Back to Journals » Drug Design, Development and Therapy » Volume 15
Formulation and Evaluation of Baclofen-Meloxicam Orally Disintegrating Tablets (ODTs) Using Co-Processed Excipients and Improvement of ODTs Performance Using Six Sigma Method
Authors Abdelmonem R , Abdellatif MM , Al-Samadi IEI, El-Nabarawi MA
Received 3 July 2021
Accepted for publication 5 October 2021
Published 16 October 2021 Volume 2021:15 Pages 4383—4402
DOI https://doi.org/10.2147/DDDT.S327193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Rehab Abdelmonem,1 Menna M Abdellatif,1 Inas Essam Ibrahim Al-Samadi,1 Mohamed A El-Nabarawi2
1Department of Industrial Pharmacy, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology (MUST), 6th of October, Giza, 12566, Egypt; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Giza, Egypt
Correspondence: Menna M Abdellatif Tel +20 1005647945
Email [email protected]
Purpose: This study aimed to formulate an orally disintegrating tablet (ODT) containing both baclofen and meloxicam together for treating osteoarthritis.
Methods: Direct compression method was used to prepare ODTs using three types of co-processed excipients (Prosolv ODT G2®, F-melt®, and Pharmaburst® 500). ODTs were evaluated according to weight variation, thickness, friability, hardness, drug content, wetting time, in-vitro disintegration time, in-vitro dissolution test, and palatability. To enhance the in-vitro dissolution of meloxicam and palatability of ODT, a six sigma methodology was used, and an improvement phase was established where ODTs were prepared using lyophilization and levigation techniques. Finally, a pharmacokinetic study of the improved ODT was accomplished in comparison to the conventional oral tablet.
Results: Pharmaburst-based formula (F4) showed the shortest wetting time and, consequently, the shortest disintegration time and the highest percentage of drug dissolved within 3 min compared to the other formulae. All the improved ODTs had a bitterness taste score vary from (0) palatable and (+1) tasteless. The current sigma level was 3.628 σ and 3.33 σ for palatability and solubility of ODT, respectively, which indicated the process was successfully improved compared with the previous sigma level of 2.342 σ of both processes. Pharmacokinetic study of the improved ODTs showed a significant decrease of Tmax to 120 and 30 min instead of 180 and 120 min for meloxicam and baclofen, respectively.
Conclusion: ODTs were successfully improved using the six sigma methodology, the pharmacokinetic parameters of both drugs were enhanced due to rapid absorption through the oral mucosa.
Keywords: baclofen, meloxicam, co-processed excipients, orally disintegrating tablet, six sigma methodology
Introduction
Around 18% of women and 10% of men over 60 years are affected by osteoarthritis (OA),1,2 which is a musculoskeletal condition characterized by severe deterioration and loss of articular cartilage and usually accompanied by structural and functional changes in the whole joint.3,4 Oral non-steroidal anti-inflammatory drugs have been associated with greater efficacy in the more severe knee and hip OA patients. In addition, many joint diseases are associated with muscle spasms; therefore, adding anti-muscle contraction (skeletal muscle relaxant) like baclofen could relieve patients’ musculoskeletal pain. Baclofen is used to relieve the spasms, cramping, and tightness of muscles by relaxing skeletal muscles in the body.5
For higher efficacy, high doses of muscle relaxants and NSAIDs are required. However, the European Medical Agency’s Committee on medicinal products for human use states that both selective and non-selective NSAIDs should be used in the lowest effective doses with the shortest possible duration of treatment sufficient to control the symptoms of the disease to minimize the risk of developing cardiovascular disease.6 Thus, an ideal fixed-dose combination (FDC) is highly desirable as it is possible to reduce the analgesic and anti-inflammatory therapy timing by combining analgesic with a muscle relaxant.7 One of these combinations is 500 mg of chlorzoxazone and 400 mg of ibuprofen. This combination was approved for short-term treatment of musculoskeletal pain in India in 2010. It has complementary actions and relieves pain and spasms in patients with musculoskeletal disorders. Furthermore, adding a skeletal muscle relaxant to an NSAID or paracetamol has provided superior pain relief than taking the pain reliever alone.8 Also, a clinical study was conducted to explore the efficacy and tolerability of the combined use of the meloxicam with baclofen in 50 patients with exacerbation of chronic musculoskeletal pain syndrome. The study concluded that using both drugs increases the effectiveness of therapy with a significant decrease in pain intensity by more than 50% by the end of the first week of treatment. Furthermore, the study revealed a reasonably high efficacy and safety of meloxicam in the complex therapy of chronic recurrent musculoskeletal pain syndromes.9
For this reason, this study aimed to combine meloxicam with baclofen in a single dosage to reduce the time to achieve a pronounced analgesic for effective relief of musculoskeletal disorders, which is extremely important for preventing the development of NSAID-associated side effects as baclofen is the gold standard of anti-spastic therapy as it induces its action mainly at the spinal level. Furthermore, unlike other NSAIDs, meloxicam does not cause damage to the articular cartilage in the treatment of OA, which was demonstrated by clinical trials.9
Orally disintegrating tablets (ODTs) were the best-selected dosage form. The medication could be absorbed partially or entirely into the systemic circulation from blood vessels in the oral mucosa, thus decreasing gastrointestinal tract side effects. In addition, the ODTs produce a faster onset of action than orally ingested tablets; ODTs could bypass the hepatic first-pass effect and destroy the drug by gastric acid and digestive enzymes, resulting in increased bioavailability of drugs.10,11 Baclofen is a good candidate for ODT formulations as it is water soluble with a suitable log p-value (1.3), small molecular weight, dose less than 20 mg, suitable half-life.12,13 Meloxicam partially fulfills the ODT formulation requirement as it possesses a small molecular weight, long half-life, dose less than 20 mg, but possesses a poor water solubility. Meloxicam is classified under class II of the Biopharmaceutics Classification System (BCS), having good permeability but low water solubility.14 Several works have been published regarding enhancing the solubility of meloxicam.15–17
Many techniques have been reported for developing fast dissolving tablets; direct compression represents the most cost-effective and straightforward tablet manufacturing technique.18
The development of highly functional co-processed excipients was influenced by increasing demands for the production of ODTs with stipulated specifications.19,20 Co-processed excipients used for ODTs often contain filler, binder, and disintegrant; therefore, simple mixing with the drug followed with compaction is sufficient to formulate ODTs with no need to add any other excipients.21 Co-processed excipients such as Pharmaburst, F-melt, Prosolv ODT G2 were the most used to prepare direct compression ODTs.22
The quality of the final product in the pharmaceutical industry has become an important topic, and there is a real need for continuous improvements of pharmaceutical products.23 Six sigma (6σ) is one of the process improvement tools based on a statistical concept that helped define the problems systematically and reduce defects using the model Define-Measure-Analyze-Improve/Design-Control/Verify. The goal of applying six sigma is to identify and eliminate the wastes and reducing process variation.24,25
Six Sigma methodologies include either an improvement phase for already existing processes falling below specification and looking for incremental improvement or an improvement phase that includes developing new processes or products at six sigma quality levels if the current process requires more than just incremental improvement.25 Six sigma is usually used by manufacturing industries where there is a large number of samples. However, Carleysmith et al26 discussed using the six sigma approach in pharmaceutical research and development, with few samples with high variability. They concluded that six sigma certainly supports efficient problem definition and problem-solving, and the dissemination of ideas.
