Back to Journals » Journal of Healthcare Leadership » Volume 15
Fostering Excellence in Knee Arthroplasty: Developing Optimal Patient Care Pathways and Inspiring Knowledge Transfer of Advanced Surgical Techniques
Authors Migliorini F , Feierabend M , Hofmann UK
Received 12 September 2023
Accepted for publication 13 November 2023
Published 21 November 2023 Volume 2023:15 Pages 327—338
DOI https://doi.org/10.2147/JHL.S383916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Pavani Rangachari
Filippo Migliorini,1,2 Martina Feierabend,1 Ulf Krister Hofmann1
1Department of Orthopaedic, Trauma, and Reconstructive Surgery, RWTH University Medical Centre, Aachen, 52074, Germany; 2Department of Orthopedics and Trauma Surgery, Academic Hospital of Bolzano (SABES-ASDAA), Teaching Hospital of Paracelsus Medical University, 39100 Bolzano, Italy
Correspondence: Filippo Migliorini, Department of Orthopaedic, Trauma, and Reconstructive Surgery, RWTH University Hospital, Pauwelsstraße 30, Aachen, 52074, Germany, Tel +49 0241 80-35529, Email [email protected]
Abstract: Osteoarthritis of the knee is common. Early sports trauma or cartilage defects are risk factors for osteoarthritis. If conservative treatment fails, partial or total joint replacement is often performed. A joint replacement aims to restore physiological biomechanics and the quality of life of affected patients. Total knee arthroplasty is one of the most performed surgeries in musculoskeletal medicine. Several developments have taken place over the last decades that have truly altered the way we look at knee arthroplasty today. Some of the fascinating aspects will be presented and discussed in the present narrative review.
Keywords: knee, arthroplasty, replacement, implant, materials, robotic, artificial intelligence
Introduction
Osteoarthritis of the knee is common.1–3 Especially after early sports traumata with persisting cartilage injuries, OA can also manifest itself much earlier.4–7 If conservative treatment fails, partial or total knee arthroplasty is often performed.8–10 A joint arthroplasty aims to generate again a stable joint with smoothly articulating surfaces to free the patient of joint pain, to correct prior deformities and thus restore the disrupted function of the degenerated joint.11–13 Total knee arthroplasty (TKA) is one of the most commonly performed surgeries in the musculoskeletal field,14–16 with 173,625 cases in 2020 in Germany alone.17,18 Several developments have taken place over the last decades that have truly altered the way we look at TKA today.19–21 Some of the fascinating aspects will be presented and discussed in the present study.
Types of Implants
Historically, TKA meant knee resurfacing of the total knee with a bicondylar femoral component, a total tibial plateau requiring the resection of the anterior cruciate ligament and potentially also the posterior cruciate ligament and, if necessary, the use of a retropatellar polyethylene implant.22,23 Hinged knee replacements with stemmed fixation were the backbone of revision surgery. Over the years, this portfolio has widened enormously.
The concept of a unicompartmental knee replacement emerged, however, in the 1940s.24,25 Due to its highly variable clinical results total implantation rates in comparison to total knee replacement were marginal for a long time.26,27 In recent years, better implants and implant positioning techniques and a better understanding of selecting the right indications for this prosthesis design have led to a strong increase in its use.28,29 For example, the incidence of unicompartmental TKA in the US ranged from 6570 implants in 1998 to 44,990 in 2005.30 Even small implants addressing focal cartilage defects have been placed on the market such as the UniCAP (Arthrosurface Inc, Franklin, MA, USA).31 Difficulties in achieving the required precision when implanting these devices led to high failure rates in the first postoperative years,32 with a cumulative revision rate of over 50% after seven years in the Australian Joint Registry.33 With the advent of patient-specific instrumentation based on preoperative 3D planning, patient-specific implants for focal cartilage lesions have been introduced (Episurf, EPISEALER, 4 Medical s.r.l., Cinisello Balsamo, Italy), the first clinical results of which appear promising with a revision rate of 2.5% after 2 years.34,35 Even isolated replacements of the trochlea have been introduced. While data are also scarce in the literature, revision rates appear to be critically high.36–38
Also in the other direction, technical developments have opened new options for surgeons. The traditional portfolio of a cruciate retaining (CR, retaining the posterior cruciate ligament), a posterior stabilised (PS, replacing also the posterior cruciate ligament) and a rotational hinge and/or total hinge design have now been augmented by various PE configurations that are intended to bridge the gap between still completely intact collateral ligaments and collateral ligament deficiency. The so-called condylar constrained coupling offers an intermediary stability that can support collateral ligaments that are still present, but not strong enough anymore to completely secure functional knee stability.39 This solution has the advantage that knee kinematics can largely be preserved while at the same time, maximum stress shear on the bone-implant interface is reduced due to the lack of a rigid coupling. Condylar-constrained coupling is usually achieved by high conformity of the PE with the femoral component using a deep dish and/or a central intercondylar strong post resembling the one from a PS design, however much more prominent. This vast portfolio of implant designs now offers the possibility to more specifically address a patient problem and it reduces the likelihood of overtreating a problem.
Fixation
Traditionally bicondylar TKA was fixed at the implant-bone interface using bone cement (polymethyl-methacrylate).40–42 For the fixation of hinged prostheses, stems were required that could either be cementless or cemented. While for decades this concept has shaped the arthroplasty landscape, powerful new ideas and implants have emerged over the last few years.
Concerning primary arthroplasty, early attempts to repeat the success of cementless hip arthroplasty were met with high rates of early failure due to implant loosening.43–45 Improvements in implant design and manufacturing technology have, however, provided surgeons with roughened or even three-dimensional porous surfaces which are usually based on titanium or tantalum alloys. These surfaces already provide substantial primary stability in cancellous bone46 thus limiting micromotion before osteointegration.47–50 Their surfaces also strongly promote osseointegration through the bone on- or ingrowth.51 One reason why the topic of cementless implant fixation in TKA is also more and more relevant is the fact, that a cemented interface never osseointegrates and thus also does not allow bone-remodelling according to functional requirements placed on it. Especially in the elderly osteoporotic bone with receding trabeculae and impaired bone metabolism, may lead to high failure rates in the long run. With more TKAs being implanted also in patients aged 65 years or younger,52 the mid-term stability of the bone-implant interface needs to be increased due to the higher functional demands placed on the implant.47 Indeed, data from the British National Joint Registry demonstrate that younger patients undergoing cemented primary TKA have a higher risk of revision when compared to their older counterparts.53 In a systematic meta-analysis Prasad et al, 2020, found that based on the present literature, there is no difference in implant survival between cemented and cementless primary TKAs.47 The authors nevertheless argue in favour of cementless fixation due to a lack of cement-induced third body war and better preservation of bone stock in case of revision. It needs to be pointed out, however, that the latest 3D surfaces have not been on the market long enough to allow for a reliable long-term follow-up analysis concerning implant survival. If cementless fixation eventually outperforms cemented techniques in TKA time will have to tell.
