoma.173 Furthermore, MAFLD is an independent risk factor for diabetes mellitus (type 2), different cardiovascular diseases, and high mortality rate.174 In 2019, Yang et al reported 0.0% anti-HEV-IgG positive results among Chinese patients with chronic NASH versus 25.0% in persons with NASH-related cirrhosis.157 American authors announced 8.81% HEV IgG seroprevalence among individuals with NAFLD.159
Schistosomiasis is a neglected tropical disease, impacting approximately 250 million infected persons in more than 70 countries.175 This infection is caused by trematode blood flukes of Schistosoma genus.176 The main clinically important Schistosoma species are as follows: S. haematobium, S. japonicum, and S. mansoni.177 Other species of the genus Schistosoma have regional and local importance – S. guineensis, S. intercalatum, and S. mekongi.178 The main lesions in Schistosoma spp. are due to the eggs laid by the female worms.175 A large proportion of eggs are trapped in the liver of the definitive host.176 In the liver, they secrete different components including proteolytic enzymes that elicit eosinophilic inflammatory immune responses.175 Furthermore, S. japonicum and S. mansoni can cause the formation of granulomas, which are progressively replaced by fibrotic deposits eventually resulting in advanced or intestinal hepatosplenic schistosomiasis.176 Abdel Rahman et al reported 31.0% HEV IgM positive results among 100 Egyptian individuals with S. mansoni infection.179 Brazilian authors found 10.0% HEV seropositivity in 30 patients with schistosomiasis.180 Passos-Castilho et al presented 18.8% anti-HEV IgG positive results among 80 patients with S. mansoni.181 In 2023, Brazilian scientists announced 3.08% anti-HEV IgG positive results in 227 individuals with chronic liver disease (alcohol-related liver disease – 17 individuals; HBV – 50; HCV – 33; mixed disease – 62; NAFLD – 49; and schistosomiasis – 16).182
A brief review of the scientific literature showed that HEV infection can occur and may be more frequent among individuals with underlying chronic liver disease. HEV-related complications and mortality could be observed more often in this population. This is important for daily clinical practice because HEV could lead to a severe clinical form in individuals with underlying chronic liver disease. In this regard, it is recommended to consider HEV infection in case of patients with chronic liver disease.
Prevention of HEV Infection
Prevention against HEV infection is focused on several basic recommendations. First, it is known from the scientific literature that HEV gt 1 and HEV gt 2 are transmitted by ingesting contaminated water.1,5 Consequently, access to safe water sources and safe water supplies is needed. Second, HEV gt 3, HEV gt 4, and HEV gt 7 are transmitted by eating raw or undercooked meat, meat products, fish, seafood, and meat from different animals (domestic pigs, wild boars, deer, rabbits, camels, etc.).1,5 In this regard, it is highly recommended that meat, meat products, fish, and seafood undergo heat treatment at 70.0°C for a minimum of 2 min.5 Third, another important preventive measure is HEV vaccination, which is mainly done in China.183 Currently, the development of several vaccines against HEV is proceeding at different stages.4 Fourth, special protective equipment for persons coming into close direct contact with animal reservoirs (veterinarians, hunters, workers in pig farms, etc.) is also recommended. Fifth, proper washing and cleaning of stem vegetables and fruits before their use. Sixth, maintaining good personal hand hygiene. Seventh, the risk of transfusion-transmitted HEV is low. However, it is recommended to conduct HEV blood screening as well as other routine tests for some infectious pathogens. In conclusion, the implementation of all these measures could reduce the risk of HEV transmission and consequently HEV infection, which could be more severe in immunocompromised persons compared to immunocompetent individuals.
Conclusion
In the past two decades, the incidence of HEV infection has increased worldwide. Moreover, HEV is a serious challenge for immunocompromised individuals. In recent years, the number of transplant recipients has increased, HIV infection continues to be a serious problem for developing countries, and cases of neoplasms are frequent in all continents. These conditions lead to an increase in the population of individuals with compromised immune status. Also, there is a group of people with disorders in the immune system (inflammatory bowel diseases, rheumatic diseases, chronic liver diseases, etc.). All these facts indicate the increment of the importance of HEV infection among immunocompromised individuals. So, public health institutions and organizations should take preventive measures against the spread of HEV in different countries. This is a health problem that cannot be overlooked by public health specialists. Physicians in different medical specialties should be involved in the surveillance and management of HEV.
Acknowledgments
We thank our families for providing us with the time and support needed to write this review in a timely manner. In addition, part of the work on this article was supported by Grant КП-06-Н33/2 from 13.12.2019, National Science Fund, Sofia, Bulgaria.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Article Publishing Charge (APC) was funded by the Bulgarian Ministry of Education and Science in the frames of the Bulgarian National Recovery and Resilience Plan, Component “Innovative Bulgaria”, Project No. BG-RRP-2.004-0006-C02 “Development of research and innovation at Trakia University in service of health and sustainable well-being”. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the manuscript.
Disclosure
The authors declare no conflicts of interest.