Therefore, this study aimed to formulate baclofen-meloxicam ODTs using co-process excipients, and the formulated ODTs were characterized in terms of weight variation, thickness, friability, hardness, drug content, wetting time (WT), in-vitro disintegration time (DT), in-vitro dissolution test, and palatability. Furthermore, the six sigma methodology was used to improve formulated ODT on the laboratory scale. Finally, stability and pharmacokinetic studies were performed to assess the formulated and improved ODT.
Experimental
Materials
Misr Company kindly gifted baclofen for Pharmaceutical Industries (Cairo, Egypt), Meloxicam was kindly gifted by Amoun Pharmaceutical Company (Cairo, Egypt), Pharmaburst 500 (contained mannitol, sorbitol, crospovidone, precipitated silicon dioxide) was kindly gifted by SPI Pharma (Wilmington, DE, USA), Prosolv ODTs G2 (contained microcrystalline cellulose (MCC), colloidal silicon dioxide, mannitol, fructose, crospovidone) was kindly gifted by JRS Pharma GmbH & Co. KG (Rosenberg, Germany), and F-melt Type C (contained mannitol, xylitol, MCC, crospovidone, dibasic calcium phosphate anhydrous): was kindly gifted by Fuji Chemical Industry Ltd. (Toyama-Pref, Japan). All other reagents and solvents were of HPLC analytical grade obtained from Fisher Scientific Company, USA.
Preparation of Baclofen-Meloxicam ODTs
Baclofen – meloxicam ODTs were prepared by the direct compression process, as shown in Table 1. The powder was blended using a v-shaped mixer (Erweka, Germany) then compressed into 70 mg tablets using a single punch tablet machine (Royal Artist, India) of compression force 400 kg using a 6 mm flat punch and die set.27
 |
Table 1 Composition of Baclofen-Meloxicam ODTs Prepared by Direct Compression Using the Selected Co-Processed Excipients |
Evaluation of ODTs
Physicochemical and Mechanical Characterization of ODTs
Evaluation of ODTs was performed on the tablets of all formulae considering the visual inspection, weight, and content uniformity, thickness using a micrometer (BDM CO., Germany), hardness using tablet hardness tester (TH3/500, Copley scientific, UK), and friability using tablet friability tester (FR 1000, Copley scientific, UK) according to the pharmacopeial requirements (USP 39-NF 34).28
Fourier Transform Infrared Spectroscopy
FTIR (Fourier Transform Infrared) spectra of pure meloxicam, baclofen, Pharmaburst 500, Prosolv ODTs G2, F-melt Type C, the physical mixture of both drugs, and physical mixture of each drug with co-processed excipients were recorded by FTIR instrument (Bruker, UK). The FTIR measurements were carried out in the scanning range of 800–3500 cm− 1 at room temperature.
Determination of the Wetting Time
A piece of tissue paper folded twice was placed in a small petri dish containing 10 mL of dye solution (methylene blue aqueous solution). A tablet was carefully placed on the paper’s surface, and the time required for the dye solution to reach the upper surface of the tablet was noted as the wetting time (WT). A slow WT was defined as a value of more than 180 seconds.29
In-vitro Disintegration Time
ODTs were placed in the baskets of the USP disintegration apparatus (Pharmatest, Hainburg, Germany). At 37 ± 0.5°C, the ODTs were added to 10 mL of a phosphate buffer solution with a pH of 6.8. The time required for complete dispersion of a tablet was recorded as the disintegration time (DT).30
In-vitro Dissolution Test
Compendial in-vitro dissolution tests with a USP dissolution tester are usually used for in-vitro dissolution studies of solid oral dosage forms as ODT. In-vitro dissolution studies were performed with a USP dissolution apparatus II tester (Heusenstamm, Germany), set with a paddle speed of 100 rpm, using 500 mL of pH 6.8 phosphate buffer at 37 ± 0.5°C as a dissolution medium. A volume of 5 mL was withdrawn from the dissolution media at specified time intervals (1, 2, 3, 4, 5, 6, 8, 10, 15, 20, 25, and 30 min) and replaced with an equal volume of fresh medium. Meloxicam and baclofen were assayed using first derivative spectrophotometric methods using UV spectrophotometer (UV-1601, Shimadzu, Japan) at 363 and 233nm, respectively, to prevent overlapping between the absorbance of both drugs, where drugs concentrations were expressed as cumulative percent drug dissolved.31
Using of First Derivative Spectrophotometric Analysis in the Simultaneous Determination of Baclofen and Meloxicam in Phosphate Buffer pH 6.8
Mixtures of baclofen and meloxicam in phosphate buffer pH = 6.8 were prepared. The concentration was determined by measuring the absorbance of each drug in different mixtures at a wavelength for baclofen and meloxicam. The recovery percent (R %) was calculated for each mixture. The degree of interference was detected from the recovery percent of the mixtures.
