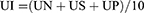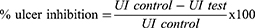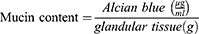Back to Journals » Journal of Experimental Pharmacology » Volume 15
Gastroprotective Activities of Aqueous and 80% Methanol Leaf Extracts of Stephania abyssinica (Quart.-Dill. and A. Rich.) Walp. (Menispermaceae) in Rats
Received 29 August 2023
Accepted for publication 9 November 2023
Published 24 November 2023 Volume 2023:15 Pages 497—512
DOI https://doi.org/10.2147/JEP.S437707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Banchayehu Firehun,1,2 Teshome Nedi2
1School of Pharmacy, Institute of Health, Jimma University, Jimma, Ethiopia; 2Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Teshome Nedi, Email [email protected]
Background: An ethnobotanical study showed that the leaf of Stephania abyssinica (S. abyssinica) is used for the treatment of gastritis, but there is no scientific investigation.
Objective: The aim of this study was to evaluate the gastroprotective activities of both aqueous and 80% methanol leaf extracts of S. abyssinica in experimental rats.
Methods: Decoction and maceration techniques were used to prepare aqueous and 80% methanol leaf extracts, respectively. The extracts were evaluated against pyloric ligation, indomethacin, and ethanol-induced gastric ulcer models at doses of 100, 200, and 400 mg/kg. Negative control received 2% tween 80, while positive controls received 20 mg/kg of omeprazole and 100 μg/kg of misoprostol. Parameters, such as ulcer index, gastric mucin content, gastric juice volume, pH, and free and total acidity were measured.
Results: In the pyloric ligation induced gastric ulcer model, all doses of both extracts significantly reduced the ulcer index and gastric juice volume, while doses of 200 and 400 mg/kg exhibited a significant increment in mucus content and gastric juice pH as well as decrease in free and total acidity as compared to negative control. In indomethacin and ethanol induced gastric ulcer models, pretreatment with both extracts significantly reduced the ulcer index and enhanced gastric mucin content in a dose-dependent manner. Phytochemical screening of both extracts showed the existence of flavonoids, phenols, tannins, saponins, alkaloids, and coumarins with high contents of phenols, flavonoids, and alkaloids in 80% methanol extract.
Conclusion: This study revealed that aqueous and 80% methanol leaf extracts of S. abyssinica possessed remarkable gastroprotective activities against experimentally induced gastric ulcer models, and this possibly justify the traditional use of S. abyssinica leaves to treat gastritis.
Keywords: gastroprotective, Stephania abyssinica, pylorus ligation, indomethacin, ethanol
Introduction
Peptic ulcer disease (PUD) is the most common gastrointestinal tract (GIT) disorder, affecting millions of people around the world,1 with an estimated incidence of 10–19% in the general population.2 It is an acid-related GIT injury that usually affects the stomach and/or proximal duodenum and causes a mucosal breach that extends to the submucosa.3 A continuous exposure of the gastric mucosa to potentially aggressive agents such as gastric acid and pepsin, bile acids, food ingredients, Helicobacter pylori infection, and drugs like non-steroidal anti- inflammatory drugs (NSAIDs) has been linked to the pathogenesis of peptic ulcers.4,5 Abdominal pain, bloating, burning sensation, nausea, vomiting, anorexia, hematemesis, and melena are some of the clinical symptoms,6 while bleeding, perforation, penetration, and gastric outlet obstruction are all life-threatening complications of PUD.7
The prevalence, recurring characteristics, and potentially serious complications of PUD cause significant morbidity, mortality, and economic loss.8,9 In addition, the majority of medications used for the management of PUD such as histamine 2 receptor antagonists, proton pump inhibitors, prostaglandins analogs, antacids, and antimicrobials have drug–drug interaction, incidence of relapse and resistance, as well as serious side effects such as bone fractures, nutritional deficiencies, enteric infections, impotence, and gynecomastia.10–12 These call for the screening of alternative sources for the development of safe, effective, and affordable gastroprotective agents.13,14 Thus, herbal medicines are becoming a viable alternative treatment to available synthetic drugs for the management of peptic ulcers, possibly due to their lower cost, perceived effectiveness, and availability, as well as the fact that they have few or no adverse effects.15–17
Stephania abyssinica (Quart.-Dill. and A. Rich.) Walp. (S. abyssinica) is a climbing shrub belonging to the family Menispermaceae containing around 65 genera and 350 species.18 It is found throughout tropical Africa, including Ethiopia, and grows on the ground or over shrubs in full sunlight and relatively high humidity, primarily on the edges of forests and distributed areas adjacent to roads. The leaves of S. abyssinica are ovate to broadly oval, rounded at the base, obtuse or subacute at the apex and glabrous with 8–10 basal nerves.19 In Ethiopia, it is known by the vernacular names ‘Etse eyesus’ or “ye ayit hareg” in Amharic and “Kalaalaa” in Afan Oromo, and it is widely utilized in traditional medicine. The leaves of the plant have been used for the treatment of various disorders, including rabies,20 abdominal pain and kuruba.21 In addition, fresh leaves decocted with water are used to treat gastritis22,23 and snake bites.22 The juice of leaves mixed with butter is given for babies’ sickness, and the juice of leaf and stem is also taken orally for stomach ache and headache.24 The roots are also used for the management of wound,25 stomach ache, and retained placenta,26 and the whole part of plant is used to treat common cold.27
Some traditional uses of S. abyssinica have been supported scientifically for its medicinal activities, including hepatoprotective,28 antimalarial,29 antihypertensive,30 analgesic and anti-inflammatory,31 antidiarrheal and antispasmodic,32 antineoplastic,33 wound healing,34 repellent activity against malaria vector Anopheles arabiensis,35 and nutritional value.36 Despite the claims of S. abyssinica leaves to treat gastritis, there is no report in the literature regarding its gastroprotective activity. Therefore, the aim of this study was to evaluate the gastroprotective activities of aqueous and 80% methanol leaf extracts of S. abyssinica in experimentally induced gastric ulcer models.
Materials and Methods
Drugs, Chemicals, and Reagents
Indomethacin (Leben Laboratories Pvt. Ltd, India), Omeprazole (Kopran Limited, India), Misoprostol (Naari Pharma Private Limited, India), Ketamine Hydrochloride USP (Neon Laboratories Limited, India), Diazepam (Cenexisas Fontenay-sous-Bois, France), distilled water (Department of Pharmaceutics and Social Pharmacy, Addis Ababa University and Kilitchestro biotech PLC, Oromia, Ethiopia), Methanol (Blulux laboratories, India), Sodium hydroxide (Ranchem industry, Turkey), Phenolphthalein (Ranchem industry and trading, India), Methyl orange (Dalul Pharmaceuticals PLC, Ethiopia), Alcian blue 8GX AR (Ozone international, India), Sucrose (Labchemical, India), Sodium acetate (Abron chemicals, India), Magnesium chloride, Diethyl ether, Glacial acetic acid, Sodium nitrite, Aluminum chloride hexahydrate, Chloroform, and Tween 80 (Lobachemie Pvt. Ltd., India), Ferric chloride hexahydrate (Techno Pharmchem Bahadurgarh, India), Sulphuric acid, Atropine, and Bromocresol green (BDH chemicals LTD, England), Hydrochloric acid (DPP laboratory reagent, Ethiopia), Quercetin dihydrate (Sigma Aldrich, Germany), Gallic acid and Folin Ciocalteu (Merck, Germany), Sodium carbonate (Neolab, India) and Ethanol (Favor Trading PLC, Ethiopia) were used in the study. Most of them were purchased from their respective suppliers in Ethiopia, while Alcian blue 8GX AR was purchased from India. All were of analytical grades.