In revision arthroplasty, Morgan-Jones et al, 2015, proposed a three different zones concept for revision-implant fixation.54 Zone 1 being the epiphysis and zone 3 being the diaphysis had long been used to fix a combination of a tibial plateau with a long stem. As the geometrical centres of the epiphysis and diaphysis are often not in line, an offset adapter may be required to match the positional requirements of the tibial plateau especially an uncemented stem.54 Epiphyseal defects had to be filled with augments consisting of metal, polyethylene (PE) or polymethyl-methacrylate (PMMA) cement to ensure a solid fixation in zone 1. The new aspect highlighted by Morgan-Jones et al was a possible fixation in zone 2, the metaphysis. The authors stipulated, that a fixation in 2 of the 3 zones would already be sufficient for good implant fixation. With the metaphysis being directly adjacent to the epiphysis, an offset correction is obsolete. When using an implant to achieve metaphyseal stability, only short stems are required to fixate the implant within the metaphyseal stabilisation. This offers the additional advantage of bone preservation and it also avoids problems with sagittal anterior translation due to the femoral bow of the diaphysis.54 While good simple cemented fixation by filling in the metaphysis is technically challenging, new implants have been brought on the market that strongly facilitate this surgical technique. These sleeves or cones can be placed press-fit into the metaphysis, thus also closing potential local defects in the bone circumference. In addition to their press-fit, their 3D surface structure usually allows fast bone ingrowth for permanent secondary stability. Once in place, the epiphyseal implant component with its distally cementless or cemented shaft is cemented onto and into the metaphyseal implant. Respecting this concept could finally break with the dogma that in knee revision surgery stems would always get longer. The popularity of this technique is clearly on the rise,55 the long-term success will still need to be evaluated. The first results are, however, highly promising.56,57
Bearing
The traditional bearing surface is that of a cobalt–chrome (CoCr-) femur against a PE insert mounted on the tibial plateau. Especially concerning polyethylene, powerful improvements could be achieved over the past decades. Denoted by the formula (C2H4)n, n can differ depending on molecular size.58 Sir John Charnley introduced the long-chained ultra-high molecular weight (UHMW) PE in the 1960s in hip arthroplasty,59 the success of which also paved the way for its use in TKA.60 As a further development to improve the wear resistance of the still soft UHMWPE, gamma or electron beams were applied to break the carbon-hydrogen chains to create highly cross-linked UHMWPE (HXLPE).61,62 This HXLPE was, however, prone to fatigue-crack propagation due to its lower elasticity.63 These XHLPE, therefore, still have to be post-treated: To reduce the concentration of free radicals created by radiation, annealing of these ends is performed at a temperature just below melting, which adequately preserves the mechanic characteristics.62 The latest development is the creation of 2nd generation HXLPE where other methods are used to eliminate free radicals. The most popular manufacturing process is the addition of the antioxidant vitamin E, which makes heat annealing after radiation obsolete and thus even better preserves the mechanic properties of the PE.61
The use of a cobalt-chrome alloy for the femur has always been highly successful from a biomechanic point of view. Yet, a relevant number of patients show a metal hypersensitivity to one of its components. Most patients with such a hypersensitivity react to nickel, while cobalt and chromium are less frequent. In dermal testing, some patients also show reactions to beryllium and less frequently to tantalum, titanium and vanadium.64 Ions released by corrosion of metallic wear debris may play a critical role and metal particles can be found in the soft tissues surrounding the implant.65 According to the 2016 Australian Arthroplasty Register, approximately 2% of revision TKAs are attributed to “metal-related pathology”.66 Moreover, one of the main reasons for knee revision surgery remains aseptic loosening67 which is often attributed to particle wear.68,69
For these main reasons, new materials were developed that would reduce particle wear and allow the successful treatment of patients hypersensitive to metal ions.70 For TKA, two main approaches have been chosen: the manufacturing of full-thickness ceramic implants and the creation of ceramic surfaces.48–50
To date, the company PETER BREHM GmbH (Weisendorf, Germany) is the only manufacturer worldwide that offers an all-ceramic primary knee endoprosthesis (BPK-S INTEGRATION Ceramic). Smith & Nephew (London, England) additionally provides a prosthesis (LEGION) where, even if not the tibial component, at least the femoral component is made of solid ceramic consisting of Zirconium and Niob. Although not reported yet in the literature, one major concern regarding a monolithic ceramic component is that the geometry of standard femoral or tibial implants is often not ideal for ceramics and the material still has a high brittleness and low tensile strength when compared to metal components. One strategy to counter this phenomenon has been modifying the countersurface rather than using a monolithic ceramic component.71
The so-called coating of the femoral component is usually performed with combinations of Zirconium, Niob, Titanium and/or Nitrid. Indeed, ceramic surfaces in knee resurfacing achieve a drastic reduction in wear rates,72 not only of metal ions but also of PE71 which is attributed to the lower coefficient of friction of ceramic surfaces. In addition, ceramic wear particles are less (bio)reactive compared to polyethylene particles or metal ions.71 A recent review article analysing 14 studies reporting on ceramic total knee replacements mid- and long-term survival and knee function were comparable to their metallic femoral counterparts.73 If expectations concerning increased long-term survival based on reduced aseptic loosening will be met future studies and registry data still have to tell.
Alignment
From its first description by Insall in 198574 but at least after Leo Whiteside publicised “Ligament Balancing of in Total Knee Arthroplasty” in 200475 it was considered standard of care to perform a mechanical alignment that would be “biomechanically friendly”. Respecting the mechanical axis in the frontal plane of a straightened leg with the Mikulicz line going centrally and perpendicularly through the joint reduces the risk of accelerated PE wear, early implant loosening at the implant-bone interface and patella instability. The axial rotational alignment of the femoral component was suggested to be externally rotated by 3° about the posterior condylar line to compensate for the then corrected 3° varus orientation of the proximal tibia joint-line found in the majority of patients.76 The remaining functional restrictions of the knee would then be balanced by an elaborate technique of soft tissue release. Being technically demanding, such a soft tissue release also always bore the risk of leading to post-operative either imbalanced or unstable conditions.
Although this standardised technique worked solidly for the majority of cases, historically up to 20% of patients complained about persistent knee pain and functional limitations even in cases of a radiologically well-aligned implant. Even modern literature still reports a figure of about 10% of patients suffering from chronic pain after TKA.77 Accompanying improvements in wear resistance of newer implant materials and better fixation techniques at the implant-bone interface, this purely mechanical approach has been increasingly challenged over the past few years.78 Alternative alignment strategies proposed try to be more anatomical and patient-specific, better-respecting ligament tension and geometry of the articulating partner’s.79 To improve at least flexion gap balancing, axial femoral rotation was adjusted to the ligament tension in flexion.80 As the usual tibiofemoral joint line is usually oriented 3° ascending from medial to lateral (called valgus orientation),81 a technique was proposed respecting this orientation thus called anatomical alignment.82 While theoretically already sound in the 1980s, this concept found no widespread use at the time. With the imprecision of traditional extramedullary tibial instrumentation being around 2° (plus outliers up to 5°)83 a targeted 3° varus position easily ended in an excessive malposition of the tibial implant with devastating effects.84
A modern way in conventional TKA that can be considered a compromise between mechanical alignment and anatomical alignment is the so-called adjusted mechanical alignment. In this case, the tibial plateau is targeted perpendicular to the mechanical axis of the tibia. At the same time, the femoral component is implanted in a way, that the preoperatively existing axis deviation is undercorrected.85,86 For an overview of different present alignment concepts see Figure 1.