Palatability Evaluation of ODTs
ODTs were estimated for their palatability assessment in six healthy volunteers (3 females and three males; 27–40 years old). An ethical committee approved this study, Faculty of Pharmacy, Cairo University, approval no. PT 1587; approval date 22/2/2016. This study was conducted according to the principles of the Declaration of Helsinki and its amendments. Volunteers were informed of the study’s aim, procedures, and risks. All volunteers provided written informed consent before undergoing any study procedure. Volunteers were asked to hold ODTs in the mouth for 30 sec. After sensing the taste for 30 sec, the solution was spat out, and the volunteer’s opinion for bitterness was noted. Rinsing of mouth with distilled water. The volunteers tasted the next ODTs for bitterness score on three successive days.32
Improvement of ODTs Using Six Sigma Methodology
Define Phase
ODTs were prepared and evaluated, then the data was collected and treated statistically, and data mapping was performed to define most of the contributing variables in the process variations. The current process performance of prepared ODTs was measured by calculating the current sigma level using software sigma XL version 7. Also, the voice of customer (VOC) was obtained.33 VOC is a term that describes volunteers’ or patients’ feedback about their experiences (current = real status = upper and lower control limits UCL, LCL) vs expectations (improved = upper and lower specification limits USL, LSL) of prepared ODTs.34
Measure Phase
The ODT process capability (Cpk) was performed. The scope of this phase measures the performance and process capability of dissolution and palatability evaluation test of prepared ODTs, to confirm whether assessment processes had an influential impact on the ODTs taste and solubility.35 Through using previously collected data and via utilizing Minitab.v.18.1.
Analyze Phase
Analysis of the data using a pareto chart prepared using Minitab v.18.1 to indicate the top reasons that cause the problem. Also, a fishbone diagram (cause and effect diagram) helped identify the problem’s root causes.
Improve Phase
Preparation of the Improved ODTs
Gelatin was first dispersed in pre-heated distilled water until a clear solution was obtained. Then, other ingredients were added and blended by a magnetic stirrer. Next, meloxicam was added, and then baclofen was incorporated into the solution after levigation with the required amount of sucralose and dextrose, as shown in Table 2. Finally, the final solution was poured into round-shaped blisters, frozen at −20 °C, then lyophilized at −80 °C and 0.2 mbar using a lyophilizer (Labconco, USA).
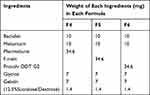 |
Table 2 Composition of Baclofen-Meloxicam ODTs Using Freeze-Dryer with Different Ratio of Matrix Former and Collapsing Agents |
Evaluation of the Improved ODTs
The improved ODTs were evaluated as mentioned before in Evaluation of ODTs. Also, in order to ensure the compatibilities of the components of the improved ODT. Differential scanning calorimetry (DSC) was used to characterize the thermal properties of meloxicam, baclofen, Pharmaburst, and the optimal ODT formula. The DSC thermograms were recorded using a thermal analyzer (TA-60, Shimadzu, Japan). The samples were hermetically sealed in aluminum pans and heated at a constant rate of 25°C/min over a temperature range of 25 to 500°C.
Control Phase
In the control phase, the sustainability of improvement was measured by performing stability studies on the improved ODTs formulae by storing the tablets in PVC blisters covered with aluminum foil and stored at 40 °C at 75% relative humidity ovens for 30 days. After 30 days, tablets were evaluated for their in-vitro dissolution and palatability. Control charts were performed, and sigma level after improvement was calculated using sigma XL version 7.36
In-vivo Study
The Faculty of Pharmacy, Cairo University’s ethical committee approved the in-vivo study (approval no. PT 1587; approval date 22/2/2016), adhering to the “Guide for the Care and Use of Laboratory Animals” declared via the Institute of Laboratory Animal Research (Washington, DC, USA). Two groups (each of 24 Wistar rats) of 200 gm were used in this study. The required animal dose was 10 mg/kg for each drug.37,38 In the first group, the ODT was cut with a sharp scalpel to 5 equal parts, each contains 2 mg of each drug; one part was allocated in the oral cavity to the rat using forceps until complete disintegration of ODT. In the second group, tablets of baclofen and meloxicam were grounded in 5 mL distilled water and were given to each rat orally. The study was used to compare the baclofen and meloxicam ODT pharmacokinetics parameters with oral market tablets (Baclofen 10 mg, Al-Delta, Egypt; Melocam 15 mg, Amoun pharmaceutical co, Egypt). Blood samples for the measurement of plasma concentrations of baclofen and meloxicam were collected in blood collection tubes containing K2 ethylenediaminetetraacetic acid at the following times: 0, 5, 10, 15, and 30 minutes; and at 1, 2, 3, 4, 5, 6, 7, and 8 hours after drug administration. Plasma was separated by centrifugation and stored frozen until analysis. Plasma concentration–time data of meloxicam and baclofen were analyzed by non-compartmental pharmacokinetic models using Kinetica® software (version 4.4.1). From the concentration–time data, the peak plasma concentrations (Cmax) and the time of its occurrence (tmax) were calculated. The linear trapezoidal rule was used to determine the area under the plasma concentration–time curve (AUC) from time zero to the last time recorded (AUC0−t). The half-life (t1/2), clearance, and volume of distribution (Vd) were also obtained. Data were statistically analyzed by unpaired t-test (two-tailed).