Plant Material
The leaves of S. abyssinica were collected from Ashewa Meda, Burayu, Sheger city administration, Oromia, Ethiopia, on July 22, 2022. The plant specimen was authenticated by a taxonomist Mr Melaku Wondafrash, and a voucher specimen (BF001) was deposited at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Experimental Animals
Healthy Sprague Dawley rats (150–250 g, either sex) were used in the experiment. They were obtained from the Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University. All of them were kept in cages at room temperature with a 12/12 h light/dark cycle and given access to pellet food and water ad libitum. Prior to experiments, they were acclimatized to the laboratory condition for seven days. Moreover, animal handling and care were carried out in accordance with the internationally accepted laboratory animal care and use guidelines,37 and approved by the Ethics Review Committee of the School of Pharmacy, College of Health Sciences, Addis Ababa University with reference number (ERB/SOP/462/14/2022).
Plant Extraction
Fresh leaves of S. abyssinica were thoroughly cleaned using water to remove dirt and then allowed to dry under shade. The dried leaves were coarsely powdered with mortar and pestle. Then, this was subjected for aqueous and 80% methanol extraction.
Preparation of Aqueous Extract
Two hundred grams of coarsely powdered leaves of S. abyssinica were boiled with distilled water (1:10 w/v) for 30 minutes, and then allowed to cool.38 After cooling, the mixture was filtered using a nylon cloth, and the filtrate was then further filtered by Whatman No. 1 filter paper using a pressurized suction filtering system. The procedure was repeated to extract the plant material exhaustively. Filtrates from each procedure were collected, placed in a deep freezer (−20 °C), and dried in a lyophilizer (Alpha 2–4 LD plus, Germany). Finally, the yield of the aqueous extract (AQ) was 29.5 g with a percentage yield of 14.75%, and it was then stored in a refrigerator until use.
Preparation of 80% Methanol Extract
The coarsely powdered leaves of the plant (300 g) were subjected to maceration with 80% methanol (1:10 (w/v)) for three days with intermittent agitation and stirring.29 The solution was filtered with a nylon cloth followed by sterile filter paper (Whatman No.1), and the residue was re-macerated twice using the same procedure. Then, the resulting filtrates were combined and concentrated in a rotary evaporator (HeidolphLaborota 4001, Germany) at 40 °C, and the remaining extract was placed in the oven at 45 °C until dried. A total of 68 g (22.67%) of 80% methanol extract (ME) was obtained, packed in a vial and stored in the refrigerator until use.
Acute Toxicity Study
Acute oral toxicity test for both extracts was conducted according to Organization for Economic Cooperation and Development (OECD) guideline 425.39 The study utilized ten female rats, five for each extract. After the period of fasting, two rats each received 2 g/kg limit test dose of an aqueous and 80% methanol extracts orally, respectively. They were closely observed for any physical or behavioral changes during the initial thirty minutes, with extra focus during the first 4 h. The observation was continued for twenty-four hours, and no deaths were noted. As a result, the remaining eight rats (four for each extract) received the same dosage and were continuously monitored for behavioral, neurological, and physical abnormalities, as well as mortality for the initial 4 h of the first day and then daily for the total of 14 days.
Grouping and Dosing of Animals
Forty-eight rats (either sex, weighing 150–250 g) were used for each model and grouped into 8, each with 6 animals. Group I assigned as negative control (NC) received 10 mL/kg of 2% tween 80 and group II assigned as positive control was treated with standard drugs: omeprazole (20 mg/kg) as anti-secretory agent for pyloric ligation induced gastric ulcer model and misoprostol (100 µg/kg) as cytoprotective agent for indomethacin and ethanol induced gastric ulcer models. Groups III–V were treated with aqueous extract of S. abyssinica at different doses of 100, 200, and 400 mg/kg, respectively, and groups VI–VIII were treated with methanol extract as dose mentioned in aqueous extract. The ME extract and standard drugs were dissolved in 10 mL/kg of 2% tween 80, while AQ extract was dissolved in distilled water. The doses for both extracts were chosen based on the results of an acute oral toxicity study as per OECD guideline, with 10% of the limit test dose serving as the medium dose. Additionally, the doses of indomethacin (40 mg/kg) and ethanol (5 mL/kg) used to induce gastric ulcers, as well as standard drugs used as positive controls (omeprazole and misoprostol) were based on scientific literature and supported by pilot study prior to the actual experiment. Administration was performed orally via oral gavage.
Gastric Ulcer Models
Pylorus Ligation-Induced Gastric Ulcer
Gastric ulcer induction by pylorus ligation was performed using the Shay method reported by Adane et al.40 Animals were fasted for 24 hours while having free access to water and treated as mentioned under grouping and dosing of animals. After one hour of administration (vehicle/standard/extracts), animals were anesthetized with ketamine (50 mg/kg) and diazepam (5 mg/kg) ip and the abdomen was opened by a small midline incision below the xiphoid process. The pyloric part of the stomach was carefully lifted out and ligated to avoid traction to the pylorus or injury to its blood supply. The stomach was replaced with care, and the wall was closed by interrupted sutures using silk and catgut. Following 4 h of pylorus ligation, the rats were sacrificed with an excess anesthetic agent (ketamine). The abdomen was opened, cardiac end of each stomach was ligated to prevent gastric contents loss, the stomach was then dissected, opened, and the contents were emptied into a centrifuge tube. Then, centrifuged and evaluated for total volume, pH, free, and total acidity. The stomach mucosa of each animal was washed with distilled water, labeled, and evaluated for ulcers. Ulcer index, % ulcer inhibition, mucin content, and % increment of mucin were determined.
Indomethacin-Induced Gastric Ulcer
Indomethacin-induced gastric ulcer in rats was performed according to Ahmed et al.41 Rats were fasted for 24 hours prior to the experiment with free access to water and treated as mentioned under grouping and dosing of animals. After 1 h of treatment, 40 mg/kg of indomethacin was administered to rats to induce gastric ulcer. After six hours, animals were sacrificed by overdose of ketamine, and their stomachs were excised and opened along the greater curvature. After that, the stomachs were washed and evaluated for lesions as described above.