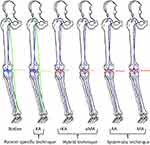 |
Figure 1 Different techniques for alignment in total knee arthroplasty. Abbreviations: KA, kinematic alignment; rKA, restricted kinematic alignment; aMA, adjusted mechanical alignment; AA, anatomic alignment; MA, mechanical alignment. Notes: Reprinted from Riviere C, Lazic S, Boughton O, Wiart Y, Villet L, Cobb J. Current concepts for aligning knee implants: patient-specific or systematic? EFORT Open Rev. 2018;3(1):1–6. Creative Commons.79. |
Modern robotic-assisted surgery techniques promise a highly precise implant positioning.83,87 Such precision but especially reliability in placing implant components has opened the window for a radically different implantation concept: kinematic alignment.88 In this concept, the patient’s anatomy and ligamentous needs are maximum respected.
Short-term clinical results of this technique seem to be excellent89,90 but there is some concern as to the long-term results due to the fear of mechanical failure of the PE or the implant-bone interface. It is also conceivable, that patients with a strong deformity preoperatively, might not benefit from restoring this anatomy at all.91
Especially among surgeons who still have to rely on conventional navigation, restricted kinematic alignment has become quite popular over recent years. First described in 2017,92 literature on that topic has strongly increased in the last three years. While generally trying a kinematic alignment, implantation follows a strict algorithm that sets clear safety limits concerning maximum implant deviation from a mechanical alignment. It is thus a hybrid technique between mechanical alignment and kinematic alignment.92 The exact safety limits are still a matter of debate. They have to take into account the safety limits of the articulating surfaces which depend on implant size, shape and used materials and their fixation technique to the bone (eg bone quality, cementation, use of stems). Due to greater variations in implant positioning with conventional techniques, safety margins have to be put narrower than when performing this technique computer navigated. In both cases, thorough preoperative planning and intraoperative evaluation are essential. Data regarding the clinical benefit of using such a technique are still scarce. The first results suggest a slight benefit when compared to mechanical alignment.93,94
Robotics and Navigation
Robotic-assisted TKA refers to the use of devices which assist the surgeon. Both image-dependent and imageless systems are available in robotic-assisted TKA. Image-dependent robotic-assisted TKA generate a virtual three-dimensional computational model of bony anatomy from pre-operative computed tomography (CT) or magnetic resonance imaging (MRI). On the other hand, imageless systems create bony surfaces and joint kinematics intra-operatively, sparring imaging costs and radiation risk.95,96 Robotic systems can be further divided into passive, semi-active, and active. Passive systems are based on navigation or computer-assisted technology which provide three-dimensional guidance via an overhead monitor but do not perform bone cuts.97–99 Semi-active systems incorporate safety constraints (eg haptic feedback) which guide the surgeon during specific tasks and advise the same if the action might result in a change in the pre-determined computational plan. On the other hand, active systems perform the bony resections independently under supervision without real-time guidance.97,100 An overview of the available robotic-assisted TKA is shown in Table 1. An important limitation of the current robotic systems is their implant specificity (closed platforms), which limits the implant choice tailored to the patient’s anatomy.101 Moreover, their cost efficacy is also debated and long-term studies are required. Despite the progress in robotic systems, whether these improve long-term outcomes and implant survivorship is still unknown.
 |
Table 1 Overview of the Available Robotic-Assisted TKA |
Artificial Intelligence
The founding father of the discipline of artificial intelligence (AI) described it as making a machine behave in ways that would be called intelligent if a human were so behaving.102 The term “artificial” contrasts it with the natural intelligence of humans or animals. AI is a cross-disciplinary approach to understanding, modelling and creating intelligence of various forms.103 It encompasses several subdisciplines such as machine learning, neural networks including deep learning, natural language processing, robotics or machine perception. The central thesis behind AI is that many aspects of learning and intelligence depend crucially on the careful probabilistic representation of uncertainty. A machine can use such AI models to make predictions about future data, and decisions that are rational given these predictions.104 AI is based on data-driven algorithms which simulate the processes of human intelligence through the creation and application of algorithms integrated into a dynamic computing environment to learn, reflect, reason, consider, and perform cognitive functions.105–107 To date, AI is still largely limited to task-specific. Numerous tools using AI are, however, available. Concerning their use in knee surgery, several applications are already in use or on the horizon for introduction. Predictive modelling, for example, estimates prognostications for specified outputs in complex systems that are otherwise difficult to grasp due to the high number of variables. Briefly, predictive models provide information to ascertain the association between variables (eg, patient characteristics) and events (eg, surgical complications) useful for decision-making, surgical planning, and postoperative rehabilitation.107–109 Computer vision has also seen powerful improvements over the past few years. Computer vision based on AI can now also extract information from images and videos. AI allows to automate of the process of image quality,110 it can screen for implant loosening on radiographs,111 it can measure knee alignment,112 and postoperatively evaluate joint replacement.113
Advances in computer vision have also accelerated developments in augmented reality. Augmented reality interacts with the human senses and provides additional visual, auditory, haptic, somatosensory or olfactory input.114 Many AR technologies have been shown to lower provider cognitive burden and reduce operative time and radiation exposure while improving surgical precision in pre-clinical cadaveric and sawbones models.115
In TKA, AI eases the choice for patient-specific implants and optimises the decision-making algorithm and resource allocation. AI-based tools can also give estimates and help with pre-TKA-planning, prediction of disease progression, and estimation of treatment outcomes.116 However, despite several published studies on the application of AI in TKA, there are still several limitations to its application in daily clinical practice. Semi-active and active robotic-assisted TKA are recent AI-based tools (Table 1). Data collected consider the three-dimensional bone surface, and computational algorithms on implant alignment and soft tissue balancing. These data are integrated to plan the surgery adjusting it to the surgeon’s and patient’s needs and goals. Semi-active and active robotic-assisted TKA indicate (semi-active) or perform (active) bone resections and suggest implant positioning with real-time haptic feedback. Doing so, implantation precision could be strongly increased.117–119 Whether this precision actually translates into a clinical benefit still needs to be evaluated.119
Outpatient Regimes
The average hospitalisation following TKA reduced from 9 days to 3–4 days in the past decades.120,121 This reduction is associated with the introduction of fast-track regimes, early mobilisation, improve in anaesthetic modalities, multimodal pain control strategies, perioperative care, and physiotherapy protocols.121–123 As a result, the concept of outpatient TKA has been introduced.124,125 Outpatient TKA requires a careful patient section and several morbidity scoring systems have been elaborated to select eligible patients.126,127 Local infiltration analgesia (LIA) and nerve blocks are associated with improvement in pain control and short recovery.128,129 The mini-subvastus exposure in TKA was associated with a shorter recovery than the traditional medial parapatellar approach.13,130,131 Older age, obesity, physical and cognitive impairment, social support at home, cardiovascular, renal and/ or hepatic disorders, and diabetes mellitus represent relative contraindications for outpatient TKA.132–135 A previous successful TKA at the contralateral side has been positively associated with an outpatient procedure.136 However, despite these ameliorations, outpatient TKA remains debated.137 In a recent meta-analysis of 159,219 TKAs, outpatient TKA did not evidence significant advantages to longer hospitalisation regimes at approximately six months of follow-up.138 There are no clear recommendations and eligibility criteria, and the current evidence is poor and controversial.120,121,127,139–146 Possible confounders are the different healthcare systems and heterogeneous admission and rehabilitation protocols. Further investigations are strongly recommended to establish consensus on the eligibility and education criteria for outpatient TKA.
