Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Forty days old infant with Pig Bronchus, presenting with recurrent pneumonia: A Case Report
Authors Rasooly AJ, Noor S, Ullah S, Baryali AT, Haidary AM
Received 10 July 2023
Accepted for publication 21 October 2023
Published 30 October 2023 Volume 2023:14 Pages 379—383
DOI https://doi.org/10.2147/PHMT.S429852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Laurens Holmes, Jr
Abdul Jamil Rasooly,1 Sahar Noor,1 Saif Ullah,2 Abdul Tawab Baryali,3 Ahmed Maseh Haidary4
1Department of Pediatrics Medicine, French Medical Institute for Mothers and Children (FMIC), Kabul, Afghanistan; 2Department of Medicine, French Medical Institute for Mothers and Children (FMIC), Kabul, Afghanistan; 3Department of Quality and Patient Safety, French Medical Institute for Mothers and Children (FMIC), Kabul, Afghanistan; 4Department of Pathology and Clinical Laboratory, French Medical Institute for Mothers and Children (FMIC), Kabul, Afghanistan
Correspondence: Ahmed Maseh Haidary, Department of Pathology and Clinical Laboratory, French Medical Institute for Mothers and Children (FMIC), Kabul, Afghanistan, Email [email protected]
Background: Pig bronchus is rare and usually asymptomatic, but it may also cause significant respiratory symptoms such as recurrent pneumonia, chronic bronchitis, atelectasis, and difficult airway management in surgical and critical care patients. This study is aimed to examine a case of pig bronchus in which the patient presented with recurrent pneumonia in her early days of life.
Case Report: A case report is the study design utilized in this assessment of a 40-days-old girl from a consanguineous marriage, who presented with cough and difficulty breathing for approximately a month. She was referred from a provincial hospital with no improvement in respiratory symptoms after three times hospitalization since birth. Radiological investigation revealed pig bronchus as the cause of recurrent pneumonia.
Conclusion: Pig bronchi, if not diagnosed on time, may result in severe lung infection that can even result in fatal disease. A high level of clinical suspicion is required to initiate an appropriate diagnostic workup. The gold standard modality for the diagnosis of pig bronchus is computed tomography (CT), ideally with multi-detector three-dimensional (3D) image reconstruction.
Keywords: pig bronchus, infant, recurrent pneumonia
Background
Bronchi on the right and left sides are air conduits that originate from the lower end of the trachea, demarcated externally on the surface of the trachea by a ridge called the carina.1 The tracheal bronchus (TRB) is a congenital anatomical variant of the bronchus that occurs as an accessory bronchus originating directly from the lateral wall of the trachea, generally located at –2–3 cm from the level of the carina.1,2 A pig bronchus (bronchus suis) is a variant of TRB in which the entire upper lobe, mostly on the right side, is supplied by this bronchus.3,4
Major tracheobronchial abnormalities are present in approximately 1% (range = 0.1–2%) of the population, with a higher prevalence in pediatric patients and those with accompanying congenital anomalies and marked right-sided predilection.2,5 TRB accounts for 7.5% of tracheobronchial anomalies, with most cases involving the right upper lobe. It is classified into two main types: the supernumerary type, in which the usual bronchial supply to the affected lung segment is concurrently present, and the displaced type, in which the usual bronchial supply to the affected lung segment is absent.5,6 The pig bronchus is named so because of its similarity to the anatomic configuration of the tracheobronchial system observed in swine.7
TRB can be an incidental finding during bronchoscopy or radiological investigations. Usually, the patients are asymptomatic. Rarely, it may cause significant respiratory symptoms owing to retained secretions, resulting in recurrent pneumonia, chronic bronchitis, atelectasis, and difficult airway management in surgical and critical care patients.5,8 Clinically sometimes, it may put an impression of other rare disorders, such as cystic fibrosis, bronchial fistulas, tracheomegally, recurrent pneumonias due to immune related disorders, etc. Imaging studies such as radiography, computed tomography, and magnetic resonance imaging are the modalities of choice for the identification and diagnosis of various types of TRB. Knowledge of the normal cross-sectional anatomy of large airway diseases and high clinical suspicion is necessary to initiate appropriate clinical correlation with radiological investigations for diagnosis.9 In this report, we present a case of a pig bronchus that caused recurrent chest infections in a 40-day-old baby girl.