Ethanol-Induced Gastric Ulcer
Absolute ethanol (1 mL/200 g) was administered to induce gastric ulcer in rats after a 24 h fast.42 Animals were given a vehicle, standard drug (misoprostol), or extracts one hour before ulcer induction as described in grouping and dosing of animals. They were sacrificed by overdose of ketamine after 60 minutes of ethanol administration, and their stomachs were dissected and opened along the larger curvature and bathed with distilled water to avoid remaining contents on the ulcerated region. Gastric ulceration was assessed by counting ulcers, calculating ulcer index, and determining mucin content as stated above for both models.
Evaluation of Gastroprotective Activity
Macroscopic Evaluation of Stomach
The stomach was opened along the greater curvature and washed using distilled water to remove any stomach contents including blood clots. It was then placed on a corkboard to assess the formation of ulcers in the glandular area of the stomach. The number of ulcers per stomach was counted and the lesions were measured with a ruler. The severity of the ulcers was scored using the following scale (0 to 5):43 almost no ulcers (0), mucosal edema and petechiae (1), 1–5 small ulcers (1–2 mm) (2), >5 small or intermediate ulcers (3–4 mm) (3), ≥2 intermediate or one gross ulcers (>4 mm) (4), perforation (5). Ulcer index and percentage ulcer inhibition were computed using the formula below.44
Where UI = ulcer index, UN = number of ulcers per animal, US = ulcer severity score per animal, UP = percentage of animals with ulcers.
Determination of Gastric Juice Volume and pH
Gastric juice from each stomach was collected and centrifuged at 1000 rpm for 10 minutes. After centrifugation, the supernatant was decanted, and the gastric juice volume and pH were measured with a measuring cylinder and pH meter (370 pH meter Jenway, England), respectively.45 Then, the gastric juice was subjected to free and total acidity determinations.
Determination of Free and Total Acidity
Free and total acidity were determined by titrations with 0.01N sodium hydroxide (NaOH) using methyl orange and phenolphthalein as indicators, respectively.46 An aliquot of gastric juice (1 mL) was pipetted into a beaker and diluted with 1 mL of distilled water. Then, 2 drops of methyl orange were added and titrated until a yellowish orange color noticed. The volume of NaOH added was recorded, which corresponds to free acidity. Similarly, total acidity was determined as well. Two to three drops of phenolphthalein indicator were added, and the mixture was titrated with NaOH until a permanent pink color appeared. The volume of NaOH required was noted, which corresponds to total acidity. Acidity was calculated as mEq/L using the following formula:
Estimation of Gastric Mucus Content
The gastric mucus content was estimated by Corne et al method described by Mekonnen et al.47 After ulcer scoring, the glandular segment of the stomach was removed, weighed, and immersed immediately in 10 mL of 0.1% Alcian blue (prepared in 0.16 M sucrose solution, buffered with 0.05 M sodium acetate at pH of 5.8) for two hours. An excess dye was then removed by two subsequent rinses in 10 mL of 0.25 M sucrose solution at 15 and 45 minutes. The remaining dye-gastric mucus complex was extracted with 10 mL of 0.5 M MgCl2 for two hours with occasional shaking (30-minute intervals). Five milliliters (5 mL) blue extract were mixed and shaken with an equal volume of diethyl ether (5 mL). The resultant emulsion was then centrifuged at 3000 rpm for 15 minutes, and the absorbance of the aqueous layer was measured at 580 nm using a UV-spectrophotometer (Unico UV-2100, USA). The amount of Alcian blue extracted per gram of glandular tissue was determined using the standard curve, which was generated by taking different concentrations of Alcian blue (0.5 1, 2, 3, 4, and 5 µg/mL) (Figure 1), and mucin content was calculated by the following formula.41
 |
Figure 1 Calibration Curve of Alcian blue 8GX. |
Phytochemical Screening
Standard methods were used to determine the major secondary metabolites such as saponins, tannins, terpenoids, flavonoids, glycosides, phenols, alkaloids, sterols, and coumarins.48–51
Quantification of Phytochemical Constituents
Total phenols, flavonoids, and alkaloids contents were quantified as follows.
Total Phenols Content
The total phenol contents (TPC) of aqueous and 80% methanol extracts were estimated by Folin-Ciocalteu method.52 A linear calibration curve (Figure 2A) was plotted using gallic acid at various concentrations of 100, 50, 25, 12.5, 6.25, and 3.125 µg/mL. About 1 mL of each extract (100 µg/mL) was mixed with 0.5 mL of Folin-Ciocalteu reagent (1:10) and incubated for 8 minutes. Then, 2 mL of sodium carbonate (7.5%) was added, and the solutions were mixed well and incubated for 30 min at ambient temperature. The absorbance was then recorded with a UV-spectrophotometer (Unico UV-2100, USA) at 765 nm. The standard (gallic acid) and blank solutions were prepared using the same method. TPC was calculated for each extract and reported as mg of gallic acid equivalents per gram of extract. The procedure was performed in triplicate and the average result was taken.
 |
Figure 2 Calibration Curve for Standard Solutions: (A) Gallic acid (B) Quercetin (C). Atropine. |
Total Flavonoids Content
Estimation of total flavonoids content (TFC) of both AQ and ME extracts was carried out by using aluminum chloride colorimetric method.53 Quercetin was used as standard and serial concentrations of 1, 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL were prepared in methanol to establish the calibration curve (Figure 2B). In each test tube containing the standard (quercetin), 0.3 mL of NaNO2 (5%) was added and incubated for five minutes. Next, 0.3 mL of 10% AlCl3 was added and incubated again for 5 minutes. Following that, 2 mL of 1M NaOH was added, and the mixture was diluted to 10 mL with distilled water. Finally, the mixture was left for 30 minutes at an ambient temperature. The same procedure was repeated for both extracts (1 mg/mL) and blank solutions. The absorbance of standard, extracts (AQ and ME), and blank solutions was measured using a UV spectrophotometer (Unico UV-2100, USA) at 510 nm. The assay was conducted in triplicate, and the average result was recorded. TFC was expressed in terms of mg of quercetin equivalents per gram of extracts.
Total Alkaloids Content
Alkaloid contents of both AQ and ME extracts were estimated using atropine as a standard.54 Atropine was dissolved in methanol (10 mg/10mL) with successive dilutions of 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL. Two milliliters of each extract (1 mg/mL) were dissolved in 2N HCl and filtered. One milliliter of this filtered extract was transferred to separating funnel, washed twice with chloroform (10 mL), and the chloroform extract was discarded. The pH of the solution was neutralized with 0.1 M NaOH. To this, 5 mL of bromocresol green (BCG) solution and 5 mL phosphate buffer were added. This solution was vigorously shaken, extracted with 8 mL of chloroform, and collected in 10 mL test tube. Chloroform was added until the volume reach to the mark. As described for extracts, a set of standard atropine solutions and a blank solution (methanol) were prepared. The absorbance of extracts, atropine and blank solutions was measured at 470 nm using a UV Spectrophotometer (Unico UV-2100, USA). The total alkaloid content was quantified as mg of atropine/g of extracts using a linear calibration curve (Figure 2C). All procedures were carried out in triplicate.