Data Sharing Statement
No dataset has been generated during the current study.
Ethical Approval
This study complies with ethical standards.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors declare that they have no competing interests for this article.
References
1. Booij MJ, van Royen BJ, Nolte PA, Twisk JWR, Harlaar J, van den Noort JC. Total knee arthroplasty improves gait adaptability in osteoarthritis patients; a pilot study. J Orthop. 2022;34:304–309. doi:10.1016/j.jor.2022.08.003
2. Singh S, Jindal D, Khanna R. Can serum MMP-3 diagnose early knee osteoarthritis? J Orthop. 2023;38:42–46. doi:10.1016/j.jor.2023.02.014
3. Voskuilen R, Boonen B, Tilman P, Schotanus M, Most J. Demographics are no clinically relevant predictors of patient-reported knee osteoarthritis symptoms - Comprehensive multivariate analysis. J Orthop. 2023;35:85–92. doi:10.1016/j.jor.2022.11.002
4. Via GG, Brueggeman DA, Lyons JG, Frommeyer TC, Froehle AW, Krishnamurthy AB. Funding has no effect on studies evaluating viscosupplementation for knee osteoarthritis: a systematic review of bibliometrics and conflicts of interest. J Orthop. 2023;39:18–29. doi:10.1016/j.jor.2023.03.015
5. Bhatia A, Bhatia S. The short-to-midterm outcomes of geniculate artery embolization for mild-to-moderate osteoarthritis of the knee: a systematic review. J Orthop. 2023;39:30–41. doi:10.1016/j.jor.2023.03.009
6. Black AL, Clark AL. Sexual dimorphism in knee osteoarthritis: biomechanical variances and biological influences. J Orthop. 2022;32:104–108. doi:10.1016/j.jor.2022.05.016
7. Migliorini F, Vecchio G, Pintore A, Oliva F, Maffulli N. The influence of athletes’ age in the onset of osteoarthritis: a systematic review. Sports Med Arthrosc Rev. 2022;1(2):97–101. doi:10.1097/JSA.0000000000000345
8. Saraf A, Hussain A, Bishnoi S, Habib H, Garg A. Serial intraarticular injections of growth factor concentrate in knee osteoarthritis: a placebo controlled randomized study. J Orthop. 2023;37:46–52. doi:10.1016/j.jor.2023.02.006
9. Mortensen JF, Mongelard KBG, Radev DI, et al. MRi of the knee compared to specialized radiography for measurements of articular cartilage height in knees with osteoarthritis. J Orthop. 2021;25:191–198. doi:10.1016/j.jor.2021.05.014
10. Hernandez-Hermoso JA, Nescolarde L, Yanez-Siller F, Calle-Garcia J, Garcia-Perdomo D, Perez-Andres R. Combined femoral and tibial component total knee arthroplasty device rotation measurement is reliable and predicts clinical outcome. J Orthop Traumatol. 2023;24(1):40. doi:10.1186/s10195-023-00718-2
11. Migliorini F, Eschweiler J, Tingart M, R B. Posterior-stabilized versus cruciate-retained implants for total knee arthroplasty: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol. 2019;29(4):937–946. doi:10.1007/s00590-019-02370-1
12. Migliorini F, Aretini P, Driessen A, et al. Better outcomes after mini-subvastus approach for primary total knee arthroplasty: a Bayesian network meta-analysis. Eur J Orthop Surg Traumatol. 2020;30:979–992. doi:10.1007/s00590-020-02648-9
13. Migliorini F, Aretini P, Driessen A, et al. Correction to: better outcomes after mini-subvastus approach for primary total knee arthroplasty: a Bayesian network meta-analysis. Eur J Orthop Surg Traumatol. 2021;31(6):1259. doi:10.1007/s00590-021-03026-9
14. Makela KT, Peltola M, Sund R, Malmivaara A, Hakkinen U, Remes V. Regional and hospital variance in performance of total Hip and knee replacements: a national population-based study. Ann Med. 2011;43:S31–38.
15. Peltola M, Juntunen M, Hakkinen U, Rosenqvist G, Seppala TT, Sund R. A methodological approach for register-based evaluation of cost and outcomes in health care. Ann Med. 2011;43:S4–13.
16. Matassi F, Pettinari F, Frascona F, Innocenti M, Civinini R. Coronal alignment in total knee arthroplasty: a review. J Orthop Traumatol. 2023;24(1):24. doi:10.1186/s10195-023-00702-w
17. Bundesamt S. Die 20 häufigsten Operationen insgesamt (OPS 5): vollstationär behandelte Patientinnen und Patienten in Krankenhäusern 2020 [The most 20 surgical interventions (OPS 5): inpatient treated patients in hospital in 2020]; 2020. Available from: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/drg-operationen-insgesamt.html;jsessionid=057935135CAAA80FFCBBC2BDF33DA20E.live741?view=main[Print].
18. Migliorini F, Weber CD, Bell A, et al. Bacterial pathogens and in-hospital mortality in revision surgery for periprosthetic joint infection of the Hip and knee: analysis of 346 patients. Eur J Med Res. 2023;28(1):177. doi:10.1186/s40001-023-01138-y
19. Pai SN, Jeyaraman M, Maffuli N, Jeyaraman N, Migliorini F, Gupta A. Evidence-based informed consent form for total knee arthroplasty. J Orthop Surg Res. 2023;18(1):156. doi:10.1186/s13018-023-03647-2
20. D’Ambrosi R, Valli F, Nuara A, et al. No difference in mobile and fixed bearing partial knee arthroplasty in octogenarians: a clinical trial. Eur J Orthop Surg Traumatol. 2023;2023:1–8.
21. Hofmann UK, Hildebrand F, Mederake M, Migliorini F. Telemedicine in orthopaedics and trauma surgery during the first year of COVID pandemic: a systematic review. BMC Musculoskelet Disord. 2023;24(1):101. doi:10.1186/s12891-023-06194-3
22. Wang R, Wang Z, Gu Y, et al. Total knee arthroplasty in patients with haemophilic arthropathy is effective and safe according to the outcomes at a mid-term follow-up. J Orthop Traumatol. 2022;23(1):31. doi:10.1186/s10195-022-00648-5
23. Xi G, Wang HH, Li H, Zhang M. Short-term outcomes of Oxford unicompartmental knee arthroplasty with coronal subluxation of the knee: a retrospective case-control study. J Orthop Traumatol. 2022;23(1):6. doi:10.1186/s10195-022-00626-x
24. Bruni D, Iacono F, Akkawi I, Gagliardi M, Zaffagnini S, Marcacci M. Unicompartmental knee replacement: a historical overview. Joints. 2013;1(2):45–47.