References
1. World Health Organization. Hepatitis E (last update: 20 July 2023). Available from: https://www.who.int/.
2. International Committee on Taxonomy of Viruses (ICTV). Family: HEPEVIRIDAE. Available from: https://ictv.global/.
3. Purdy MA, Drexler JF, Meng XJ, et al. ICTV Virus Taxonomy Profile: HEPEVIRIDAE 2022. J Gen Virol. 2022;103(9):001778. doi:10.1099/jgv.0.001778
4. Zahmanova G, Takova K, Tonova V, et al. The re-emergence of hepatitis E virus in Europe and vaccine development. Viruses. 2023;15(7):1558. doi:10.3390/v15071558
5. Dalton HR, Kamar N, Baylis SA, Moradpour D, Wedemeyer H, Negro F, European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256–1271. doi:10.1016/j.jhep.2018.03.005
6. Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20(1):23–31. doi:10.1159/000149370
7. Songtanin B, Molehin AJ, Brittan K, et al. Hepatitis E virus infections: epidemiology, genetic diversity, and clinical considerations. Viruses. 2023;15(6):1389. doi:10.3390/v15061389
8. Pepovich R, Baymakova M, Pishmisheva M, et al. Current knowledge on hepatitis E virus infection. Vojnosanit Pregl. 2019;76(7):733–739. doi:10.2298/VSP170815159P
9. Khuroo MS. Discovery of hepatitis E and its impact on global health: a journey of 44 years about an incredible human-interest story. Viruses. 2023;15(8):1745. doi:10.3390/v15081745
10. Sayed IM. Dual infection of hepatitis A virus and hepatitis E virus – what is known? Viruses. 2023;15(2):298. doi:10.3390/v15020298
11. Baymakova M, Popov GT, Pepovich R, et al. Hepatitis E virus infection in Bulgaria: a brief analysis of the situation in the country. Open Access Maced J Med Sci. 2019;7(3):458–460. doi:10.3889/oamjms.2019.073
12. Raji YE, Toung OP, Taib NM, et al. Hepatitis E virus: an emerging enigmatic and underestimated pathogen. Saudi J Biol Sci. 2022;29(1):499–512. doi:10.1016/j.sjbs.2021.09.003
13. Velavan TP, Pallerla SR, Johne R, et al. Hepatitis E: an update on One Health and clinical medicine. Liver Int. 2021;41(7):1462–1473. doi:10.1111/liv.14912
14. Prpic J, Baymakova M. Hepatitis E virus (HEV) infection among humans and animals: epidemiology, clinical characteristics, treatment, and prevention. Pathogens. 2023;12(7):931. doi:10.3390/pathogens12070931
15. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. doi:10.1186/s41073-019-0064-8
16. Cancela F, Noceti O, Arbiza J, et al. Structural aspects of hepatitis E virus. Arch Virol. 2022;167(12):2457–2481. doi:10.1007/s00705-022-05575-8
17. Ahmed R, Nasheri N. Animal reservoirs for hepatitis E virus within the Paslahepevirus genus. Vet Microbiol. 2023;278:109618. doi:10.1016/j.vetmic.2022.109618
18. Willauer AN, Sherman KE. Hepatitis E virus: has anything changed? Curr Opin Gastroenterol. 2023;39(3):169–174. doi:10.1097/MOG.0000000000000918
19. Aslan AT, Balaban HY. Hepatitis E virus: epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26(37):5543–5560. doi:10.3748/wjg.v26.i37.5543
20. Huang F, Li Y, Yu W, et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology. 2016;64(2):350–359. doi:10.1002/hep.28668
21. Li P, Liu J, Li Y, et al. The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis. Liver Int. 2020;40(7):1516–1528. doi:10.1111/liv.14468
22. Perisetti A, Laoveeravat P, Inamdar S, et al. Hepatitis E virus infection in liver transplant recipients: a descriptive literature review. Eur J Gastroenterol Hepatol. 2020;32(8):916–922. doi:10.1097/MEG.0000000000001682
23. Markakis GE, Papatheodoridis GV, Cholongitas E. Epidemiology and treatment of hepatitis E in the liver transplantation setting: a literature review. J Viral Hepat. 2022;29(9):698–718. doi:10.1111/jvh.13709
24. Wasuwanich P, Sirisreetreerux P, Ingviya T, et al. Hepatitis E virus infection and rejection in kidney transplant recipients. Transpl Immunol. 2022;70:101517. doi:10.1016/j.trim.2021.101517
25. Carter M, Solsrud K, Yeddula S, et al. Hepatitis E diagnosis and management after liver, kidney, or heart transplant: a single-center experience. Transplant Proc. 2022;54(7):1737–1741. doi:10.1016/j.transproceed.2022.04.025
26. Owada Y, Oshiro Y, Inagaki Y, et al. A nationwide survey of hepatitis E virus infection and chronic hepatitis in heart and kidney transplant recipients in Japan. Transplantation. 2020;104(2):437–444. doi:10.1097/TP.0000000000002801
27. Harritshoj LH, Hother CE, Sengelov H, et al. Epidemiology of hepatitis E virus infection in a cohort of 4023 immunocompromised patients. Int J Infect Dis. 2020;91:188–195. doi:10.1016/j.ijid.2019.11.014
28. Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358(8):811–817. doi:10.1056/NEJMoa0706992
29. Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150(2):355–7.e3. doi:10.1053/j.gastro.2015.10.048
30. Buescher G, Ozga AK, Lorenz E, et al. Hepatitis E seroprevalence and viremia rate in immunocompromised patients: a systematic review and meta-analysis. Liver Int. 2021;41(3):449–455. doi:10.1111/liv.14695
31. Ankcorn MJ, Ijaz S, Poh J, et al. Toward systematic screening for persistent hepatitis E virus infections in transplant patients. Transplantation. 2018;102(7):1139–1147. doi:10.1097/TP.0000000000002097
32. Hansrivijit P, Trongtorsak A, Puthenpura MM, et al. Hepatitis E in solid organ transplant recipients: a systematic review and meta-analysis. World J Gastroenterol. 2021;27(12):1240–1254. doi:10.3748/wjg.v27.i12.1240
33. Gorris M, van der Lecq BM, van Erpecum KJ, et al. Treatment for chronic hepatitis E virus infection: a systematic review and meta-analysis. J Viral Hepat. 2021;28(3):454–463. doi:10.1111/jvh.13456
34. Wang Y, Zhou X, Debing Y, et al. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146(7):1775–1783. doi:10.1053/j.gastro.2014.02.036
35. De Winter BCM, Hesselink DA, Kamar N. Dosing ribavirin in hepatitis E-infected solid organ transplant recipients. Pharmacol Res. 2018;130:308–315. doi:10.1016/j.phrs.2018.02.030
36. Kamar N, Abravanel F, Behrendt P, et al. Ribavirin for hepatitis E virus infection after organ transplantation: a large European retrospective multicenter study. Clin Infect Dis. 2020;71(5):1204–1211. doi:10.1093/cid/ciz953
37. Todt D, Gisa A, Radonic A, et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut. 2016;65(10):1733–1743. doi:10.1136/gutjnl-2015-311000
38. Wang B, Mahsoub HM, Li W, et al. Ribavirin treatment failure-associated mutation, Y1320H, in the RNA-dependent RNA polymerase of genotype 3 hepatitis E virus (HEV) enhances virus replication in a rabbit HEV infection model. mBio. 2023;14(2):e0337222. doi:10.1128/mbio.03372-22
39. Lhomme S, DebRoy S, Kamar N, et al. Plasma hepatitis E virus kinetics in solid organ transplant patients receiving ribavirin. Viruses. 2019;11(7):630. doi:10.3390/v11070630
40. Mangia A, Piazzolla V. Overall efficacy and safety results of sofosbuvir-based therapies in Phase II and III studies. Dig Liver Dis. 2014;46:S179–85. doi:10.1016/j.dld.2014.09.026
41. Dao Thi VL, Debing Y, Wu X, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016;150(1):82–85.e4. doi:10.1053/j.gastro.2015.09.011
42. Cornberg M, Pischke S, Muller T, et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E – the HepNet SofE pilot study. J Hepatol. 2020;73(3):696–699. doi:10.1016/j.jhep.2020.05.020
43. UNAIDS. Global HIV & AIDS statistics – fact sheet. Available from: https://www.unaids.org/en.
44. The official U.S. Government website managed by the U.S. Department of Health & Human Services and supported by the Minority HIV/AIDS Fund. Global statistics (last update: 20 July 2023). Available from: https://www.hiv.gov/.
45. Soriano V, Moreno-Torres V, Mendoza C, et al. Viral hepatitis in persons living with HIV in the post-COVID era. AIDS Rev. 2023;25(1):1–13. doi:10.24875/AIDSRev.M23000061
46. Rivero-Juarez A, Lopez-Lopez P, Frias M, et al. Hepatitis E infection in HIV-infected patients. Front Microbiol. 2019;10:1425. doi:10.3389/fmicb.2019.01425
47. Pawlotsky JM, Belec L, Gresenguet G, et al. High prevalence of hepatitis B, C, and E markers in young sexually active adults from the Central African Republic. J Med Virol. 1995;46(3):269–272. doi:10.1002/jmv.1890460318
48. Psichogiou M, Tzala E, Boletis J, et al. Hepatitis E virus infection in individuals at high risk of transmission of non-A, non-B hepatitis and sexually transmitted diseases. Scand J Infect Dis. 1996;28(5):443–445. doi:10.3109/00365549609037936
49. Balayan MS, Fedorova OE, Mikhailov MI, et al. Antibody to hepatitis E virus in HIV-infected individuals and AIDS patients. J Viral Hepat. 1997;4(4):279–283. doi:10.1046/j.1365-2893.1997.00050.x
50. Fainboim H, Gonzalez J, Fassio E, et al. Prevalence of hepatitis viruses in an anti-human immunodeficiency virus-positive population from Argentina. A multicentre study. J Viral Hepat. 1999;6(1):53–57. doi:10.1046/j.1365-2893.1999.t01-1-6120135.x
51. Ng KP, He J, Saw TL, et al. A seroprevalence study of viral hepatitis E infection in human immunodeficiency virus type 1 infected subjects in Malaysia. Med J Malaysia. 2000;55(1):58–64.