Assay of Baclofen
Baclofen and internal standard (levetiracetam solution) were separated from plasma by precipitating protein with methanol. Then the sample solution was filtered and injected onto HPLC with a C18 column. The mobile phase consisting of PBS (pH = 3) and an equal mixture of acetonitrile and methanol (65:35) solution, the temperature of the column was 30 °C with a flow rate of 0.8 mL/min.39
Assay of Meloxicam
Meloxicam and internal standard (piroxicam) were separated from plasma by precipitating protein with methanol then the sample solution was filtered and injected into HPLC with a C-18 column. The mobile phase consisted of 1:1 v/v phosphate buffer (pH 3.0) and methanol. The temperature of the column was 40 °C with a flow rate of 1 mL/min.40
Statistical Analysis of Data
All the data are reported as mean ± standard deviation where the sample size of each evaluated ODT formula was 20 tablets (n = 20). Shapiro–Wilk test was used to assess data distribution. One-way ANOVA with Tukey’s post-hoc analysis was applied to check the difference in mean values, and the level of significance was set at 0.05, and (p < 0.05) was statistically significant. Except for the in-vivo pharmacokinetic parameters (total sample size, n = 48), which were reported as mean± standard deviation and were statistically analyzed using an unpaired t-test (two-tailed). For the palatability test, the Friedman test with Dunn’s pairwise post hoc test was used to assess the difference between different formulae in bitterness score mean rank, while Wilcoxon signed-rank test was used to assess the difference in median bitterness score for each formula before and after the enhancement phase.
Results and Discussion
Evaluation of ODTs
Physicochemical and Mechanical Characterization of ODTs
Table 3 shows that all the prepared tablets achieved the pharmaceutical specification for weight variation. The average thickness of prepared ODTs was from 3.09 ± 0.05mm, to 2.92 ± 0.06mm. The reproducibility of the results confirmed the consistency of thickness and weights of all formulae. Furthermore, all ODTs did not break or show any capping, cracking, or chipping during the friability.41 All ODTs showed an optimum range of hardness (from 4 ± 0.26 kg to 4.10 ± 0.36 kg) as it can provide enough strength and porosity and at the same time ensure rapid wetting and disintegration of the tablets.42 There was no significant difference between formulae in hardness and thickness (p > 0.05). For drug content, all formulae complied with the United States Pharmacopoeia (USP 39-NF 34) limits.28 As the average drug content ranged from 91.65 ± 0.02 to 115.28 ± 0.05% for baclofen and from 93.52 ± 0.02% to 110.45 ± 0.05% for meloxicam.
 |
Table 3 Physical Evaluation of the ODTs Using Different Co-Processed Excipients |
Fourier Transform Infrared Spectroscopy
Compatibility study was made using FTIR, and major peaks assigned to baclofen due to its functional groups (-COOH and -NH2) are 1530 and 1627 cm−1, respectively. These characteristic peaks were also observed in the combination formulation, indicating no significant interaction between the functional groups of baclofen with meloxicam and other ingredients, as shown in Figure 1. Pure meloxicam showed its functional groups at 1347 cm−1 (-S=O), 1530 cm−1 (aromatic-C-C), 1620 cm−1 (-N-H-), 2930 cm−1 and 3293 cm-1 (-S-N-). FTIR spectra of meloxicam and its physical mixture with baclofen or any excipients showed the same characteristic bands in the same regions indicating the absence of any significant interaction.
Wetting Time
For the WT, it was found that all prepared formulae had acceptable WT (<180sec). By comparing different types of co-processed excipients-based formulae with the mixture, data revealed that while Prosolv ODT-based formula (F3) showed relatively longer WT than other formulae (p < 0.05). These results were attributed to the complicated matrix of Prosolv ODT, which contained mannitol and fructose and crospovidone and MCC, thus increasing its matrix’s strength and increased its WT. This result agrees with El-Nabarawi et al43 where the Prosolv ODT-based formula showed relatively longer WT than other formulae although crospovidone’s presence. Also, Sunada and Bi studied the WT of tablets containing MCC, and they found that tablets containing MCC showed lower porosity (lower water uptake) than other formulae and longer WT.44 Although both Prosolv and F-melt contain MCC. There was a significant difference (p < 0.05) in the WT of (F2) and (F3) where Prosolv based formula (F3) showed longer WT than of F-melt-based formula (F2), as Prosolv contains a higher content of MCC (15–30%) than F-melt (10–25%). On the contrary, there was no significant difference (p > 0.05) between Pharmaburst-based formula (F1) and F-melt-based formula (F2). Pharmaburst-based formula (F1) showed shorter WT than Prosolv-based formula (F3), this may be due to the excellent hydration capacity of sorbitol as the equatorial OH on the C-2 atom in sorbitol results in better hydration and high wetting capacity than an axial OH on the C-2 atom in mannitol which exists in Prosolv as the equatorial OH groups have two hydrogen-bonded contacts.45,46
In-vitro Disintegration Time
Pharmaburst 500 based formula (F1) showed shorter DT than other formulae (p < 0.05). This could be explained by the higher capacity of crospovidone as a super disintegrant, as it had rapid capillary activity and pronounced hydration with a slight tendency to gel formation.47 Although F-melt contains crospovidone besides insoluble inorganic salt (dibasic calcium phosphate anhydrous), water-insoluble inorganic excipients enhance the disintegration time than most commonly used water-soluble sugars or salts. In general, tablets composed mainly of water-soluble components tend to dissolve rather than disintegrate, resulting in a much longer disintegration time. The soluble components dissolve on the tablet’s outer layer, decreasing the water diffusion into the tablet core due to the formation of viscous, concentrated solutions.48 F-melt showed longer DT than Pharmaburst; this may be due to the lower specific surface area of F-melt than Pharmaburst 500, which was the reason for the delay of DT in F-melt based formula (F2) than Pharamaburst formula (F1).49 On the other hand, ODTs containing Prosolv had prolonged DT; as mentioned before, Prosolv consists of crospovidone, MCC, and mannitol, which was likely to cause DT delay. These results agree with Jacob et al50 who observed similar results and stated that MCC and mannitol exhibit non-wetting properties due to the formation of central rigid core leading to delaying the disintegration.