25. Campbell WC. Interposition of vit allium plates in arthroplasties of the knee: preliminary report. Am J Surg. 1940;47(3):639–641. doi:10.1016/S0002-9610(40)90176-3
26. Migliorini F, Tingart M, Niewiera M, Rath B, Eschweiler J. Unicompartmental versus total knee arthroplasty for knee osteoarthritis. Eur J Orthop Surg Traumatol. 2019;29(4):947–955. doi:10.1007/s00590-018-2358-9
27. Migliorini F, Maffulli N, Cuozzo F, et al. Mobile bearing versus fixed bearing for unicompartmental arthroplasty in monocompartmental osteoarthritis of the knee: a meta-analysis. J Clin Med. 2022;11(10):2837. doi:10.3390/jcm11102837
28. Migliorini F, Driessen A, Oliva F, Maffulli GD, Tingart M, Maffulli N. Better outcomes and reduced failures for arthroplasty over osteotomy for advanced compartmental knee osteoarthritis in patients older than 50 years. J Orthop Surg Res. 2020;15(1):545. doi:10.1186/s13018-020-02079-6
29. Cao Z, Guo J, Li Q, Wu J, Li Y. Comparison of efficacy and safety of different tourniquet applications in total knee arthroplasty: a network meta-analysis of randomized controlled trials. Ann Med. 2021;53(1):1816–1826. doi:10.1080/07853890.2021.1991588
30. Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplasty. 2008;23(3):408–412. doi:10.1016/j.arth.2007.04.012
31. Miniaci A. UniCAP as an alternative for unicompartmental arthritis. Clin Sports Med. 2014;33(1):57–65. doi:10.1016/j.csm.2013.06.002
32. Malahias MA, Chytas D, Thorey F. The clinical outcome of the different HemiCAP and UniCAP knee implants: a systematic and comprehensive review. Orthop Rev (Pavia). 2018;10(2):7531. doi:10.4081/or.2018.7531
33. Elbardesy H, Nagle M, Simmons L, Harty J. The partial femoral condyle focal resurfacing (HemiCAP-UniCAP) for treatment of full-thickness cartilage defects, systematic review and meta-analysis. Acta Orthop Belg. 2021;87(1):93–102. doi:10.52628/87.1.12
34. Al-Bayati M, Martinez-Carranza N, Roberts D, Hogstrom M, Stalman A. Good subjective outcome and low risk of revision surgery with a novel customized metal implant for focal femoral chondral lesions at a follow-up after a minimum of 5 years. Arch Orthop Trauma Surg. 2022;142(10):2887–2892. doi:10.1007/s00402-021-04160-z
35. Holz J, Spalding T, Boutefnouchet T, et al. Patient-specific metal implants for focal chondral and osteochondral lesions in the knee; excellent clinical results at 2 years. Knee Surg Sports Traumatol Arthrosc. 2021;29(9):2899–2910. doi:10.1007/s00167-020-06289-7
36. Elbardesy H, McLeod A, Gul R, Harty J. Midterm results of modern patellofemoral arthroplasty versus total knee arthroplasty for isolated patellofemoral arthritis: systematic review and meta-analysis of comparative studies. Arch Orthop Trauma Surg. 2022;142(5):851–859. doi:10.1007/s00402-021-03882-4
37. Johnson DS, Turner PG. Replacement for patellofemoral arthritis. Knee. 2019;26(6):1166–1170. doi:10.1016/j.knee.2019.10.016
38. Charalambous CP, Abiddin Z, Mills SP, Rogers S, Sutton P, Parkinson R. The low contact stress patellofemoral replacement: high early failure rate. J Bone Joint Surg Br. 2011;93(4):484–489. doi:10.1302/0301-620X.93B4.25899
39. Kader D, Caplan N, Kokkinakis M, Refaie R, Loughead J. Constrained condylar knee systems: a review of five commonly used brands. J Arthrosc Jt Surg. 2015;2(1):23–32. doi:10.1016/j.jajs.2014.12.001
40. Chen C, Li R. Cementless versus cemented total knee arthroplasty in young patients: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2019;14(1):262. doi:10.1186/s13018-019-1293-8
41. Liu Y, Zeng Y, Wu Y, Li M, Xie H, Shen B. A comprehensive comparison between cementless and cemented fixation in the total knee arthroplasty: an updated systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):176. doi:10.1186/s13018-021-02299-4
42. Chen C, Shi Y, Wu Z, et al. Long-term effects of cemented and cementless fixations of total knee arthroplasty: a meta-analysis and systematic review of randomized controlled trials. J Orthop Surg Res. 2021;16(1):590. doi:10.1186/s13018-021-02762-2
43. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kisslinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378–381. doi:10.1007/s002640100294
44. Regner L, Carlsson L, Karrholm J, Herberts P. Clinical and radiologic survivorship of cementless tibial components fixed with finned polyethylene pegs. J Arthroplasty. 1997;12(7):751–758. doi:10.1016/S0883-5403(97)90004-8
45. Matassi F, Carulli C, Civinini R, Innocenti M. Cemented versus cementless fixation in total knee arthroplasty. Joints. 2013;1(3):121–125. doi:10.11138/jts/2013.1.3.121
46. Aprato A, Risitano S, Sabatini L, Giachino M, Agati G, Masse A. Cementless total knee arthroplasty. Ann Transl Med. 2016;4(7):129. doi:10.21037/atm.2016.01.34
47. Prasad AK, Tan JHS, Bedair HS, Dawson-Bowling S, Hanna SA. Cemented vs. cementless fixation in primary total knee arthroplasty: a systematic review and meta-analysis. EFORT Open Rev. 2020;5(11):793–798. doi:10.1302/2058-5241.5.200030
48. Migliorini F, Eschweiler J, Maffulli N, Hildebrand F, Schenker H. Functionalised high-performance oxide ceramics with bone morphogenic protein 2 (BMP-2) induced ossification: an in vivo study. Life. 2022;12:6.
49. Migliorini F, Marsilio E, Oliva F, Eschweiler J, Hildebrand F, Maffulli N. Chondral injuries in patients with recurrent patellar dislocation: a systematic review. J Orthop Surg Res. 2022;17(1):63. doi:10.1186/s13018-022-02911-1
50. Migliorini F, Schenker H, Betsch M, et al. Silica coated high performance oxide ceramics promote greater ossification than titanium implants: an in vivo study. J Orthop Surg Res. 2023;18(1):31. doi:10.1186/s13018-022-03494-7
51. Dalury DF. Cementless total knee arthroplasty: current concepts review. Bone Joint J. 2016;98(7):867–873. doi:10.1302/0301-620X.98B7.37367
52. Losina E, Katz JN. Total knee arthroplasty on the rise in younger patients: are we sure that past performance will guarantee future success? Arthritis Rheum. 2012;64(2):339–341. doi:10.1002/art.33371
53. National Joint Registry for England W. Northern Ireland and the Isle of Man. 16th Annual Report; 2019. Available from: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/.