Statistical Analysis
The results of the experiment were analyzed with a Statistical Package for the Social Sciences (SPSS) window version 26 statistical software and expressed as mean ± standard error of the mean (SEM). A one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test was employed to compare differences between groups. At 95% confidence interval, p-value less than 0.05 (p<0.05) was considered as statistically significant. Linear regression was used to determine correlation coefficient (R2) where applicable. The data were presented in tables and figures.
Results
Acute Toxicity Study
The acute oral toxicity study for both aqueous and 80% methanol extracts of the leaves of S. abyssinica did not show any sign of toxicity or mortality at a limit test dose of 2000 mg/kg during the first 24 h as well as the subsequent 14 days of follow-up period.
Effects of Both Extracts of S. abyssinica Leaf in Pylorus Ligation-Induced Gastric Ulcer
Pylorus ligation resulted visible ulcers in the negative control with an ulcer index of 11.20 ± 0.07. Pretreatment of rats with both AQ (R2 = 0.969; p<0.05) and ME (R2 = 0.959; p<0.05) extracts of S. abyssinica resulted a dose-dependent ulcer index reduction. All doses of AQ and ME extracts showed a significant (p<0.001) reduction in ulcer index as compared to negative control. Ulcer index for aqueous extract was found to be 9.03 ± 0.21, 5.55 ± 0.21, and 3.45 ± 0.08 at doses of 100, 200, and 400 mg/kg, respectively. Similarly, the ulcer index for methanol extract was found to be 8.87 ± 0.12, 5.23 ± 0.08, and 3.42 ± 0.05 at the dose indicated for aqueous extract, respectively. Moreover, 200 and 400 mg/kg of both extracts and OMP20 exhibited a significant (p<0.001) ulcer index reduction in comparison to 100 mg/kg of extracts. Regarding mucin content, the doses of 200 mg/kg (p<0.01) and 400 mg/kg (p<0.001) showed a significant increment with percentage increments of 52.42% and 59.20% in AQ, and 53.71% and 60.66% in ME extracts, respectively, as compared to the negative control. There was a dose-dependent stomach mucin content increment in AQ (R2 = 0.955; p<0.05) and ME (R2 = 0.973; p<0.05) extracts. The standard drug OMP20 increased gastric wall mucus content significantly (p<0.001), which is statistically comparable with higher doses (Table 1).
 |
Table 1 Effects of S. abyssinica Leaf Extracts on Ulcer Index and Mucin Content Against Pylorus Ligation-Induced Gastric Ulcer |
Both extracts of S. abyssinica at all doses significantly reduced the gastric juice volume as compared to negative control, and the reduction was highly significant (p<0.001) in higher dose of ME extract (ME400), which is comparable to OMP20 (p<0.001). Both AQ and ME extracts at 200 (p<0.05), and 400 mg/kg (p<0.001), as well as OMP20 (p<0.001) were able to significantly rise the gastric juice pH when compared to the negative control. Furthermore, AQ400 (p<0.01) and ME400 (p<0.001) provided a substantial rise of gastric juice pH compared to AQ100. In comparison to ME100, higher doses of both extracts AQ400 (p < 0.05) and ME400 (p < 0.01) caused a considerable rise in gastric juice pH, and similarly, the standard drug (p < 0.001) caused higher gastric juice pH than both AQ100 and ME100. Administration of both extracts at doses of 200 mg/kg (p<0.05) and 400 mg/kg (p<0.01) resulted in a substantial decrease in free and total acidity when compared to negative control, and the effect of higher doses was comparable with standard drug (p<0.01) (Table 2).
 |
Table 2 Effects of S. abyssinica Leaf Extracts on Gastric Juice Volume, pH, Free and Total Acidity against Pylorus Ligation-Induced Gastric Ulcer |
Effects of Both Extracts of S. abyssinica Leaf in Indomethacin-Induced Gastric Ulcer
As presented in Table 3, indomethacin caused massive gastric ulcers with an ulcer index of 12.25 ± 0.23 in the control group. It was significantly reduced in rats pretreated with AQ and ME extracts of S. abyssinica at doses of 100 (p<0.05), 200, and 400 (p<0.001) mg/kg, and the reduction was dose-dependent with R2 of 0.928; p<0.05 in AQ extract and R2 of 0.977; p<0.01 in ME extract. The higher doses of both extracts have comparable ulcer index reduction with standard drug, M100 µg/kg. Concerning gastric mucin content, AQ200, and ME200 (p<0.01), AQ400, ME400, and M100 (p<0.001) showed significant increment as compared to that of negative control. The percent increment of mucin content for AQ extract was 38.29% and 45.52% at the doses of 200 and 400 mg/kg, respectively, but for ME extract it was 42.24% and 49.51% at the similar doses. ME400 (p<0.05), and M100 (p<0.01) produced a noticeable difference in mucin content when compared to AQ100 and ME100. The mucin content increment by extracts was dose-dependent in AQ (R2 of 0.992; p<0.01) and ME (R2 of 0.988; p<0.05).
 |
Table 3 Effects of S. abyssinica Leaf Extracts on Ulcer Index and Mucin Content Against Indomethacin-Induced Gastric Ulcer |
Effects of Both Extracts of S. abyssinica Leaf in Ethanol-Induced Gastric Ulcer
Table 4 summarizes the data obtained from ethanol-induced gastric ulcer model. Accordingly, oral administration of absolute ethanol (99.9%) exhibited deep or superficial erosions and hemorrhagic streaks as observed in vehicle treated group with an ulcer index of 12.90 ± 0.17, whereas pretreatment with extracts produced a statistically significant (p<0.001) reduction at doses of 200 and 400 mg/kg, with ulcer indexes of 9.33 ± 0.26 and 5.38 ± 0.20 in AQ extract, and ulcer indexes of 9.13 ± 0.19 and 5.31 ± 0.17 in ME extract. An ulcer index reduction was dose-dependent in AQ (R2 of 0.976, p<0.05) and ME (R2 of 0.983, p<0.01) extracts. The ulcer index for the standard drug, M100, was 3.65 ± 0.20. As compared to low dose (100 mg/kg) of extracts, 200 and 400 mg/kg of both extracts and OMP20 showed a significant (p<0.001) reduction in ulcer index. Furthermore, ethanol administration significantly decreased gastric mucin content in vehicle received rats. However, pretreatment with both extracts at doses of 200 (p<0.05) and 400 (p<0.001), as well as standard drug M100 (p<0.001), significantly enhanced the gastric wall mucin content compared to negative control. Those higher dose of extracts also showed a significant (p<0.01) enhancement in gastric mucin content when compared to AQ100 and M100. M100 also increased mucin content much more than AQ100 (p<0.001) and ME100 (p<0.01). Both extracts showed dose-dependent gastric wall mucus content increment in AQ and ME with R2 of 0.911, p<0.05 and R2 of 0.952, p<0.05, respectively.