Using of First Derivative Spectrophotometric Analysis in the Simultaneous Determination of Baclofen and Meloxicam in Phosphate Buffer pH = 6.8
Derivative spectroscopy for simultaneous determination of both baclofen and meloxicam in phosphate buffer pH6.8 was where meloxicam at λ 363 nm had no reading for baclofen, at this λmax of meloxicam, baclofen had zero absorption so, and there was no interference for measurement of meloxicam in the presence of baclofen. On the other hand, at λmax of baclofen, there was a reading for meloxicam, which means overlapping the investigated drugs, making the first derivative methodology to measure baclofen in the presence of meloxicam. The absorbance and first derivative spectra were recorded for both baclofen and meloxicam solution in phosphate buffer pH = 6.8: Using the zero-crossing method, the suitable wavelengths were detected. The spectra obtained revealed that the zero-crossing was at 233 nm (peak amplitude) for baclofen, and the recovery percent (R %) was (95.67 ± 0.5 to 109.75 ± 0.9) for meloxicam and (91.10 ± 0.56–96.88 ± 0.74) for baclofen. The degree of interference was detected from the recovery percent of the mixtures. No significant interference was detected in different mixtures.
In-vitro Dissolution Test
Pharmaburst (F1) based formula showed a high percentage of drug dissolved (100.12 ± 0.061% for meloxicam) at 15 min and (99.83 ± 0.032% for baclofen) at 4 min as shown in Figures 2 and 3. This can be explained by the fast WT and DT when compared with the other formulae. F-melt-based formula (F2) showed (83.13 ± 0.056% for meloxicam) at 15 min and (89.54 ±0.029% for baclofen) at 4 min. Prosolv-based formula (F3) showed (95.38 ±0.43% for meloxicam) at 15 min and (87.2 ± 0.52% for baclofen) at 4 min. As mentioned previously, the F-melt-based formula (F2) showed prolonged dissolution compared with Pharmaburst 500 (F1) because of slow DT due to specific surface area. Prosolv-based formulation (F3) showed less drug dissolved compared with Pharmaburst-based formula (F1). The slow WT and DT can explain this due to the prementioned reasons regarding the composition of Prosolv. In general, these results showed that Pharmaburst revealed an excellent result with water-soluble drugs (baclofen), and it could relatively enhance the dissolution of water-insoluble drugs (meloxicam). In general, co-processed excipients enhance the release of poorly soluble drugs from ODTs, and this can be attributed to the hydrophilic components incorporated within the co-processed excipients. These hydrophilic agents induce faster drug wetting, solubilization, and enhanced drug release from ODTs. The imparted hydrophilicity allowed dissipation of drug particles upon their contact with the dissolution medium.51 However, the superiority of Pharmaburst is that it contains additional sorbitol and possesses a high specific area.
 |
Figure 2 In-vitro dissolution profile of baclofen from prepared ODTs. |
 |
Figure 3 In-vitro dissolution profile of meloxicam from prepared ODTs. |
Palatability Assessment of ODT
The six volunteers had tasted the palatability of ODTs formulae (F1, F2, and F3) on three successive days. They showed a very strong bitterness taste score (+3) difficult to drink and (+4) very difficult to drink, as shown in Table 4. Using the Friedman test, there was no significant difference between different formulae in bitterness score mean rank (p = 0.1893, Friedman statistics = 4.33). So co-processed excipients failed to mask the bitter taste of baclofen. These results comply with our aim of the work in the following steps where further improvement applied of taste using six sigma.
 |
Table 4 Bitterness Taste Score of Baclofen-Meloxicam ODTs by Volunteers on Three Successive Days |
Improvement of ODTs Using the Six Sigma Methodology
Define Phase
Process mapping was performed as shown in Figure 4. The current sigma levels were calculated. The sigma level for discrete data for ODTs taste was 2.342 σ and 2.342 for ODTs solubility. As sigma levels were 2.34; hence, the process performance needs further improvement to achieve 6σ level. Table 5 illustrates the taste score of the prepared ODTs. The volunteer’s experiences (UCL, LCL) record did not comply with volunteer expectations (USL, LSL), so in this step, VOC can identify current upper and lower control limits of ODTs taste, which has shown a need for further improvement.
 |
Table 5 VOC Experience vs Expectation |
 |
Figure 4 Process map of recording activity. |
Measurement Phase
The process capability Cpk of palatability assessment of ODTs was 2.52 as shown in Figure 5, while for meloxicam in-vitro dissolution test was 3.75 as shown in Figure 6. The process capability Cpk of palatability assessment and meloxicam dissolution were >1, indicating both processes were capable and sufficient to meet the volunteer’s specifications.52 So, palatability assessment and dissolution method were excluded from being a cause of previously mentioned problems.