54. Morgan-Jones R, Oussedik SI, Graichen H, Haddad FS. Zonal fixation in revision total knee arthroplasty. Bone Joint J. 2015;97(2):147–149. doi:10.1302/0301-620X.97B2.34144
55. Carender CN, An Q, Tetreault MW, De A, Brown TS, Bedard NA. Use of cementless metaphyseal fixation in revision total knee arthroplasty in the United States. J Arthroplasty. 2022;37(3):554–558. doi:10.1016/j.arth.2021.11.027
56. Denehy KM, Abhari S, Krebs VE, et al. Metaphyseal fixation using highly porous cones in revision total knee arthroplasty: minimum two year follow up study. J Arthroplasty. 2019;34(10):2439–2443. doi:10.1016/j.arth.2019.03.045
57. Heidenreich MJ, Lanting BA, McCalden RW, et al. Survivorship of metaphyseal cones and sleeves in revision total knee arthroplasty. J Arthroplasty. 2022;37(6S):S263–S269. doi:10.1016/j.arth.2022.02.074
58. Chakrabarty G, Vashishtha M, Leeder D. Polyethylene in knee arthroplasty: a review. J Clin Orthop Trauma. 2015;6(2):108–112. doi:10.1016/j.jcot.2015.01.096
59. Charnley J. Arthroplasty of the Hip. A new operation. Lancet. 1961;1(7187):1129–1132. doi:10.1016/S0140-6736(61)92063-3
60. R C. History of Total Knee Replacement. The Southern Orthopaedic Association and Data Trace Publishing Company; 2006.
61. Jacobs CA, Christensen CP, Greenwald AS, McKellop H. Clinical performance of highly cross-linked polyethylenes in total Hip arthroplasty. J Bone Joint Surg Am. 2007;89(12):2779–2786. doi:10.2106/JBJS.G.00043
62. Ries MD, Pruitt L. Effect of cross-linking on the microstructure and mechanical properties of ultra-high molecular weight polyethylene. Clin Orthop Relat Res. 2005;440:149–156. doi:10.1097/01.blo.0000185310.59202.e5
63. Bradford L, Baker D, Ries MD, Pruitt LA. Fatigue crack propagation resistance of highly crosslinked polyethylene. Clin Orthop Relat Res. 2004;429:68–72. doi:10.1097/01.blo.0000150124.34906.34
64. Frigerio E, Pigatto PD, Guzzi G, Altomare G. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64(5):273–279. doi:10.1111/j.1600-0536.2011.01886.x
65. Solarino G, Piconi C, De Santis V, Piazzolla A, Moretti B. ceramic total knee arthroplasty: ready to go? Joints. 2017;5(4):224–228. doi:10.1055/s-0037-1607428
66. Association AO. National joint replacement registry - annual report; 2016. Available from: https://aoanjrr.sahmri.com/annual-reports-2016.
67. Grimberg A, Jansson V, Lützner J, Melsheimer O, Morlock M, Steinbrück A. The German Arthroplasty Registry (EPRD): annual report 2021; 2021.
68. Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77(2):177–197. doi:10.1080/17453670610045902
69. Jones MD, Buckle CL. How does aseptic loosening occur and how can we prevent it? Orthop Trauma. 2020;34(3):146–152. doi:10.1016/j.mporth.2020.03.008
70. Migliorini F, Pilone M, Bell A, Merfort R, Giorgino R, Maffulli N. Serum cobalt and chromium concentration following total Hip arthroplasty: a Bayesian network meta-analysis. Sci Rep. 2023;13(1):6918. doi:10.1038/s41598-023-34177-w
71. Bal BS, Garino J, Ries M, Oonishi H. Ceramic bearings in total knee arthroplasty. J Knee Surg. 2010;20(4):261–270. doi:10.1055/s-0030-1248055
72. Rahaman MN, Yao A, Bal BS, Garino JP, Ries MD. Ceramics for prosthetic hip and knee joint replacement. J Am Ceram Soc. 2007;90:1965–1988. doi:10.1111/j.1551-2916.2007.01725.x
73. Xiang S, Zhao Y, Li Z, Feng B, Weng X. Clinical outcomes of ceramic femoral prosthesis in total knee arthroplasty: a systematic review. J Orthop Surg Res. 2019;14(1):57. doi:10.1186/s13018-019-1090-4
74. Insall JN, Binazzi R, Soudry M, Mestriner LA. Total knee arthroplasty. Clin Orthop Relat Res. 1985;192:13–22. doi:10.1097/00003086-198501000-00003
75. Whiteside L. Ligament Balancing in Total Knee Arthroplasty - an Instructional Manual. Berlin Heidelber New York: Springer-Verlag; 2004.
76. Whiteside LA. Soft tissue balancing: the knee. J Arthroplasty. 2002;17(4 Suppl 1):23–27. doi:10.1054/arth.2002.33264
77. DeFrance MJ, Scuderi GR. Are 20% of patients actually dissatisfied following total knee arthroplasty? A systematic review of the literature. J Arthroplasty. 2023;38(3):594–599. doi:10.1016/j.arth.2022.10.011
78. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89–95. doi:10.1007/s12178-014-9218-y
79. Riviere C, Lazic S, Boughton O, Wiart Y, Villet L, Cobb J. Current concepts for aligning knee implants: patient-specific or systematic? EFORT Open Rev. 2018;3(1):1–6. doi:10.1302/2058-5241.3.170021
80. Daines BK, Dennis DA. Gap balancing vs. measured resection technique in total knee arthroplasty. Clin Orthop Surg. 2014;6(1):1–8. doi:10.4055/cios.2014.6.1.1
81. Bellemans J, Colyn W, Vandenneucker H, Victor J. The chitranjan ranawat award: is neutral mechanical alignment normal for all patients? The concept of constitutional varus. Clin Orthop Relat Res. 2012;470(1):45–53. doi:10.1007/s11999-011-1936-5
82. Hungerford DS, Kenna RV, Krackow KA. The porous-coated anatomic total knee. Orthop Clin North Am. 1982;13(1):103–122. doi:10.1016/S0030-5898(20)30270-4
83. Zahn RK, Graef F, Conrad JL, Renner L, Perka C, Hommel H. Accuracy of tibial positioning in the frontal plane: a prospective study comparing conventional and innovative techniques in total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(6):793–800. doi:10.1007/s00402-020-03389-4
84. Srivastava A, Lee GY, Steklov N, Colwell CW, Ezzet KA, D’Lima DD. Effect of tibial component varus on wear in total knee arthroplasty. Knee. 2012;19(5):560–563. doi:10.1016/j.knee.2011.11.003
85. Ranawat AS, Ranawat CS, Elkus M, Rasquinha VJ, Rossi R, Babhulkar S. Total knee arthroplasty for severe valgus deformity. J Bone Joint Surg Am. 2005;87(Pt 2):271–284. doi:10.2106/JBJS.E.00308
86. Vanlommel L, Vanlommel J, Claes S, Bellemans J. Slight undercorrection following total knee arthroplasty results in superior clinical outcomes in varus knees. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2325–2330. doi:10.1007/s00167-013-2481-4
87. Deckey DG, Rosenow CS, Verhey JT, et al. Robotic-assisted total knee arthroplasty improves accuracy and precision compared to conventional techniques. Bone Joint J. 2021;103-B(6):74–80. doi:10.1302/0301-620X.103B6.BJJ-2020-2003.R1
88. Howell SM, Hull ML. Kinematic alignment in total knee arthroplasty. In: Insall and Scott Surgery of the Knee.
89. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117–2124. doi:10.1007/s00264-015-2743-5
90. Howell SM, Roth JD, Hull ML. Kinematic alignment in total knee arthroplasty. definition, history, principle, surgical technique, and results of an alignment option for TKA. Arthropaedia. 2014;1:44–53.