 |
Table 4 Effects of S. abyssinica Leaf Extracts on Ulcer Index and Mucin Content Against Ethanol-Induced Gastric Ulcer |
Phytochemical Screening and Quantification of Phytochemical Constituents
The phytochemical screening of both AQ and ME extracts of S. abyssinica leaf showed the presence of saponins, phenols, flavonoids, tannins, alkaloids, and coumarins. Moreover, the total phenols, flavonoids, and alkaloids content were quantified. Accordingly, the total phenols content was found to be 146.58 mg GAE/g and 189.00 mg GAE/g in AQ and ME extracts, respectively. Total flavonoids content was found to be 125.88 mg QE/g in AQ and 160.84 mg QE/g in ME extract. In addition, AQ and ME extracts contained 53.90 mg AE/g and 165.09 mg AE/g of total alkaloids, respectively (Table 5).
 |
Table 5 Phytochemical Screening of Both Extracts of S. abyssinica Leaf and Quantification of Total Phenols, Flavonoids, and Alkaloids Contents |
Discussion
In Ethiopia, several medicinal plants have been identified and reported for their use in the treatment of PUD, but only a small number of plants have been scientifically studied for safety and efficacy in animal models.55 Therefore, the current study was conducted to evaluate the safety and efficacy of S. abyssinica leaf extracts claimed for the management of gastritis using animal models.
The acute toxicity study confirmed that both AQ and ME extracts of S. abyssinica leaf did not show any acute toxicity or mortality up to 14 days at a dose of 2000 mg/kg. Thus, ingestion of S. abyssinica leaf for any of its medicinal effects would be supposed to be safe. Furthermore, the data suggested that this plant’s leaf had a wider safety margin in which lethal dose is much more than 2000 mg/kg.
There are several models for evaluating antiulcer drugs.56 The pylorus ligation, indomethacin, and ethanol induced gastric ulcer models were selected to evaluate the gastroprotective effects of both AQ and ME extracts at three doses of 100, 200, and 400 mg/kg on parameters such as ulcer index, mucin content, gastric juice volume, pH, free, and total acidity.
Pylorus ligation is a commonly employed model for assessing the anti-secretory and cytoprotective effects of drugs that reduce gastric aggressive factor secretion and increase mucus production, respectively. Ulcers induced by pyloric ligation are due to accumulation of gastric secretion such as acid and pepsin, resulting to auto-digestion of stomach mucosa.56 In this model, both extracts showed a dose-dependent reduction in ulcer index, and the effect of higher doses was more significant and comparable to a standard drug, omeprazole. In terms of mucin content, both extracts failed to show a significant increase in gastric mucin content at lower dose, but the medium and higher doses exhibited a substantial increase in gastric mucin content compared to the negative control. The effect is more significant with higher doses, probably due to increased concentration of active ingredients. It is interesting to note that the plant seems to have cytoprotective effects.
Accumulation of gastric acid/pepsin is an important aggressive agent for the formation of ulcers in the pyloric ligation model. This was observed in the vehicle administered group of animals, which had increased gastric juice volume and acidity as well as decreased gastric juice pH. However, oral administration of both extracts at all doses exhibited a statistically significant reduction of gastric juice secretion as compared to the negative control. Concerning pH and acidity, a low dose of extracts failed to show a meaningful increase in gastric juice pH and a reduction in free and total acidity, while medium and higher doses showed a remarkable increment in pH and a decrease in acidity when compared to the vehicle received group. This demonstrates that the low dose of extracts might not be sufficient for gastric acid neutralization compared to the middle and higher doses, and the effect offered by the higher dose of extracts was comparable to antiulcer drug omeprazole, which is an irreversible proton pump inhibitor. This suggests that this plant may have potential anti-secretory activity.
As revealed in the phytochemical study, both extracts comprised different phytoconstituents including flavonoids, alkaloids, coumarins, and phenols, which could justify the anti-secretory activity of S. abyssinica. Flavonoids have been well studied for their gastroprotective potential such as anti-secretory, cytoprotective, antioxidant, anti-inflammatory, and antibacterial effects.57,58 Their anti-secretory effect may be due to their antihistaminic properties, which reduce histamine levels while also limiting histamine release from gastric mast cells by inhibition of histidine decarboxylase and inhibiting gastric H+/K+ ATPase, resulting in decreased gastric acid secretion.59–61 Likewise, alkaloids have the same effect via H2-receptor antagonism and anti-cholinergic action.62 Another mechanism by which alkaloids might have anti-secretory effect is mainly by blocking H+/K+-ATPase activity and gastrin secretion.63 In addition, phenolic substances have the capability to inhibit the H+, K+-ATPase action.64 The other phytoconstituents, such as coumarins, may reduce gastric secretion through an anticholinergic mechanism or by interfering with intracellular processes that are related to acid secretion.65
NSAIDs are known to induce gastric ulcers by inhibiting cyclooxygenase, which is important in the biosynthesis of prostaglandins involved in maintaining the integrity of the gastric mucosa,66 and indomethacin is a commonly used NSAID to induce gastric ulcer in the experimental animal.67 In this study, 40 mg/kg of indomethacin was administered for gastric ulcer induction. The model was employed to assess the cytoprotective effects of extracts.
Following ulcer induction with indomethacin, there were visible and noticeable gastric ulcers in the negative control as confirmed by a higher ulcer index and a low mucin content. However, extract pretreated groups showed a dose-dependent decrease in ulcer index and an increase in mucin content compared to ulcerated group. The fact that indomethacin administration makes the stomach more susceptible to mucosal damage may be due to the suppression of endogenous prostaglandin (PG) synthesis.68 Nevertheless, exogenous PG, particularly the E series, protect gastrointestinal mucosa from injury induced by a broad range of irritants.69 This was observed in the positive control group of rats treated with misoprostol. In accordance with this, the gastroprotective activity offered by extracts of AQ400 and ME400 was comparable to standard medication (M100) pretreated animals. Hence, cytoprotection could be a plausible mechanism for S. abyssinica’s gastric ulcer preventive activity.
The gastroprotective effect of the plant might be due to its effects in enhancing the mucus and HCO3 secretion, stimulation of PG production, and gastric blood flow. These may be arbitrated through phenols, flavonoids, tannins, and saponins found in the extracts. Phenolic substances boost PGE synthesis by acting as co-substrates in the peroxidase reaction.70 Similarly, flavonoids are highly gastroprotective, probably by increasing gastric PG content, gastric mucus secretion and gastric blood flow.59 Saponins may contribute to antiulcer activity by promoting PG and mucus formation in the stomach mucosa.9,71 Moreover, tannins have astringent properties and react with the tissue proteins with which they come into contact. They produce a tannin-protein complex that protects the stomach mucosa by enhancing resistance to chemical and mechanical harm or irritation.72
Ethanol-induced gastric ulcer model was also used to assess the gastroprotective activity of both extracts of S. abyssinica. It is a typical experimental model for the preclinical assessment of agents with potential anti-ulcer activity73 which also have cytoprotective and/or antioxidant activities. Ethanol easily penetrates the stomach mucosa due to its capacity to dissolve the protective mucus and expose the mucosa to the hydrolytic and proteolytic effects of HCl and pepsin.56 Additionally, ethanol administration causes necrotic gastric injury and inflammatory cell infiltration, as well as a decrease in the secretion of gastric mucus, bicarbonate, and nitric oxide.42,74
In this ethanol induced gastric ulcer model, ulcer index was found to be high in the control group. However, pretreatment with medium and higher dosages of extracts dramatically reduced ulcer index compared to the negative control. In addition, the determination of gastroprotective mucin content in this model revealed a significant decrease in the vehicle administered group. Meanwhile, a considerable increment of gastric mucin was observed in rats treated with misoprostol and extracts at the doses of 200 and 400 mg/kg. This indicates that the antiulcer effect of S. abyssinica is mediated partly by preserving gastric mucus.