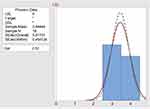 |
Figure 5 Process capability Cpk of palatability assessment of prepared ODTs. |
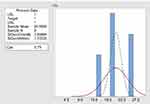 |
Figure 6 Process capability Cpk of meloxicam dissolution test of prepared ODTs. |
Analysis Phase
As shown in Figure 7, A Pareto chart illustrated that 56.3% of the defects were attributed to tablet palatability assessment, and 28.1% were attributed to the in-vitro dissolution test. Where weight variation, thickness, friability, hardness, drug content, and wetting time ratios were 3.1%. The sorting of ODTs data revealed that 56.3% and 28.1% were related to baclofen (bitter taste) and meloxicam (water-insoluble) drug nature must be given priority for improvement and corrective actions.53
 |
Figure 7 Pareto chart for process analysis of evaluation test of ODTs. |
Whereas Figure 8 illustrates the cause-and-effect diagram where the material section indicated that using co-process excipients to prepare ODTs inadequate to enhance the ODTs solubility caused by water-insoluble drug meloxicam and to mask the bitter taste caused by baclofen.
 |
Figure 8 Cause–effect diagram of ODTs preparation. |
Improve Phase
Through the preparation of ODT using lyophilization and levigation techniques, then evaluation of the improved ODT. Table 6 shows that all formulations achieved the pharmaceutical specification for weight variation.54 Friability ODTs did not lose more than 1% of the tablet weight.55 For drug content, all formulae complied with the United States Pharmacopoeia (USP 39-NF 34) limits.28 For in-vitro DT, it is reasonable to expect that using the freeze-drying technique led to the formation of more porous ODTs; hence, faster disintegration was obtained because of the faster penetration of dissolution medium into it.56 Therefore, the DT and WT were reduced compared with previous prepared ODTs (two-tailed t-test, p-value < 0.0001).
 |
Table 6 Physical Evaluation of the Improved Baclofen-Meloxicam ODTs After Applying Six Sigma Using Lyophilization Technique |
Freeze-dried ODTs exhibited high porosity and light texture, quick disintegration that ensures quick saliva penetration in pores when placed in the oral cavity. Therefore, the data presented enhancement of the dissolution after the improvement phase, as shown in Figures 9 and 10. Improved Pharmaburst formula (F4) showed complete dissolution of both drugs within 3 min. (100.07% ± 0.46) for meloxicam and (100.15 ± 0.63) while it took from Pharmaburst-based formula before the improvement phase (F1) 15 min for a complete dissolution of meloxicam (100.12 ± 0.061%) and 4 min for baclofen (99.83 ± 0.032%).
 |
Figure 9 In-vitro dissolution profile of baclofen from improved ODTs. |
 |
Figure 10 In-vitro dissolution profile of meloxicam from improved ODTs. |
Minitab v.18.1 software was used to interpret the dissolution profile result compared with the previous one via select control charts Np-chart recommended for the attribute and repetitive sampling. In addition, it indicated the number of nonconforming units of a process.57 As shown in Figures 11 and 12, UCL and LCL were inside USL and LSL after improvement, indicating the process was successfully improved.
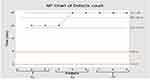 |
Figure 11 NP-chart of meloxicam dissolution in ODTs before improvement. |
 |
Figure 12 NP-chart of meloxicam dissolution in ODTs after improvement. |
The six volunteers had tasted the different ODTs formulae for bitterness score records on three successive days. All optimized ODTs had a bitterness taste score vary from (0) very easy to drink (palatable) and (+1) easy to drink (tasteless). There was a significant difference in the median bitterness score of each formula after the enhancement phase compared to the median bitterness score before the enhancement phase (two-tailed Wilcoxon signed-rank test, p-value <0.05). By applying control chart where control chart considered one of the statistical processes controlling (SPC) methods used in six sigma.58 Np-chart can interpret the result of improved ODTs compared with ODTs before improvement where Np-chart was selected to illustrate the number of defects (attributes) as shown in Figures 13 and 14.
 |
Figure 13 NP-chart of defect count of ODTs taste before improvement. |
 |
Figure 14 NP-chart of defect count of ODTs taste after improvement. |
The DSC studies of meloxicam, baclofen, and excipients revealed no change in the melting points of meloxicam and baclofen in the presence of each other and the presence of the excipients, indicating no interaction between both drugs and between any of the drugs and the excipients shown as in Figure 15.
 |
Figure 15 Differential scanning calorimetry thermograms of the raw materials and the optimal ODT formula (F4). |
Control Phase
The stability studies were carried out where the tablets were withdrawn after 30 days and analyzed for in-vitro dissolution and palatability using a control chart to compare with previous results as shown in Figures 16 and 17. The sigma level for ODTs taste after one month was 3.62 σ, while the sigma level for ODTs Solubility was 3.33 σ indicating the sustainability of the improvement.
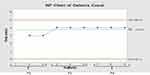 |
Figure 16 NP-chart of meloxicam dissolution from ODTS after one month. |
 |
Figure 17 NP-chart of defect count of ODTs taste after one month. |
In-vivo Study
The plasma concentration–time profiles from the improved formulation of baclofen-meloxicam Pharmaburst 500 ODT (F4) and the marketed oral tablet (Baclofen 10 mg, Al-Delta, Egypt; Melocam 15 mg, Amoun pharmaceutical co, Egypt) are represented in Figures 18 and 19. The values of Cmax, tmax and AUC (0–8) are summarized in Table 7 for both drugs from these formulations. The results indicated that improved ODTs enhance the bioavailability of both drugs compared with the marketed tablet. The oral absorption of meloxicam from ODTs was higher when compared with the marketed tablets, which was evident from the value of C max that increased significantly (two-tailed p-value = 0.0482) from 2.81 µg/mL for the marketed tablet to 3.07 µg/mL from ODTs. In contrast, there is no significant difference (two-tailed p-value = 0.9134) between Cmax of baclofen from ODT, and commercial oral tablet, the tmax of baclofen and meloxicam was shortened to 30 and 120, respectively, when compared with tmax of 120 and 180 min for the marketed tablets, respectively, which indicated that the onset of action of both drugs from ODTs was accelerated in comparison with the marketed tablets. Improvement of ODTS by six sigma methodology enhances both bitter taste of baclofen and the solubility and dissolution rate of poorly soluble drug meloxicam that reflects the availability of meloxicam ready for absorption.