Case Presentation
A 40-day-old girl was referred from provincial hospital with fever, cough, and dyspnea. On general physical examination, she was awake, alert, and febrile with a rectal temperature of 38°C. Both fontanelles were open and flat, and the head was normocephalic and atraumatic. The heart had a regular rhythm with no murmur, whereas respiratory examination revealed a respiratory rate of 70 beats/min with 75% SPO2. She had severe subcostal and intercostal retractions and coarse crackles were audible on auscultation. The results of the remaining physical examination were normal. She was the product of a consanguineous marriage with an unremarkable family history. The symptoms were recurrent since birth, which had led to hospitalization of the patient three times in provisional hospital. During the previous admissions, the patient presented with signs and symptoms associated with pneumonia, thus receiving empirical antibiotic therapy. Accordingly, the patient would respond to antibiotics but would develop symptoms again once the antibiotics were stopped.
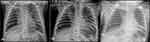
She was admitted to the neonatal intensive care unit (NICU) because of her unstable condition. Chest radiography revealed left lung hyperinflation and reduced right lung volume along with upper lobe haziness (Figure 1A). The patient was treated with antibiotics, regular nebulization, and chest physiotherapy for approximately 12 days. As there was significant clinical improvement, the patient was shifted to the inpatient ward, where she required 1 L of supplemental oxygen. While in the ward, the chest X-ray was repeated that showed significant radiological resolution, as shown in Figure 1B. After 2–3 days on the ward, her condition deteriorated. Repeated chest radiography showed worsening of the radiological features (Figure 1C). Thus, the antibiotic was empirically upgraded to cover healthcare-associated pneumonia without clinical improvement. Considering the poor response to therapy and the history of recurrent pneumonia, a chest CT scan was performed, which revealed emphysematous changes in the right upper and ground-glass opacities in the middle and lower zones of the right lung, suggestive of pneumonitis. An extra bronchus was revealed to be originating above the carina, along with atelectasis and consolidation of the upper lobe of the right lung, shown in Figure 2A. Similarly, the three-dimensional multiplanar reconstruction of the lungs and bronchi leads to definitive diagnosis of pig bronchus, shown in Figure 2A and B. Accordingly, the department of paediatrics referred the patient for surgery and post ablation, the patient is completely stable and healthy, right now being 18 months of age.
Discussion
The TRB was initially described by Sandifort in 1785.10 TRB either can be displaced or supernumerary. TRB can be classified into three types. In Type I TRB, the bronchus originates between the junction of the middle and lower one-third of the trachea. In Type II, TRB is small and originates from the lower third of the trachea. Similarly, in Type III TRB, the bronchus arises from the lateral wall, almost at the level of the carina, giving rise to a trifurcated carina.11 If the supernumerary bronchus ends blindly, it is called tracheal diverticulum. Similarly, if they terminate in aerated or bronchiectatic lung tissue, this is called apical accessory lungs or tracheal lobe.12,13 Arterial supply and venous drainage are usually through the pulmonary artery and veins in the displaced bronchus, respectively.10 In the case of extralobar supernumerary bronchus, if it shares the pleura of the upper lobe, a TRB may have its own arterial supply arising from the systemic circulation.14
Pig bronchus is a variant of TRB, in which the entire right upper lobe is aerated by an accessory bronchus. It can be an incidental finding during radiological investigations, such as during bronchoscopy, or can be associated with recurrent chest infections due to retained secretions, causing decreased ventilation, recurrent pneumonia, chronic bronchitis, and atelectasis.12,15 Therefore, a supernumerary pig bronchus should be considered in the differential diagnosis when evaluating patients with recurrent pneumonia. Our patient presented with recurrent lung infection since birth that would recur after the cessation of antibiotics. CT scan of the chest was the key to identification of the supernumerary pig bronchus as the cause of recurrent lung infections.