52. Madejon A, Vispo E, Bottecchia M, et al. Lack of hepatitis E virus infection in HIV patients with advanced immunodeficiency or idiopathic liver enzyme elevations. J Viral Hepat. 2009;16(12):895–896. doi:10.1111/j.1365-2893.2009.01138.x
53. Renou C, Lafeuillade A, Cadranel JF, et al. Hepatitis E virus in HIV-infected patients. AIDS. 2010;24(10):1493–1499. doi:10.1097/QAD.0b013e32833a29ab
54. Sellier P, Mazeron MC, Tesse S, et al. Hepatitis E virus infection in HIV-infected patients with elevated serum transaminases levels. Virol J. 2011;8:171. doi:10.1186/1743-422X-8-171
55. Kenfak-Foguena A, Schoni-Affolter F, Burgisser P, et al. Hepatitis E virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17(6):1074–1078. doi:10.3201/eid/1706.101067
56. Kaba M, Richet H, Ravaux I, et al. Hepatitis E virus infection in patients infected with the human immunodeficiency virus. J Med Virol. 2011;83(10):1704–1716. doi:10.1002/jmv.22177
57. Keane F, Gompels M, Bendall R, et al. Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 2012;13(1):83–88. doi:10.1111/j.1468-1293.2011.00942.x
58. Crum-Cianflone NF, Curry J, Drobeniuc J, et al. Hepatitis E virus infection in HIV-infected persons. Emerg Infect Dis. 2012;18(3):502–506. doi:10.3201/eid1803.111278
59. Maylin S, Stephan R, Molina JM, et al. Prevalence of antibodies and RNA genome of hepatitis E virus in a cohort of French immunocompromised. J Clin Virol. 2012;53(4):346–349. doi:10.1016/j.jcv.2012.01.001
60. Jardi R, Crespo M, Homs M, et al. HIV, HEV and cirrhosis: evidence of a possible link from eastern Spain. HIV Med. 2012;13(6):379–383. doi:10.1111/j.1468-1293.2011.00985.x
61. Caron M, Bouscaillou J, Kazanji M. Acute risk for hepatitis E virus infection among HIV-1-positive pregnant women in Central Africa. Virol J. 2012;9:254. doi:10.1186/1743-422X-9-254
62. Feldt T, Sarfo FS, Zoufaly A, et al. Hepatitis E virus infections in HIV-infected patients in Ghana and Cameroon. J Clin Virol. 2013;58(1):18–23. doi:10.1016/j.jcv.2013.05.004
63. Mateos-Lindemann ML, Diez-Aguilar M, Galdamez AL, et al. Patients infected with HIV are at high-risk for hepatitis E virus infection in Spain. J Med Virol. 2014;86(1):71–74. doi:10.1002/jmv.23804
64. Scotto G, Martinelli D, Centra M, et al. Epidemiological and clinical features of HEV infection: a survey in the district of Foggia (Apulia, Southern Italy). Epidemiol Infect. 2014;142(2):287–294. doi:10.1017/S0950268813001167
65. Jacobs C, Chiluba C, Phiri C, et al. Seroepidemiology of hepatitis E virus infection in an urban population in Zambia: strong association with HIV and environmental enteropathy. J Infect Dis. 2014;209(5):652–657. doi:10.1093/infdis/jit409
66. Junaid SA, Agina SE, Abubakar KA. Epidemiology and associated risk factors of hepatitis E virus infection in plateau state, Nigeria. Virology. 2014;5:15–26. doi:10.4137/VRT.S15422
67. Riveiro-Barciela M, Buti M, Homs M, et al. Cirrhosis, liver transplantation and HIV infection are risk factors associated with hepatitis E virus infection. PLoS One. 2014;9(7):e103028. doi:10.1371/journal.pone.0103028
68. Pineda JA, Cifuentes C, Parra M, et al. Incidence and natural history of hepatitis E virus coinfection among HIV-infected patients. AIDS. 2014;28(13):1931–1937. doi:10.1097/QAD.0000000000000378
69. Sherman KE, Terrault N, Barin B, et al. Hepatitis E infection in HIV -infected liver and kidney transplant candidates. J Viral Hepat. 2014;21(8):e74–7. doi:10.1111/jvh.12233
70. Hassing RJ, van der Eijk AA, Lopes VB, et al. Hepatitis E prevalence among HIV infected patients with elevated liver enzymes in the Netherlands. J Clin Virol. 2014;60(4):408–410. doi:10.1016/j.jcv.2014.05.009
71. Yong MK, Paige EK, Anderson D, et al. Hepatitis E in Australian HIV-infected patients: an under-recognised pathogen? Sex Health. 2014;11(4):375–378. doi:10.1071/SH13198
72. Dakovic Rode O, Jemersic L, Brnic D, et al. Hepatitis E in patients with hepatic disorders and HIV-infected patients in Croatia: is one diagnostic method enough for hepatitis E diagnosis? Eur J Clin Microbiol Infect Dis. 2014;33(12):2231–2236. doi:10.1007/s10096-014-2187-7
73. Bura M, Michalak M, Chojnicki M, et al. Seroprevalence of anti-HEV IgG in 182 Polish patients. Postepy Hig Med Dosw. 2015;69:320–326. doi:10.5604/17322693.1143051
74. Joulaei H, Rudgari O, Motazedian N, et al. Hepatitis E virus seroprevalence in HIV positive individuals in Shiraz, Southern Iran. Iran J Microbiol. 2015;7(2):103–108.
75. Rivero-Juarez A, Martinez-Duenas L, Martinez-Peinado A, et al. High hepatitis E virus seroprevalence with absence of chronic infection in HIV-infected patients. J Infect. 2015;70(6):624–630. doi:10.1016/j.jinf.2014.10.016
76. Taha TE, Rusie LK, Labrique A, et al. Seroprevalence for hepatitis E and other viral hepatitides among diverse populations, Malawi. Emerg Infect Dis. 2015;21(7):1174–1182. doi:10.3201/eid2107.141748
77. Politou M, Boti S, Androutsakos T, et al. Seroprevalence of hepatitis E in HIV infected patients in Greece. J Med Virol. 2015;87(9):1517–1520. doi:10.1002/jmv.24214
78. Scotto G, Grisorio B, Filippini P, et al. Hepatitis E virus co-infection in HIV-infected patients in Foggia and Naples in Southern Italy. Infect Dis. 2015;47(10):707–713. doi:10.3109/23744235.2015.1049658
79. Merchante N, Parra-Sanchez M, Rivero-Juarez A, et al. High prevalence of antibodies against hepatitis E virus in HIV-infected patients with unexplained liver disease. Enferm Infecc Microbiol Clin. 2015;33(8):532–535. doi:10.1016/j.eimc.2014.10.018
80. Nouhin J, Barennes H, Madec Y, et al. Low frequency of acute hepatitis E virus (HEV) infections but high past HEV exposure in subjects from Cambodia with mild liver enzyme elevations, unexplained fever or immunodeficiency due to HIV-1 infection. J Clin Virol. 2015;71:22–27. doi:10.1016/j.jcv.2015.07.304
81. Pischke S, Schwarze-Zander C, Bremer B, et al. Hepatitis E virus seroprevalence rate in HIV-infected patients in Germany: a comparison of two commercial assays. Intervirology. 2015;58(5):283–287. doi:10.1159/000441472
82. Lopez-Fabal MF, Gomez-Garces JL. Seroprevalencia del virus de la hepatitis E en pacientes con hepatitis C y/o infeccion por el virus de la inmunodeficiencia humana [Seroprevalence of hepatitis E virus in patients with hepatitis C and / or infected with HIV]. Rev Esp Quimioter. 2015;28(6):314–316. Spanish.