 |
Table 7 Summary of the Pharmacokinetic Parameters of Meloxicam and Baclofen Following the Administration of Commercial Oral Tablets and the Baclofen-Meloxicam ODT |
 |
Figure 18 Mean plasma concentration–time curve of meloxicam following the oral administration of the reference Melocam tablets and the selected ODT. |
 |
Figure 19 Mean plasma concentration–time curve of baclofen following the oral administration of the reference Baclofen tablets and the selected ODT. |
Conclusion
Six sigma methodology was successfully used to improve already existing processes, the improvement was reflected in the results obtained from the in-vivo study where the Cmax, AUC0–8 of meloxicam was increased, and the TMAX was shortened when compared to oral tablets indicating that formulation of the poorly soluble drug in the ODTs enhances its solubility and dissolution. Also, the data retrieved from the palatability assessment confirmed that the taste of ODTs was improved. Moreover, the control phase, the sustainability of improvement, was proved. However, an accelerated stability study for six months is required to confirm this improvement; thus, six sigma is a promising methodology to address, solve, and improve any predominant problem in the pharmaceutical industry.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Neuprez A, Neuprez AH, Kurth W, Gillet P, Bruyère O, Reginster JY. Profile of osteoarthritic patients undergoing hip or knee arthroplasty, a step toward a definition of the “need for surgery.”. Aging Clin Exp Res. 2018;30(4):315–321. doi:10.1007/s40520-017-0780-1
2. Samvelyan HJ, Hughes D, Stevens C, Staines KA. Models of osteoarthritis: relevance and new insights. Calcif Tissue Int. 2020;1–14. doi:10.1007/s00223-020-00670-x
3. Mobasheri A, Bay-Jensen A-C, Van Spil WE, Larkin J, Levesque MC. Osteoarthritis year in review 2016: biomarkers (biochemical markers). Osteoarthr Cartil. 2017;25(2):199–208. doi:10.1016/j.joca.2016.12.016
4. Machado GC, Abdel-Shaheed C, Underwood M, Day RO. Non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain. BMJ. 2021;372. doi:10.1136/bmj.n104
5. Ibrahim M, Sarhan HA, Naguib YW, Abdelkader H. Design, characterization and in vivo evaluation of modified release baclofen floating coated beads. Int J Pharm. 2020;582:119344. doi:10.1016/j.ijpharm.2020.119344
6. Magni A, Agostoni P, Bonezzi C, et al. Management of osteoarthritis: expert opinion on NSAIDs. Pain Ther. 2021:1–26. doi:10.1007/s40122-021-00260-1.
7. Patel HD, Uppin RB, Naidu AR, Rao YR, Khandarkar S, Garg A. Efficacy and safety of combination of NSAIDs and muscle relaxants in the management of acute low back pain. Pain Ther. 2019;8(1):121–132. doi:10.1007/s40122-019-0112-6
8. Rani S, Kumar S, Joyti VP, Lamba D, Saini R. To compare the efficacy and safety of eperisone with thiocolchicoside in patients with acute lower backache associated with muscle spasm. Indian J Pharm Pharmacol. 2016;3(2):79. doi:10.5958/2393-9087.2016.00018.2
9. Karneev AN, Solovyova EY, Fedin AI. Efficacy and tolerability of the combined therapy with mesipol and baclosan in chronic recurrent vertebrogenic pain syndrome. Zhurnal Nevrol I Psihiatr Im SS Korsakova. 2008;108(9):48–51.
10. Kumar JNS, Gunda RK. Design, formulation and evaluation of pravastatin fast dissolving tablets. Pharm Methods. 2017;9(1):16–23. doi:10.5530/phm.2018.1.4
11. Singh K, Sharma S. Development of nifedipine orodispersible tablets by different techniques using natural and synthetic superdisintegrants. Res J Pharm Technol. 2021;14(2):715–724. doi:10.5958/0974-360x.2021.00125.6
12. Edwards P, Shihab N, Scott HW. Treatment of a case of feline baclofen toxicosis with intravenous lipid therapy. Vet Rec Case Rep. 2014;2(1):e000059. doi:10.1136/vetreccr-2014-000059
13. Yoon S, Lee SH, Yu KS, Yim SV, Kim BH. Pharmacokinetic comparison and bioequivalence evaluation of two 10-mg baclofen formulations in healthy male subjects. Int J Clin Pharmacol Ther. 2017;55(2):194–200. doi:10.5414/CP202760
14. Emam MF, Taha NF, Mursi NM, Emara LH. Preparation, characterization and in-vitro/in-vivo evaluation of meloxicam extruded pellets with enhanced bioavailability and stability. Drug Dev Ind Pharm. 2021;47(1):163–175. doi:10.1080/03639045.2020.1862175
15. Khan KU, Minhas MU, Sohail M, et al. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev Ind Pharm. 2021;47(3):465–476. doi:10.1080/03639045.2021.1892738
16. Namburu K, Kolapalli VRM, Yalavarthi PR, Vadlamudi HC, Peesa JP. Pharmacokinetic profiling and bioavailability assessment of meloxicam solid dispersion tablets. Jordan J Pharm Sci. 2018;11(2):85–92.