Furthermore, the cytoprotective effect of this plant could be related to its antioxidant activities. Indeed, exposure of gastric mucosa to ethanol induces oxidative stress by increasing malondialdehyde (MDA) production and reducing antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) formation.74,75 Conversely, a report from Zhou et al74 showed that pretreatment of rats with gallic acid, a phenolic compound, revealed a significant rise of SOD, GSH, and CAT levels, which suggests its antioxidant potential against ethanol induced gastric ulcer. The antioxidant properties of phenols are mainly related to their ability to scavenge free radicals due to the presence of a hydroxyl functional group. Thus, phenolic content of plants might contribute its pharmacological activity due to its antioxidant action.76 Another bioactive component, flavonoids, also exert an antioxidant effect by scavenging free radicals, protecting and activating antioxidant enzymes, inhibiting oxidizing enzymes, and preventing lipid peroxidation.58,77 In line with these, S. abyssinica may have antioxidant activities due to the presence of high content of flavonoids and phenols in both AQ and ME extracts.
Gastric mucus serves a crucial role in the defensive mechanism against stomach ulcers. It forms adherent layer that covers the gastric mucosa, preserves a near neutral pH at the mucosal surface, and provides a physical barrier against luminal pepsin.78 The weakening of mucus barrier is directly responsible for gastric mucosa damage.79 The quality and quantity of gastric mucus secretion are the crucial indicators of mucosal defense barrier status against undesirable effects of acid and pepsin.80 In the present study, as mentioned above, gastric mucin content was determined in the three models. The pylorus ligation, indomethacin, and ethanol significantly decreased gastric mucus/mucin production, but administration of each extract significantly increased the amount of mucus secretion, specifically, at the middle and higher doses, indicating increased mucin production might be one of the important factor in the gastroprotective activity of S. abyssinica.
The therapeutic effect of medicinal plants is mainly associated with the presence of phytochemical constituents, such as flavonoids, tannins, phenols, terpenoids, saponins, and alkaloids.15 As a result, the phytochemical screening test used to identify those constituents revealed the existence of saponins, flavonoids, phenols, alkaloids, tannins, and coumarins in both AQ and ME extracts. In addition, quantitative analysis of both extracts showed the presence of high contents of total phenols, flavonoids, and alkaloids. ME extract, on the other hand, had more flavonoids, phenols, and alkaloids than AQ extract. This may explain, at least in part, the slight difference in activity between the two extracts.
It has been well documented that gastroprotective activity in rodents predicts gastroprotective activities in human beings. Consequently, the gastroprotective activities of both extracts in humans could be associated with antisecretory and cytoprotective properties. In addition, antioxidant properties of extracts might be related due to the presence potent antioxidant compounds including phenols and flavonoids. The gastroprotective effect offered by the higher dose of extracts was comparable to those antiulcer drugs omeprazole and misoprostol. Thus, S. abyssinica might be used as the alternative source of antiulcer drug, but need further study.
As limitations, this study did not conduct long-term safety and efficacy and ulcer healing effect of plant extracts. Additionally, the isolation of active compound and exact molecular mechanism of plant extracts were not determined.
Conclusion
The aqueous and 80% methanol leaf extracts of S. abyssinica possessed remarkable gastroprotective activities in pylorus ligation, indomethacin, and ethanol-induced gastric ulcer models. The findings of this study provide a scientific support for the traditional use of S. abyssinica leaf to treat gastritis. Further studies are recommended to conduct the ulcer healing effect and to determine the precise mechanism of plant extracts.
Data Sharing Statement
The data sets used during the present work are included in this article.
Ethics Approval
The protocol was approved by the Ethics Review Committee of the School of Pharmacy, College of Health Sciences, Addis Ababa University with reference number of ERB/SOP/462/14/2022.
Acknowledgments
We would like to thank Addis Ababa University for material and financial support. This research was done with funding obtained from Addis Ababa University for MSc dissertation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Singh AK, Singh SK, Singh PP, et al. Biotechnological aspects of plants metabolites in the treatment of ulcer: a new prospective. Biotechnol Rep. 2018;18:e00256. doi:10.1016/j.btre.2018.e00256
2. Alsinnari YM, Alqarni MS, Attar M, et al. Risk factors for recurrence of peptic ulcer disease: a retrospective study in tertiary care referral center. Cureus. 2022;14(2):e22001. doi:10.7759/cureus.22001
3. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390(10094):613–624. doi:10.1016/S0140-6736(16)32404-7
4. Raju D, Ilango K, Chitra V, Ashish K. Evaluation of Anti-ulcer activity of methanolic extract of Terminalia chebula fruits in experimental rats. J Pharm Sci Res. 2009;1(3):101.
5. Magaji R, Tanko Y, Magaji G. The role of an aggressive factor in peptic ulcer disease (pud). AfrJ Infect Dis. 2008;2(2):80–84.
6. Dunlap JJ, Patterson S. Peptic ulcer disease. Gastroenterol Nurs. 2019;42(5):451–454. doi:10.1097/SGA.0000000000000478
7. Milosavljevic T, Kostic-Milosavljevic M, Jovanovic I, Krstic M. Complications of peptic ulcer disease. Dig Dis. 2011;29(5):491–493. doi:10.1159/000331517
8. Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. 2022;22(1):58. doi:10.1186/s12876-022-02130-2
9. Yismaw YE, Abdelwuhab M, Ambikar DB, Yismaw AE, Derebe D, Melkam W. Phytochemical and antiulcer activity screening of seed extract of cordia africana lam (Boraginaceae) in pyloric ligated rats. Clin Pharmacol. 2020;12:67–73. doi:10.2147/CPAA.S245672
10. Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol. 2009;2(3):295–314. doi:10.1586/ecp.09.8
11. Gupta M, Kapoor B, Gupta R, Singh N. Plants and phytochemicals for treatment of peptic ulcer: an overview. S Afr J Bot. 2021;138:105–114.
12. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors-evidence and plausibility.Int. J Mol Sci. 2019;20(20):5203. doi:10.3390/ijms20205203
13. Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M. Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med. 2019;8(2):179. doi:10.3390/jcm8020179
14. Chinnasamy V, Subramaniyan V, Chandiran S, et al. Antiarthritic activity of achyranthes aspera on formaldehyde - induced arthritis in rats. Open Access Maced J Med Sci. 2019;7(17):2709–2714. doi:10.3889/oamjms.2019.559
15. Sharifi-Rad M, Fokou PVT, Sharopov F, et al. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23(7):1751. doi:10.3390/molecules23071751
16. Akinwumi IA, Sonibare MA. Use of medicinal plants for the treatment of gastric ulcer in some parts of Southwestern Nigeria. Afr J Pharm Pharmacol. 2019;13(15):223–235.
17. Fuloria S, Subramaniyan V, Karupiah S, et al. A comprehensive review on source, types, effects, nanotechnology, detection, and therapeutic management of reactive carbonyl species associated with various chronic diseases. Antioxidants. 2020;9(11):1075. doi:10.3390/antiox9111075
18. Semwal DK, Badoni R, Semwal R, Kothiyal SK, Singh GJ, Rawat U. The genus Stephania (Menispermaceae): chemical and pharmacological perspectives. J Ethnopharmacol. 2010;132(2):369–383. doi:10.1016/j.jep.2010.08.047
19. De Wet H, Struwig M, Van Wyk BE. Taxonomic notes on the genus Stephania (Menispermaceae) in Southern Africa. S Afr J Bot. 2014;95:146–151.
20. Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010;132(1):75–85. doi:10.1016/j.jep.2010.07.046
21. Birhanu Z, Endale A, Shewamene Z. An ethnomedicinal investigation of plants used by traditional healers of Gondar town, North-Western Ethiopia. J Med Plants Stud. 2015;3(2):36–43.
22. Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte Town, East Wellega (Oromia), Ethiopia. J Herbs Spices Med Plants. 2012;18(1):34–57. doi:10.1080/10496475.2011.645188
23. Regassa T. Vascular plant diversity and ethnobotanical study of medicinal and wild edible plants in Jibat, Gedo and Chilimo Forests, West Shewa Zone of Oromia Region, Ethiopia [Doctoral dissertation]. Addis Ababa Universty; 2016.
24. Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):1–12. doi:10.1186/1746-4269-3-12
25. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525. doi:10.1016/j.jep.2006.10.011
26. Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124(3):513–521. doi:10.1016/j.jep.2009.05.009
27. Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka district, east Welega zone of Oromia regional state, West Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):18. doi:10.1186/1746-4269-9-68
28. Washe A, Fanta D. Hepatoprotective activities and bioactive constituents of Stephania abyssinica. Br JPharm Res. 2016;10(6):1–9. doi:10.9734/BJPR/2016/24840
29. Zemene M, Geta M, Huluka SA, Birru EM. Antimalarial activity of the 80% methanol leaf extract and solvent fractions of stephania abyssinica (Dill. & A. Rich.) Walp. Against plasmodium berghei infection in mice. Ethio Pharm J. 2021;36(2):109–120. doi:10.4314/epj.v36i2.4
30. Fodem C, Nguelefack-Mbuyo EP, Ndjenda Ii MK, Kamanyi A, Nguelefack TB. Vasorelaxant-mediated antihypertensive effect of the leaf aqueous extract from stephania abyssinica (Dillon & A. Rich) Walp (Menispermaceae) in rat. Biomed Res Int. 2021;2021:4730341. doi:10.1155/2021/4730341
31. Leyikun T. Evaluation of the analgesic and anti-inflammatory activities of 80% methanol leaf extract of stephania abyssinica (Quart.-Dill. & A. Rich.) Walp.(Menispermaceae) in Mice [MSc Thesis]. Addis Ababa University; 2015.
32. Deneke T. Antidiarrheal and Antispasmodic Activities of Stephania Abyssinica (Minspermaseae) Used in Ethiopian Traditional Medicine [MSc Thesis]. Addis Ababa University; 2010.
33. Abebe W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. Eur J Medplants. 2016;14(4):1–16. doi:10.9734/EJMP/2016/25670
34. Yiblet TG, Tsegaw A, Ahmed N, Dagnew SB, Tadesse TY, Kifle ZD. Evaluation of wound healing activity of 80% methanol root crude extract and solvent fractions of Stephania abyssinica (Dill. & A. Rich.) Walp. (Menispermaceae) in Mice. J Exp Pharmacol. 2022;14:255–273. doi:10.2147/JEP.S364282
35. Jemberie W, Tadie A, Enyew A, Debebe A, Raja N. Repellent activity of plant essential oil extracts against malaria vector Anopheles arabiensis Patton (Diptera: Culicidae). Entomon. 2016;41(2):91–98.
36. Ndemanou Feudjio Y, Raymond Simplice M, Monique Odette K, Jules-Roge RK, Christopher T. Effect of stephania abyssinica leaves powder supplementation on some biochemical parameters and immune-nutritional status of malnourish early-weaned rats. J Food Stab. 2020;3(1):67–80. doi:10.36400/J.Food.Stab.3.1.2020-0018
37. National Research Council. Guide for the Care and Use of Laboratory Animals.
38. Kaneria M, Kanani B, Chanda S. Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants. Asian Pac J Trop Biomed. 2012;2(3):195–202. doi:10.1016/S2221-1691(12)60041-0
39. Guideline O. Acute oral Toxicity: up and down procedure. Guide Test Chemicals. 2008;425:1–2.
40. Adane H, Atnafie SA, Kifle ZD, Ambikar D. Evaluation of in vivo antiulcer activity of hydro-methanol extract and solvent fractions of the stem bark of ficus thonningii (Moraceae) on rodent models. Biomed Res Int. 2021;2021:6685395. doi:10.1155/2021/6685395
41. Ahmed O, Nedi T, Yimer EM. Evaluation of anti-gastric ulcer activity of aqueous and 80% methanol leaf extracts of Urtica simensis in rats. Metabol Open. 2022;14:100172. doi:10.1016/j.metop.2022.100172
42. Sistani Karampour N, Arzi A, Rezaie A, Pashmforoosh M, Kordi F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina. 2019;55(3):64. doi:10.3390/medicina55030064
43. Bhattamisra SK, Yean Yan VL, Koh Lee C, et al. Protective activity of geraniol against acetic acid and Helicobacter pylori- induced gastric ulcers in rats. J TraditComplement Med. 2019;9(3):206–214. doi:10.1016/j.jtcme.2018.05.001
44. Sisay Zewdu W, Jemere Aragaw T. Evaluation of the anti-ulcer activity of hydromethanolic crude extract and solvent fractions of the root of Rumex nepalensis in rats. J Exp Pharmacol. 2020;12:325–337. doi:10.2147/JEP.S258586
45. Gundamaraju R, Maheedhar K, Hwi KK. Exploiting the phenomenal anti-ulcerogenic potential of Talinum portulacifolium ethanolic extract whole plant on Albino Rats: the therapeutic potential of Chinese Herb-mǎ chǐ xiàn kē (Portulacaceae). Pharmacognosy Res. 2014;6(3):227–233. doi:10.4103/0974-8490.132600
46. Zakaria ZA, Balan T, Mamat SS, Mohtarrudin N, Kek TL, Salleh MZ. Mechanisms of gastroprotection of methanol extract of Melastoma malabathricum leaves. BMC Complement Altern Med. 2015;15:135. doi:10.1186/s12906-015-0638-z
47. Mekonnen AN, Asrade Atnafie S, Wahab Atta MA. Evaluation of antiulcer activity of 80% methanol extract and solvent fractions of the root of croton macrostachyus hocsht: ex Del. (Euphorbiaceae) in rodents. Evid Based Complement Alternat Med. 2020;2020:2809270. doi:10.1155/2020/2809270
48. Santhi K, Sengottuvel R. Qualitative and quantitative phytochemical analysis of moringa concanensis Nimmo. Int J Curr Microbiol Appl Sci. 2016;5(1):633–640. doi:10.20546/ijcmas.2016.501.064
49. Sheel R, Nisha K, Kumar J. Preliminary phytochemical screening of methanolic extract of Clerodendron infortunatum. IOSR J Appl Chem. 2014;7(1):10–13. doi:10.9790/5736-07121013
50. Yamuna P, Abirami P, Sharmila M, Vijayashalini P. Qualitative phytochemical analysis of Gomphrena globosa Linn. and Gomphrena decumbens Jacq. Int J Biol Res. 2017;2(3):20–22.