83. Kuniholm MH, Ong E, Hogema BM, et al. Acute and chronic hepatitis E virus infection in human immunodeficiency virus-infected U.S. women. Hepatology. 2016;63(3):712–720. doi:10.1002/hep.28384
84. Debes JD, Martinez Wassaf M, Pisano MB, et al. Increased hepatitis E virus seroprevalence correlates with lower CD4+ cell counts in HIV-infected persons in Argentina. PLoS One. 2016;11(7):e0160082. doi:10.1371/journal.pone.0160082
85. Abravanel F, Lhomme S, Fougere M, et al. HEV infection in French HIV-infected patients. J Infect. 2017;74(3):310–313. doi:10.1016/j.jinf.2016.12.004
86. Bura M, Bukowska A, Bura A, et al. Hepatitis E virus antibodies in HIV-infected patients and blood donors from Western Poland: a preliminary report. Adv Clin Exp Med. 2017;26(4):577–579. doi:10.17219/acem/62353
87. Bura M, Lagiedo M, Michalak M, et al. Hepatitis E virus IgG seroprevalence in HIV patients and blood donors, West-Central Poland. Int J Infect Dis. 2017;61:20–22. doi:10.1016/j.ijid.2017.05.014
88. Zeng H, Wang L, Liu P, et al. Seroprevalence of hepatitis E virus in HIV-infected patients in China. AIDS. 2017;31(14):2019–2021. doi:10.1097/QAD.0000000000001585
89. Rivero-Juarez A, Cuenca-Lopez F, Martinez-Peinado A, et al. Rural habitat as risk factor for hepatitis E virus seroconversion in HIV-infected patients: a prospective longitudinal study. Zoonoses Public Health. 2017;64(7):e60–e64. doi:10.1111/zph.12347
90. Shrestha A, Adhikari A, Bhattarai M, et al. Prevalence and risk of hepatitis E virus infection in the HIV population of Nepal. Virol J. 2017;14(1):228. doi:10.1186/s12985-017-0899-x
91. Ferreira AC, Gomes-Gouvea MS, Lisboa-Neto G, et al. Serological and molecular markers of hepatitis E virus infection in HIV-infected patients in Brazil. Arch Virol. 2018;163(1):43–49. doi:10.1007/s00705-017-3562-3
92. Boon D, Redd AD, Laeyendecker O, et al. Hepatitis E virus seroprevalence and correlates of anti-HEV IgG antibodies in the Rakai district, Uganda. J Infect Dis. 2018;217(5):785–789. doi:10.1093/infdis/jix610
93. Debes JD, Pas SD, Groothuismink ZMA, et al. A mutation in the progesterone receptor predisposes to HEV infection in HIV-positive patients. Liver Int. 2018;38(5):792–796. doi:10.1111/liv.13678
94. Zhou S, Ren L, Xia X, et al. Hepatitis E virus infection in HIV-infected patients: a large cohort study in Yunnan province, China. J Med Virol. 2018;90(6):1121–1127. doi:10.1002/jmv.25060
95. Harritshoj LH, Theilgaard ZP, Mannheimer E, et al. Hepatitis E virus epidemiology among HIV-infected women in an urban area in Tanzania. Int J Infect Dis. 2018;73:7–9. doi:10.1016/j.ijid.2018.05.010
96. Greco L, Uceda Renteria SC, Guarneri D, et al. HEV and HAV seroprevalence in men that have sex with men (MSM): an update from Milan, Italy. J Med Virol. 2018;90(8):1323–1327. doi:10.1002/jmv.25052
97. Salvio AL, Lopes AO, Almeida AJ, et al. Detection and quantification of hepatitis E virus in the absence of IgG and IgM anti-HEV in HIV-positive patients. J Appl Microbiol. 2018;125(4):1208–1215. doi:10.1111/jam.14024
98. Demi Sibiro OA, Manirakiza A, Komas NP. Seroprevalence of hepatitis E virus infection among people living with HIV in the Central African Republic. Open Forum Infect Dis. 2018;5(12):ofy307. doi:10.1093/ofid/ofy307
99. Alberts CJ, Schim van der Loeff MF, Sadik S, et al. Hepatitis E virus seroprevalence and determinants in various study populations in the Netherlands. PLoS One. 2018;13(12):e0208522. doi:10.1371/journal.pone.0208522
100. Parfieniuk-Kowerda A, Jaroszewicz J, Lapinski TW, et al. High prevalence of anti-HEV antibodies among patients with immunosuppression and hepatic disorders in Eastern Poland. Arch Med Sci. 2018;17(3):675–681. doi:10.5114/aoms.2018.79958
101. Bezerra LA, de Oliveira-Filho EF, Silva JVJ, et al. Risk analysis and seroprevalence of HEV in people living with HIV/AIDS in Brazil. Acta Trop. 2019;189:65–68. doi:10.1016/j.actatropica.2018.09.026
102. Shah SM, Baniya JB, Gupta BP, et al. Short article: association between liver fibrosis and hepatitis E seroprevalence among HIV-positive individuals in Nepal. Eur J Gastroenterol Hepatol. 2019;31(4):503–505. doi:10.1097/MEG.0000000000001308
103. Harritshoj LH, Kirkegaard-Klitbo DM, Mejer N, et al. Prevalence of anti-hepatitis E virus immunoglobulin G in HIV-infected individuals over three decades. Int J Infect Dis. 2019;84:67–72. doi:10.1016/j.ijid.2019.04.029