Some studies have demonstrated that occasional patients with pig bronchi may present with wheezing due to a narrowed aberrant bronchus and are often misdiagnosed with asthma. It is often found as a part of multiple congenital abnormalities such as the syndrome associated with abnormalities of vertebrae, anus, heart, trachea, esophagus, kidney and limbs, known as the VATER syndrome, laryngomalacia, tracheomalacia, tracheal stenosis, congenital heart disease, esophageal atresia, neural tube defects, and hypoplastic lung.15–21 Such syndromic presentations may affect prognosis, lengthen the disease duration, and alter the selection of therapeutic options.
The gold standard modality for the diagnosis of TRB, especially pig bronchus, is computed tomography (CT), especially when coupled with multi-detector CT (MDCT) 3D image reconstruction. It allows direct and non-invasive diagnosis with a faster reporting time, extended anatomic coverage of the area, and minimal need for anesthesia compared to bronchoscopy.13 The diagnosis of our patient was also concluded by CT chest supported by three-dimensional multiplanar reconstruction, which helped to further elaborate the anomaly.
When discovered as an incidental finding, extra caution should be taken while intubating the patient because the endotracheal tube can obstruct the TRB or cause pneumothorax, post-obstructive pneumonia, respiratory failure, or even migration to the bronchus itself, resulting in atelectasis, hypoxemia, or both.12,15 For such cases, one-sided lung ventilation with the use of bronchial blocker in cases of unilateral thoracotomy or use of double-lumen endotracheal tube is an option for intubation of such patients.22
Patient management is dictated by the presence or absence of symptoms. In the absence of clinical symptoms, no specific therapy is needed, whereas when symptoms are present, treatment options are decided based on the severity of symptoms. Since patients can have a broad range of symptoms, bronchodilators, inhaled corticosteroids, muscarinic antagonists, and antibiotics are selectively prescribed, but if associated with recurrent pneumonia or other complications, resection of the aberrant bronchus and the lobe it supplies is mandatory.13,23,24
Conclusion
Our patient presented with recurrent and symptomatic lung infection immediately after birth due to an underlying pig bronchus. Had such an abnormal airway been overlooked, serious complications could have occurred. The possibility of TRB should be considered in infants presenting with recurrent pneumonia, atelectasis, and wheezing.
Abbreviations
FMIC, French Medical Institute for Mothers and Children; TRB, Tracheal bronchus; NICU, Neonatal intensive care unit; CT Scan, Computed Tomography scan; 3D, Three Dimensional; MDCT, Multi detector CT scan.
Data Sharing Statement
All generated data are included in this article.
Ethical Approval and Consent to Participate
Ethical approval was obtained from the hospital’s ethical review committee. Informed consent for participation in this case report was obtained from the patient’s legal guardian (father).
Consent
Written informed consent was obtained from the patient’s legal guardian for the publication of this case report.
Funding
The authors received no funding for current writing.
Disclosure
The authors declare to have no competing interest.
References
1. Amador C, Weber C, Varacallo M. Anatomy, Thorax, Bronchial. Treasure Island (FL): StatPearls Publishing; 2022.
2. Doolittle A, Mair E. tracheal bronchus: classification, endoscopic analysis, and airway management. Otolaryngeal Head Neck Surg. 2002;126(3):240–243. doi:10.1067/mhn.2002.122703