51. Ali S, Khan MR, Sajid M, Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med. 2018;18(1):43. doi:10.1186/s12906-018-2114-z
52. Maria R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi:10.1016/j.jksus.2017.03.009
53. Durai M, Balamuniappan G, Anandalakshmi R, Geetha S, Kumar NS. Qualitative and quantitative analysis of phytochemicals in crude extract of big-leaf mahogany (Swietenia macrophylla King). Int J Herb Med. 2016;4(6):88–91.
54. Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc Sci Life. 2012;31(4):198–201. doi:10.4103/0257-7941.107361
55. Tadesse TY, Zeleke MM, Dagnew SB. Review of ethnobotanical and ethnopharmacological evidence of some Ethiopian medicinal plants traditionally used for peptic ulcer disease treatment. Clin ExpGastroenterol. 2022;15:171–187. doi:10.2147/CEG.S384395
56. Adinortey MB, Ansah C, Galyuon I, Nyarko A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013;2013:1–12. doi:10.1155/2013/796405
57. Perveen S, Fawzy GA, Al-Taweel AM, et al. Antiulcer activity of different extracts of Anvillea garcinii and isolation of two new secondary metabolites. OpenChem. 2018;16(1):437–445.
58. Zhang W, Lian Y, Li Q, et al. Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules. 2020;25(20):4626. doi:10.3390/molecules25204626
59. Mota KS, Dias GE, Pinto ME, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14(3):979–1012. doi:10.3390/molecules14030979
60. Pyloric PU. screening for anti ulcer activity of convolvulus pluricaulis using pyloric ligation method in Wister rats. Int J Pharm Sci Res. 2015;6(1):89–99.
61. Borrelli F, Izzo AA. The plant kingdom as a source of anti‐ulcer remedies. Phytother Res. 2000;14(8):581–591. doi:10.1002/1099-1573(200012)14:8<581::AID-PTR776>3.0.CO;2-S
62. Mosaddik MA, Alam KMF. The anti‐ulcerogenic effect of an alkaloidal fraction from mikania cordata on diclofenac sodium‐induced gastrointestinal lesions in rats. J Pharm Pharmacol. 2000;52(9):1157–1162. doi:10.1211/0022357001774930
63. Zhang S-L, Li H, He X, et al. Alkaloids from Mahonia bealei posses anti-H+/K+-ATPase and anti-gastrin effects on pyloric ligation-induced gastric ulcer in rats. Phytomedicine. 2014;21(11):1356–1363. doi:10.1016/j.phymed.2014.07.007
64. Siddaraju MN, Dharmesh SM. Inhibition of gastric H+, K+‐ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res. 2007;51(3):324–332. doi:10.1002/mnfr.200600202
65. Bighetti AE, Antonio MA, Kohn LK, et al. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from mikania laevigata Schultz bip. Phytomedicine. 2005;12(1–2):72–77. doi:10.1016/j.phymed.2003.09.006
66. Takeuchi K. Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol. 2012;18(18):2147–2160. doi:10.3748/wjg.v18.i18.2147
67. Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–234. doi:10.1007/s10753-009-9176-5
68. Inas Z, Hala A, Gehan HH. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 2011;8(3):433–445.
69. Brzozowski T, Konturek P, Konturek S, Brzozowska I, Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol. 2005;56(5):33–55.
70. Bansal VK, Goel RK. Gastroprotective effect of Acacia nilotica young seedless pod extract: role of polyphenolic constituents. Asian Pac J Trop Med. 2012;5(7):523–528. doi:10.1016/S1995-7645(12)60092-3
71. Andargie Y, Sisay W, Molla M, Norahun A, Singh P. Evaluation of the antiulcer activity of methanolic extract and solvent fractions of the leaves of Calpurnia aurea (Ait.) Benth. (Fabaceae) in Rats. EvidBased Complement Alternat Med. 2022;2022:4199284. doi:10.1155/2022/4199284
72. De Jesus NZT, Falcao HS, Gomes IF, et al. Tannins, peptic ulcers and related mechanisms. Int J Mol Sci. 2012;13(3):3203–3228. doi:10.3390/ijms13033203
73. Arab HH, Salama SA, Omar HA, Arafa El SA, Maghrabi IA. Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One. 2015;10(3):e0122417. doi:10.1371/journal.pone.0122417
74. Zhou D, Yang Q, Tian T, et al. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother. 2020;126:110075. doi:10.1016/j.biopha.2020.110075
75. Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One. 2013;8(5):e64751. doi:10.1371/journal.pone.0064751
76. Tosun M, Ercisli S, Sengul M, et al. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res. 2009;42(2):175–181. doi:10.4067/S0716-97602009000200005
77. Cheng YT, Wu CH, Ho CY, Yen GC. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem. 2013;24(2):475–483. doi:10.1016/j.jnutbio.2012.01.010
78. Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288(1):C1–19. doi:10.1152/ajpcell.00102.2004
79. Sidahmed HMA, Vadivelu J, Loke MF, et al. Anti-ulcerogenic activity of dentatin from clausena excavata Burm.f. against ethanol-induced gastric ulcer in rats: possible role of mucus and anti-oxidant effect. Phytomedicine. 2019;55(1):31–39. doi:10.1016/j.phymed.2018.06.036
80. Zakaria ZA, Balan T, Suppaiah V, Ahmad S, Jamaludin F. Mechanism(s) of action involved in the gastroprotective activity of Muntingia calabura. J Ethnopharmacol. 2014;151(3):1184–1193. doi:10.1016/j.jep.2013.12.045
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.