104. da Silva C M, Oliveira JM, Mendoza-Sassi RA, et al. Detection and characterization of hepatitis E virus genotype 3 in HIV-infected patients and blood donors from Southern Brazil. Int J Infect Dis. 2019;86:114–121. doi:10.1016/j.ijid.2019.06.027
105. Modiyinji AF, Amougou-Atsama M, Monamele CG, et al. Seroprevalence of hepatitis E virus antibodies in different human populations of Cameroon. J Med Virol. 2019;91(11):1989–1994. doi:10.1002/jmv.25545
106. Lin KY, Lin PH, Sun HY, et al. Hepatitis E virus infections among Human Immunodeficiency Virus-positive individuals during an outbreak of acute hepatitis A in Taiwan. Hepatology. 2019;70(6):1892–1902. doi:10.1002/hep.30771
107. Shinohara N, Owada T, Tanaka A, et al. Hepatitis A virus and hepatitis E virus prevalence relates to human immunodeficiency virus infection in Japanese male blood donors. Microbiol Immunol. 2020;64(5):392–395. doi:10.1111/1348-0421.12780
108. Shahriarirad R, Erfani A, Rastegarian M, et al. Seroprevalence of anti-hepatitis E antibodies and antigens among HIV-infected patients in Fars Province, Southern Iran. Virol J. 2020;17(1):109. doi:10.1186/s12985-020-01384-0
109. Oz S, Altindis M, Toptan H, et al. Investigation of hepatitis E seroprevalence in HIV positive patients by a novel enzyme linked fluorescent assay test. Clin Lab. 2020;66(7). doi:10.7754/Clin.Lab.2019.191122
110. Osundare FA, Klink P, Akanbi OA, et al. Hepatitis E virus infection in high-risk populations in Osun State, Nigeria. One Health. 2021;13:100256. doi:10.1016/j.onehlt.2021.100256
111. Golkocheva-Markova E, Kevorkyan A, Raycheva R, et al. Assessment of hepatitis E seropositivity among HIV-infected patients in Bulgaria. Braz J Infect Dis. 2022;26(1):102329. doi:10.1016/j.bjid.2022.102329
112. Filipe R, Prista-Leao B, Silva-Pinto A, et al. Hepatitis E in a Portuguese cohort of human immunodeficiency virus positive patients: high seroprevalence but no chronic infections. Health Sci Rep. 2022;5(3):e624. doi:10.1002/hsr2.624
113. Lopez-Lopez P, Frias M, Camacho A, et al. Seroreversion of IgG anti-HEV in HIV cirrhotic patients: a long-term multi-sampling longitudinal study. Transbound Emerg Dis. 2022;69(5):e1541–e1548. doi:10.1111/tbed.14486
114. Zahedi MJ, Shafieipour S, Hayatbakhsh Abassi MM, et al. Higher risk of chronic hepatitis E virus infection in patients with Human Immunodeficiency Virus-1: an Iranian cross-sectional study. Iran J Pathol. 2023;18(2):125–133. doi:10.30699/IJP.2023.551657.2870
115. Golkocheva-Markova E, Ismailova C, Kevorkyan A, et al. Age and gender trends in the prevalence of markers for hepatitis E virus exposure in the heterogeneous Bulgarian population. Life. 2023;13(6):1345. doi:10.3390/life13061345
116. Biello KB, Mimiaga MJ, Santostefano CM, et al. MSM at highest risk for HIV acquisition express greatest interest and preference for injectable antiretroviral PrEP compared to daily, oral medication. AIDS Behav. 2018;22(4):1158–1164. doi:10.1007/s10461-017-1972-6
117. Chaix ML, Leturque N, Gabassi A, et al. Prevalence and incidence of HEV among men using HIV pre-exposure prophylaxis: a sub-study of the ANRS IPERGAY trial. J Clin Virol. 2023;160:105380. doi:10.1016/j.jcv.2023.105380
118. Kupke P, Werner JM. Hepatitis E virus infection – immune responses to an underestimated global threat. Cells. 2021;10(9):2281. doi:10.3390/cells10092281
119. Kemming J, Gundlach S, Panning M, et al. Mechanisms of CD8+ T-cell failure in chronic hepatitis E virus infection. J Hepatol. 2022;77(4):978–990. doi:10.1016/j.jhep.2022.05.019
120. Dalton HR, Keane FE, Bendall R, et al. Treatment of chronic hepatitis E in a patient with HIV infection. Ann Intern Med. 2011;155(7):479–480. doi:10.7326/0003-4819-155-7-201110040-00017
121. Neukam K, Barreiro P, Macias J, et al. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis. 2013;57(3):465–468. doi:10.1093/cid/cit224
122. Todesco E, Demeret S, Calin R, et al. Chronic hepatitis E in HIV/HBV coinfected patient: lack of power of sofosbuvir-ribavirin. AIDS. 2017;31(9):1346–1348. doi:10.1097/QAD.0000000000001474
123. Mahale P, Kontoyiannis DP, Chemaly RF, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57(6):1177–1185. doi:10.1016/j.jhep.2012.07.031