3. Muller NL, Silva CI Imaging of the chest. (2008) ISBN:141604048X.
4. Weerakkody Y, El-Feky M, Bickle L, et al. Tracheal bronchus. Reference article, Radiopaedia.org.
5. Cheng L, Liu S, Qi W, Dong Y. The Incidence of Tracheal Bronchus in Thoracic Surgery Patients and Its Implication for Lung Isolation: a Retrospective Cohort Study. J Cardiothorac Vasc Anesth. 2020;34(11):3068–3072. doi:10.1053/j.jvca.2020.06.073
6. Wong LM, Cheruiyot I, Santos de Oliveira MH, et al. Congenital anomalies of the tracheobronchial tree: a meta-analysis and clinical considerations. Ann Thorac Surg. 2020;112(1):315–325. doi:10.1016/j.athoracsur.2020.08.060
7. Qureshi R, Soorae A. Foreign body in tracheal bronchus simulating bronchogenic cancer. Eur J Cardio-Thoracic. 2001;20(3):639–641. doi:10.1016/S1010-7940(01)00838-7
8. Kuriakose N, Al-Ismaili M, Raniga S, Date R, Al-Abady A, Al-Balushi Z. Airway and Anaesthetic Challenges in a Child With Bronchus Suis and Superimposed Double Aortic Arch: a case report. Sultan Qaboos Univ Med J. 2021;21:132–136. doi:10.18295/squmj.2021.21.01.020
9. Lee DK, kim YM, Kim HZ, Lim SH. Right upper lobe tracheal bronchus: anesthetic challenge in one lung-ventilated patients- a report of three cases. Korean J Anesthesiol. 2013;63(5):448–450. doi:10.4097/kjae.2013.64.5.448
10. Prountzos S, Papakonstantinou O, Bizimi V, et al. Large airway diseases in pediatrics: a pictorial essay. Acta Radiol open. 2020;9(12):2058460120972694. doi:10.1177/2058460120972694
11. Ghaye B, Szapiro D, Franchamps JM, Dondelinger RF. Congenital bronchial abnormalities revisited. Radiographic. 2001;21(1):105–119. doi:10.1148/radiographics.21.1.g01ja06105
12. Conacher ID. Implications of a tracheal bronchus for adult anaesthetic practice. Br J Anaesth. 2000;85(2):317–320. doi:10.1093/bja/85.2.317
13. Berrocal T, Madrid C, Novo S, Gutierrez J, Arjonilla A, Gomez-Leon N. Congenital anomalies of the tracheobronchial tree, lung and mediastinum: embryology, radiology, and pathology. Radiographic. 2004;24(1):e17. doi:10.1148/rg.e17
14. Jamil A, Soos M. Tracheal Bronchus. Treasure Island (FL): StatPearls Publishing; Jan 2023.
15. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations; the spectrum of anomalies. Ann Surg. 1986;203(5):517–524. doi:10.1097/00000658-198605000-00011
16. Lai KM, Hsieh MH, Lam F, Chen CY, Chen TL, Chang CC. Anesthesia for patients with tracheal bronchus. Asian J Anesthesiol. 2017;55(4):87–88. doi:10.1016/j.aja.2017.09.002
17. Kairamkonda V, Thorburn K, Sarginson R. Tracheal bronchus associated with VACTERL. Eur J Pediatr. 2003;162(3):165–167. doi:10.1007/s00431-002-1109-3
18. Lee SL, Cheung YF, Leung MP, et al. Airway obstruction in children with congenital heart disease: assessment by flexible bronchoscopy. Pediatr Pulmonol. 2002;34(4):304–311. doi:10.1002/ppul.10164
19. Nose K, Kamata S, Sawai T, et al. Airway anomalies in patients with congenital diaphragmatic hernia. J Pediatr Surg. 2000;35(11):1562–1565. doi:10.1053/jpsu.2000.18310
20. Bertrand P, Navarro H, Caussade S, et al. Airway anomalies in children with Down syndrome: endoscopic findings. Pediatr Pulmonol. 2003;36(2):137–141. doi:10.1002/ppul.10332
21. Sanchez I, Navarro H, Mendez M, et al. Clinical characteristics of children with tracheobronchial anomalies. Pediatr Pulmonol. 2003;35(4):288–291. doi:10.1002/ppul.10256
22. Kabra NS, Bowen JR, Allen H. porcine bronchus’ diagnosed in neonatal period. Indian J Pediatr. 2001;68:681–684. doi:10.1007/BF02752287
23. Moon YJ, Kim SH, Park SW, Lee YM. The implications of a tracheal bronchus on one-lung ventilation and fibreoptic bronchoscopy in a patient undergoing thoracic surgery: a case report. Can J Anaesth. 2015;62(4):399–402. doi:10.1007/s12630-014-0293-8
24. Barat M, Konrad H. Tracheal bronchus. Am J Otolaryngol. 1987;8(2):118–122. doi:10.1016/S0196-0709(87)80034-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.