91. Riviere C, Iranpour F, Auvinet E, et al. Alignment options for total knee arthroplasty: a systematic review. Orthop Traumatol Surg Res. 2017;103(7):1047–1056. doi:10.1016/j.otsr.2017.07.010
92. Almaawi AM, Hutt JRB, Masse V, Lavigne M, Vendittoli P-A. The impact of mechanical and restricted kinematic alignment on knee anatomy in total knee arthroplasty. J Arthroplasty. 2017;32(7):2133–2140. doi:10.1016/j.arth.2017.02.028
93. Ma R, Chen X, Li H, et al. Computer navigation assisted restricted kinematic alignment improves short-term outcomes in total knee arthroplasty: an ambispective cohort study. Orthop Surg. 2023;15(2):460–470. doi:10.1111/os.13603
94. Miura T, Takahashi T, Watanabe J, et al. Postoperative clinical outcomes for kinematically, restricted kinematically, or mechanically aligned total knee arthroplasty: a systematic review and network meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2023;24(1):322. doi:10.1186/s12891-023-06448-0
95. Mont MA, Cool C, Gregory D, Coppolecchia A, Sodhi N, Jacofsky DJ. Health care utilization and payer cost analysis of robotic arm assisted total knee arthroplasty at 30, 60, and 90 days. J Knee Surg. 2021;34(3):328–337. doi:10.1055/s-0039-1695741
96. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–2086. doi:10.1001/archinternmed.2009.427
97. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054–1059. doi:10.1016/j.arth.2007.05.036
98. Antonios JK, Korber S, Sivasundaram L, et al. Trends in computer navigation and robotic assistance for total knee arthroplasty in the United States: an analysis of patient and hospital factors. Arthroplast Today. 2019;5(1):88–95. doi:10.1016/j.artd.2019.01.002
99. Konyves A, Willis-Owen CA, Spriggins AJ. The long-term benefit of computer-assisted surgical navigation in unicompartmental knee arthroplasty. J Orthop Surg Res. 2010;5:94. doi:10.1186/1749-799X-5-94
100. Mancino F, Cacciola G, Malahias MA, et al. What are the benefits of robotic-assisted total knee arthroplasty over conventional manual total knee arthroplasty? A systematic review of comparative studies. Orthop Rev (Pavia). 2020;12(Suppl 1):8657. doi:10.4081/or.2020.8657
101. Banerjee S, Cherian JJ, Elmallah RK, Pierce TP, Jauregui JJ, Mont MA. Robot-assisted total Hip arthroplasty. Expert Rev Med Devices. 2016;13(1):47–56. doi:10.1586/17434440.2016.1124018
102. McCarthy J, Minsky ML, Shannon CE. A proposal for the Dartmouth summer research project on artificial intelligence, August 31, 1955. AI Mag. 2006;27(4):12.
103. Frankish K, Ramsey WM. The Cambridge Handbook of Artificial Intelligence. Cambridge University Press; 2014.
104. Ghahramani Z. Probabilistic machine learning and artificial intelligence. Nature. 2015;521:7553):452–459. doi:10.1038/nature14541
105. Jones LD, Golan D, Hanna SA, Ramachandran M. Artificial intelligence, machine learning and the evolution of healthcare: a bright future or cause for concern? Bone Joint Res. 2018;7(3):223–225. doi:10.1302/2046-3758.73.BJR-2017-0147.R1
106. Panchmatia JR, Visenio MR, Panch T. The role of artificial intelligence in orthopaedic surgery. Br J Hosp Med. 2018;79(12):676–681. doi:10.12968/hmed.2018.79.12.676
107. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358–2361. doi:10.1016/j.arth.2018.02.067
108. Bini SA, Shah RF, Bendich I, Patterson JT, Hwang KM, Zaid MB. Machine learning algorithms can use wearable sensor data to accurately predict six-week patient-reported outcome scores following joint replacement in a prospective trial. J Arthroplasty. 2019;34(10):2242–2247. doi:10.1016/j.arth.2019.07.024
109. Shahid N, Rappon T, Berta W. Applications of artificial neural networks in health care organizational decision-making: a scoping review. PLoS One. 2019;14(2):e0212356. doi:10.1371/journal.pone.0212356
110. Sun H, Wang W, He F, et al. An AI-based image quality control framework for knee radiographs. J Digit Imaging. 2023;36(5):2278–2289. doi:10.1007/s10278-023-00853-6
111. Kim MS, Cho RK, Yang SC, Hur JH, In Y. Machine learning for detecting total knee arthroplasty implant loosening on plain radiographs. Bioengineering. 2023;10:6.