124. Cong W, Liu GH, Meng QF, et al. Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 2015;359(2):307–313. doi:10.1016/j.canlet.2015.01.036
125. Vittecoq M, Elguero E, Lafferty KD, et al. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol. 2012;12(2):496–498. doi:10.1016/j.meegid.2012.01.013
126. Zella D, Gallo RC. Viruses and bacteria associated with cancer: an overview. Viruses. 2021;13(6):1039. doi:10.3390/v13061039
127. Elkhalifa AME, Nabi SU, Shah OS, et al. Insight into oncogenic viral pathways as drivers of viral cancers: implication for effective therapy. Curr Oncol. 2023;30(2):1924–1944. doi:10.3390/curroncol30020150
128. Chiu CY, Zhang HC, Westin J, et al. Hepatitis E virus infection in cancer patients. Transplant Cell Ther. 2022;28(11):788.e1–788.e5. doi:10.1016/j.jtct.2022.08.020
129. Ollier L, Tieulie N, Sanderson F, et al. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150(6):430–431. doi:10.7326/0003-4819-150-6-200903170-00026
130. le Coutre P, Meisel H, Hofmann J, et al. Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut. 2009;58(5):699–702. doi:10.1136/gut.2008.165571
131. von Felden J, Alric L, Pischke S, et al. The burden of hepatitis E among patients with haematological malignancies: a retrospective European cohort study. J Hepatol. 2019;71(3):465–472. doi:10.1016/j.jhep.2019.04.022
132. Bettinger D, Schlabe S, Pischke S, et al. Ribavirin in acute hepatitis E infection in patients with gynecological cancer: a case series. J Clin Transl Hepatol. 2018;6(2):237–240. doi:10.14218/JCTH.2017.00063
133. Bai MJ, Zhou N, Dong W, et al. Seroprevalence and risk factors of hepatitis E virus infection in cancer patients in Eastern China. Int J Infect Dis. 2018;71:42–47. doi:10.1016/j.ijid.2018.04.003
134. Arruga F, Gyau BB, Iannello A, et al. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci. 2020;21(5):1825. doi:10.3390/ijms21051825
135. Allegra A, Tonacci A, Musolino C, et al. Secondary immunodeficiency in hematological malignancies: focus on multiple myeloma and chronic lymphocytic leukemia. Front Immunol. 2021;12:738915. doi:10.3389/fimmu.2021.738915
136. Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358(8):859–860. doi:10.1056/NEJMc0708687
137. Borentain P, Colson P, Bolon E, et al. Hepatocellular carcinoma complicating hepatitis E virus-related cirrhosis. Hepatology. 2018;67(1):446–448. doi:10.1002/hep.29508
138. Amougou Atsama M, Atangana PJA, Noah Noah D, et al. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: preliminary observations. Int J Infect Dis. 2017;64:4–8. doi:10.1016/j.ijid.2017.08.010
139. Klohn M, Schrader JA, Bruggemann Y, et al. Beyond the usual suspects: hepatitis E virus and its implications in hepatocellular carcinoma. Cancers. 2021;13(22):5867. doi:10.3390/cancers13225867
140. Meister TL, Bruening J, Todt D, et al. Cell culture systems for the study of hepatitis E virus. Antiviral Res. 2019;163:34–49. doi:10.1016/j.antiviral.2019.01.007
141. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi:10.1007/s00535-021-01784-1
142. Plevris N, Lees CW. Disease monitoring in inflammatory bowel disease: evolving principles and possibilities. Gastroenterology. 2022;162(5):1456–1475.e1. doi:10.1053/j.gastro.2022.01.024
143. Senosiain C, Gonzalez-Tallon AA, Lopez-Sanroman A, et al. Hepatitis E seroprevalence in inflammatory bowel disease. Gastroenterol Hepatol. 2016;39(3):185–190. doi:10.1016/j.gastrohep.2015.06.004
144. Hoffmann P, Behnisch R, Gsenger J, et al. Hepatitis E seroprevalence in a German cohort of patients with inflammatory bowel diseases. PLoS One. 2020;15(10):e0239825. doi:10.1371/journal.pone.0239825
145. Grigas J, Montoya M, Simkute E, et al. Molecular characterization and seroprevalence of hepatitis E virus in inflammatory bowel disease patients and solid organ transplant recipients. Viruses. 2021;13(4):670. doi:10.3390/v13040670
146. Kounis I, Renou C, Nahon S, et al. Hepatitis E virus infection in patients with chronic inflammatory bowel disease treated with immunosuppressive therapy. Pathogens. 2023;12(2):332. doi:10.3390/pathogens12020332
147. American College of Rheumatology (ACR). Clinical Practice Guidelines. Available from: https://rheumatology.org/.
148. European Alliance of Associations for Rheumatology (EULAR). Free learning materials. Available from: https://www.eular.org/.
149. Bauer H, Luxembourger C, Gottenberg JE, et al. Outcome of hepatitis E virus infection in patients with inflammatory arthritides treated with immunosuppressants: a French retrospective multicenter study. Medicine. 2015;94(14):e675. doi:10.1097/MD.0000000000000675
150. Zheng JR, Wang ZL, Feng B. Hepatitis B functional cure and immune response. Front Immunol. 2022;13:1075916. doi:10.3389/fimmu.2022.1075916
151. Chang ML, Liaw YF. Hepatitis B flare in hepatitis B e antigen-negative patients: a complicated cascade of innate and adaptive immune responses. Int J Mol Sci. 2022;23(3):1552. doi:10.3390/ijms23031552
152. Wang ZL, Zheng JR, Yang RF, et al. An ideal hallmark closest to complete cure of chronic hepatitis B patients: high-sensitivity quantitative HBsAg loss. J Clin Transl Hepatol. 2023;11(1):197–206. doi:10.14218/JCTH.2022.00289
153. Lampertico P, Agarwal K, Berg T, European Association for the Study of the Liver (EASL). EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
154. Bayram A, Eksi F, Mehli M, et al. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50(4):281–286. doi:10.1159/000103916
155. Zhang X, Ke W, Xie J, et al. Comparison of effects of hepatitis E or A viral superinfection in patients with chronic hepatitis B. Hepatol Int. 2010;4(3):615–620. doi:10.1007/s12072-010-9204-4
156. McGivern DR, Lin HS, Wang J, et al. Prevalence and impact of hepatitis E virus infection among persons with chronic hepatitis B living in the US and Canada. Open Forum Infect Dis. 2019;6(5):ofz175. doi:10.1093/ofid/ofz175
157. Yang H, Wu J, Yuan Y, et al. Retrospectively seroprevalence study on anti-HEV-IgG antibody in patients with chronic hepatitis or liver cirrhosis in a Chinese teaching hospital. J Med Virol. 2019;91(3):437–443. doi:10.1002/jmv.25335
158. Kilonzo SB, Wang YL, Jiang QQ, et al. Superinfective hepatitis E virus infection aggravates hepatocytes injury in chronic hepatitis B. Curr Med Sci. 2019;39(5):719–726. doi:10.1007/s11596-019-2097-0
159. Wong RJ, Cheung R, Gish RG, et al. Prevalence of hepatitis E infection among adults with concurrent chronic liver disease. J Viral Hepat. 2021;28(11):1643–1655. doi:10.1111/jvh.13597
160. Stuart JD, Salinas E, Grakoui A. Immune system control of hepatitis C virus infection. Curr Opin Virol. 2021;46:36–44. doi:10.1016/j.coviro.2020.10.002
161. Hei L, Zhong J. Laboratory of genetics and physiology 2 (LGP2) plays an essential role in hepatitis C virus infection-induced interferon responses. Hepatology. 2017;65(5):1478–1491. doi:10.1002/hep.29050
162. Mellgren Å, Karlsson M, Karlsson M, et al. High seroprevalence against hepatitis E virus in patients with chronic hepatitis C virus infection. J Clin Virol. 2017;88:39–45. doi:10.1016/j.jcv.2017.01.005
163. Bricks G, Senise JF, Pott-Jr H, et al. Previous hepatitis E virus infection, cirrhosis and insulin resistance in patients with chronic hepatitis C. Braz J Infect Dis. 2019;23(1):45–52. doi:10.1016/j.bjid.2019.02.002
164. Zitelli PMY, Gomes-Gouvea M, Mazo DF, et al. Hepatitis E virus infection increases the risk of diabetes and severity of liver disease in patients with chronic hepatitis C virus infection. Clinics. 2021;76:e3270. doi:10.6061/clinics/2021/e3270
165. Jang ES, Choi GH, Kim YS, et al. Prevalence, incidence, and outcomes of hepatitis E virus coinfection in patients with chronic hepatitis C. Sci Rep. 2023;13(1):13632. doi:10.1038/s41598-023-39019-3
166. Hasa E, Hartmann P, Schnabl B. Liver cirrhosis and immune dysfunction. Int Immunol. 2022;34(9):455–466. doi:10.1093/intimm/dxac030
167. Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385–1396. doi:10.1016/j.jhep.2014.08.010
168. Albillos A, Martin-Mateos R, Van der Merwe S, et al. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19(2):112–134. doi:10.1038/s41575-021-00520-7
169. Kumar Acharya S, Kumar Sharma P, Singh R, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46(3):387–394. doi:10.1016/j.jhep.2006.09.016
170. Fantilli AC, Trinks J, Marciano S, et al. Unexpected high seroprevalence of hepatitis E virus in patients with alcohol-related cirrhosis. PLoS One. 2019;14(10):e0224404. doi:10.1371/journal.pone.0224404
171. Choi JW, Son HJ, Lee SS, et al. Acute hepatitis E virus superinfection increases mortality in patients with cirrhosis. BMC Infect Dis. 2022;22(1):62. doi:10.1186/s12879-022-07050-w
172. Eslam M, Sanyal AJ, George J, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
173. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
174. Zhou XD, Cai J, Targher G, et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc Diabetol. 2022;21(1):270. doi:10.1186/s12933-022-01697-0
175. McManus DP, Dunne DW, Sacko M, et al. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):13. doi:10.1038/s41572-018-0013-8
176. Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi:10.1016/S0140-6736(13)61949-2
177. Sanchez-Marques R, Mas-Coma S, Salas-Coronas J, et al. Research on schistosomiasis in the era of the COVID-19 pandemic: a bibliometric analysis. Int J Environ Res Public Health. 2022;19(13):8051. doi:10.3390/ijerph19138051
178. Carbonell C, Rodriguez-Alonso B, Lopez-Bernus A, et al. Clinical spectrum of schistosomiasis: an update. J Clin Med. 2021;10(23):5521. doi:10.3390/jcm10235521
179. Abdel Rahman MM, Massoud AM, Kamel MA, et al. Risk of hepatitis “E” virus infection among some schistosomiasis patients in Egypt. J Egypt Soc Parasitol. 1995;25(1):115–123.
180. Parana R, Cotrim HP, Cortey-Boennec ML, et al. Prevalence of hepatitis E virus IgG antibodies in patients from a referral unit of liver diseases in Salvador, Bahia, Brazil. Am J Trop Med Hyg. 1997;57(1):60–61. doi:10.4269/ajtmh.1997.57.60
181. Passos-Castilho AM, de Sena A, Domingues AL, et al. Hepatitis E virus seroprevalence among schistosomiasis patients in Northeastern Brazil. Braz J Infect Dis. 2016;20(3):262–266. doi:10.1016/j.bjid.2016.03.001
182. de Araujo LR, Batista AD, Coelho MR, et al. Seroprevalence of hepatitis E virus in patients with chronic liver disease. Braz J Microbiol. 2023. doi:10.1007/s42770-023-01197-7
183. Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, Phase 3 trial. Lancet. 2010;376(9744):895–902. doi:10.1016/S0140-6736(10)61030-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