112. Simon S, Schwarz GM, Aichmair A, et al. Fully automated deep learning for knee alignment assessment in lower extremity radiographs: a cross-sectional diagnostic study. Skeletal Radiol. 2022;51(6):1249–1259. doi:10.1007/s00256-021-03948-9
113. Bonnin M, Muller-Fouarge F, Estienne T, Bekadar S, Pouchy C, Ait Si Selmi T. Artificial intelligence radiographic analysis tool for total knee arthroplasty. J Arthroplasty. 2023;38(7):S199–S207 e192. doi:10.1016/j.arth.2023.02.053
114. Cipresso P, Giglioli IAC, Raya MA, Riva G. The past, present, and future of virtual and augmented reality research: a network and cluster analysis of the literature. Front Psychol. 2018;9:2086. doi:10.3389/fpsyg.2018.02086
115. Furman AA, Hsu WK. Augmented Reality (AR) in orthopedics: current applications and future directions. Curr Rev Musculoskelet Med. 2021;14(6):397–405. doi:10.1007/s12178-021-09728-1
116. Lee LS, Chan PK, Wen C, et al. Artificial intelligence in diagnosis of knee osteoarthritis and prediction of arthroplasty outcomes: a review. Arthroplasty. 2022;4(1):16. doi:10.1186/s42836-022-00118-7
117. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268–271. doi:10.1016/j.knee.2012.11.001
118. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141–146. doi:10.1007/s11999-009-0977-5
119. Pailhe R. Total knee arthroplasty: latest robotics implantation techniques. Orthop Traumatol Surg Res. 2021;107(1S):102780. doi:10.1016/j.otsr.2020.102780
120. Kolisek FR, McGrath MS, Jessup NM, Monesmith EA, Mont MA. Comparison of outpatient versus inpatient total knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1438–1442. doi:10.1007/s11999-009-0730-0
121. Rodriguez-Merchan EC. Outpatient total knee arthroplasty: is it worth considering? EFORT Open Rev. 2020;5(3):172–179. doi:10.1302/2058-5241.5.180101
122. Migliorini F, Cipollaro L, Cuozzo F, Oliva F, Marino AV, Maffulli N. Outpatient total hip arthroplasty: a meta-analysis. App Sci. 2021;11(5):6853. doi:10.3390/app11156853
123. den Hertog A, Gliesche K, Timm J, Muhlbauer B, Zebrowski S. Pathway-controlled fast-track rehabilitation after total knee arthroplasty: a randomized prospective clinical study evaluating the recovery pattern, drug consumption, and length of stay. Arch Orthop Trauma Surg. 2012;132(8):1153–1163. doi:10.1007/s00402-012-1528-1
124. Yang J, Olsen AS, Serino J, Terhune EB, DeBenedetti A, Della Valle CJ. Similar 90-day outcomes among inpatient and outpatient arthroplasties: a single-surgeon matched cohort analysis. Bone Joint J. 2021;103(7):84–90. doi:10.1302/0301-620X.103B7.BJJ-2020-2341.R1
125. Fassihi SC, Malahias MA, Gu A, et al. Hospital discharge within a day after total knee arthroplasty does not affect 1-year complications compared with rapid discharge. J Am Acad Orthop Surg. 2021;29(9):397–405. doi:10.5435/JAAOS-D-20-00187
126. Edwards PK, Milles JL, Stambough JB, Barnes CL, Mears SC. Inpatient versus Outpatient Total Knee Arthroplasty. J Knee Surg. 2019;32(8):730–735. doi:10.1055/s-0039-1683935
127. Otero JE, Gholson JJ, Pugely AJ, Gao Y, Bedard NA, Callaghan JJ. Length of hospitalization after joint arthroplasty: does early discharge affect complications and readmission rates? J Arthroplasty. 2016;31(12):2714–2725. doi:10.1016/j.arth.2016.07.026
128. Song MH, Kim BH, Ahn SJ, et al. Peri-articular injections of local anaesthesia can replace patient-controlled analgesia after total knee arthroplasty: a randomised controlled study. Int Orthop. 2016;40(2):295–299. doi:10.1007/s00264-015-2940-2
129. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925–933. doi:10.1007/s00264-015-2998-x
130. Gillis ME, Dobransky J, Dervin GF. Defining growth potential and barriers to same day discharge total knee arthroplasty. Int Orthop. 2019;43(6):1387–1393. doi:10.1007/s00264-018-4100-y
131. Li Z, Cheng W, Sun L, et al. Mini-subvastus versus medial parapatellar approach for total knee arthroplasty: a prospective randomized controlled study. Int Orthop. 2018;42(3):543–549. doi:10.1007/s00264-017-3703-z
132. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443–1449. doi:10.1007/s11999-009-0736-7
133. Argenson JN, Husted H, Lombardi A, Booth RE, Thienpont E. Global forum: an international perspective on outpatient surgical procedures for adult hip and knee reconstruction. J Bone Joint Surg Am. 2016;98(13):e55. doi:10.2106/JBJS.15.00998
134. Courtney PM, Boniello AJ, Berger RA. Complications Following Outpatient Total Joint Arthroplasty: an Analysis of a National Database. J Arthroplasty. 2017;32(5):1426–1430. doi:10.1016/j.arth.2016.11.055
135. Kort NP, Bemelmans YFL, van der Kuy PHM, Jansen J, Schotanus MGM. Patient selection criteria for outpatient joint arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2668–2675. doi:10.1007/s00167-016-4140-z
136. Keulen MHF, Asselberghs S, Boonen B, Hendrickx RPM, van Haaren EH, Schotanus MGM. Predictors of (Un)successful same-day discharge in selected patients following outpatient hip and knee arthroplasty. J Arthroplasty. 2020;35(8):1986–1992. doi:10.1016/j.arth.2020.03.034
137. Lan RH, Samuel LT, Grits D, Kamath AF. Contemporary outpatient arthroplasty is safe compared with inpatient surgery: a propensity score-matched analysis of 574,375 procedures. J Bone Joint Surg Am. 2021;103(7):593–600. doi:10.2106/JBJS.20.01307
138. Migliorini F, Pintore A, Cipollaro L, Oliva F, Maffulli N. Outpatient total knee arthroplasty: a meta-analysis. Appl Sci. 2021;11(20):9376. doi:10.3390/app11209376
139. Carey K, Morgan JR, Lin MY, Kain MS, Creevy WR. Patient outcomes following total joint replacement surgery: a comparison of hospitals and ambulatory surgery centers. J Arthroplasty. 2020;35(1):7–11. doi:10.1016/j.arth.2019.08.041
140. Courtney PM, Froimson MI, Meneghini RM, Lee GC, Della Valle CJ. Can total knee arthroplasty be performed safely as an outpatient in the medicare population? J Arthroplasty. 2018;33(7s):S28–S31. doi:10.1016/j.arth.2018.01.003
141. Gromov K, Jorgensen CC, Petersen PB, et al. Complications and readmissions following outpatient total Hip and knee arthroplasty: a prospective 2-center study with matched controls. Acta Orthop. 2019;90(3):281–285. doi:10.1080/17453674.2019.1577049
142. Husted C, Gromov K, Hansen HK, Troelsen A, Kristensen BB, Husted H. Outpatient total Hip or knee arthroplasty in ambulatory surgery center versus arthroplasty ward: a randomized controlled trial. Acta Orthop. 2020;91(1):42–47. doi:10.1080/17453674.2019.1686205
143. Kelly MP, Calkins TE, Culvern C, Kogan M, Della Valle CJ. Inpatient versus outpatient hip and knee arthroplasty: which has higher patient satisfaction? J Arthroplasty. 2018;33(11):3402–3406. doi:10.1016/j.arth.2018.07.025
144. Lovald ST, Ong KL, Malkani AL, et al. Complications, mortality, and costs for outpatient and short-stay total knee arthroplasty patients in comparison to standard-stay patients. J Arthroplasty. 2014;29(3):510–515. doi:10.1016/j.arth.2013.07.020
145. Lovecchio F, Alvi H, Sahota S, Beal M, Manning D. Is outpatient arthroplasty as safe as fast-track inpatient arthroplasty? A propensity score matched analysis. J Arthroplasty. 2016;31(9 Suppl):197–201. doi:10.1016/j.arth.2016.05.037
146. Schotanus MGM, Bemelmans YFL, Grimm B, Heyligers IC, Kort NP. Physical activity after outpatient surgery and enhanced recovery for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(11):3366–3371. doi:10.1007/s00167-016-4256-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