17. Parekh VJ, Desai ND, Shaikh MS, Shinde UA. Self nanoemulsifying granules (SNEGs) of meloxicam: preparation, characterization, molecular modeling and evaluation of in vivo anti-inflammatory activity. Drug Dev Ind Pharm. 2017;43(4):600–610. doi:10.1080/03639045.2016.1275665
18. Gulsun T, Akdag Cayli Y, Izat N, Cetin M, Oner L, Sahin S. Development and evaluation of terbutaline sulfate orally disintegrating tablets by direct compression and freeze drying methods. J Drug Deliv Sci Technol. 2018;46:251–258. doi:10.1016/j.jddst.2018.05.014
19. Gangurde A, Patole RK, Sav AK, Amin PD. A novel directly compressible co-processed excipient for sustained release formulation. J Appl Pharm Sci. 2013;3(9):89–97. doi:10.7324/JAPS.2013.3917
20. Conceição J, Adeoye O, Cabral-Marques H, Concheiro A, Alvarez-Lorenzo C, Sousa Lobo JM. Orodispersible carbamazepine/Hydroxypropyl-β-cyclodextrin tablets obtained by direct compression with five-in-one co-processed excipients. AAPS PharmSciTech. 2020;21(2):1–10. doi:10.1208/s12249-019-1579-5
21. Drašković M, Djuriš J, Ibrić S, Parojčić J. Functionality and performance evaluation of directly compressible co-processed excipients based on dynamic compaction analysis and percolation theory. Powder Technol. 2018;326:292–301. doi:10.1016/j.powtec.2017.12.021
22. Thulluru A, Madhavi C, Nandini K, Sirisha S, Spandana D. Co-processed excipients: new era in pharmaceuticals. Asian J Res Pharm Sci. 2019;9(1):1. doi:10.5958/2231-5659.2019.00001.8
23. Haleem RM, Salem MY, Fatahallah FA, Abdelfattah LE. Quality in the pharmaceutical industry - A literature review. Saudi Pharm J. 2015;23(5):463–469. doi:10.1016/j.jsps.2013.11.004
24. Chugani N, Kumar V, Garza-Reyes JA, Rocha-Lona L, Upadhyay A. Investigating the green impact of lean, six sigma and lean six sigma: a systematic literature review. Int J Lean Six Sigma. 2017;8(1):7–32. doi:10.1108/IJLSS-11-2015-0043
25. Sharma OP, Gupta V, Rathore GS, Saini NK, Sachdeva K. Six sigma in pharmaceutical industry and regulatory affairs: a review. J Nat Conscientia. 2011;2(1):273–293.
26. Carleysmith SW, Dufton A, Altria K. Implementing Lean Sigma in pharmaceutical research and development: a review by practitioners. RD Manag. 2009;39(1):95–106. doi:10.1111/j.1467-9310.2008.00542.x
27. Jeevanandham S, Dhachinamoorthi D, Chandra Sekhar KBC, Muthukumaran M, Sriram N, Joysaruby J. Formulation and evaluation of naproxen sodium orodispersible tablets - A sublimation technique. Asian J Pharm. 2010;4(1):48–51. doi:10.4103/0973-8398.63985
28. United States Pharmacopeial Convention. General chapter <905> uniformity of dosage unit In: USP 39-NF 34. Rockville, MD USA: United States Pharmacopeial Convention. Vol. 1; 2016:736. ISBN ISBN-13: 978-3769265606.
29. Thakur RR, Kashi M. An unlimited scope for novel formulations as orally disintegrating systems: present and future prospects. J Appl Pharm Sci. 2011;1(1):13–19.
30. Bhardwaj S, Jain V, Jat RC, Mangal A, Jain S. Formulation and evaluation of fast dissolving tablet of aceclofenac. Int J Drug Deliv. 2010;2(1):93–97. doi:10.5138/ijdd.2010.0975.0215.02017
31. Bashiri-Shahroodi A, Nassab PR, Szabó-Révész P, Rajkó R. Preparation of a solid dispersion by a dropping method to improve the rate of dissolution of meloxicam. Drug Dev Ind Pharm. 2008;34(7):781–788. doi:10.1080/03639040801925735
32. Srinivasan K, Muthu S, Devadasan SR, Sugumaran C. Six Sigma through DMAIC phases: a literature review. Int J Product Qual Manag. 2016;17(2):236–257. doi:10.1504/IJPQM.2016.074462
33. Rimantho D, Rahman TA, Cahyadi B, Tina Hernawati S. Application of six sigma and AHP in analysis of variable lead time calibration process instrumentation.
34. Gures N, Arslan S, Yucel Tun S. Customer expectation, satisfaction and loyalty relationship in Turkish airline industry. Int J Mark Stud. 2014;6(1):66. doi:10.5539/ijms.v6n1p66
35. Gijo EV, Antony J. Reducing patient waiting time in outpatient department using lean six sigma methodology. Qual Reliab Eng Int. 2014;30(8):1481–1491. doi:10.1002/qre.1552
36. Ullah M, Hussain I, Sun CC. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev Ind Pharm. 2016;42(6):969–976. doi:10.3109/03639045.2015.1096281
37. Weyna DR, Cheney ML, Shan N, et al. Improving solubility and pharmacokinetics of meloxicam via multiple-component crystal formation. Mol Pharm. 2012;9(7):2094–2102. doi:10.1021/mp300169c
38. Priano L, Zara GP, El-Assawy N, et al. Baclofen-loaded solid lipid nanoparticles: preparation, electrophysiological assessment of efficacy, pharmacokinetic and tissue distribution in rats after intraperitoneal administration. Eur J Pharm Biopharm. 2011;79(1):135–141. doi:10.1016/j.ejpb.2011.02.009
39. Dukova OA, Krasnov EA, Efremov AA. Development of an HPLC method for determining baclofen. Pharm Chem J. 2015;48(10):687–689. doi:10.1007/s11094-015-1172-5
40. Bae JW, Kim MJ, Jang CG, Lee SY. Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci. 2007;859(1):69–73. doi:10.1016/j.jchromb.2007.09.004
41. Sharma D, Singh M, Kumar D, Singh G, Rathore MS. Formulation development and evaluation of fast disintegrating tablets of Ambroxol hydrochloride for pediatrics- a novel approach for drug delivery. Indian J Pharm Educ Res. 2014;48(4):40–48. doi:10.5530/ijper.48.4s.6
42. Yadav AK, Sharma A, Saxena S, Kesarwani A. Mouth dissolving tablets: general overview and formulation aspects. Bull Pharm Res. 2014;4(1):43–57.
43. El-Nabarawi MA, Teaima MH, Hamid MMA, Shoman NA, Mohamed AI, El-Sahar A. Formulation, evaluation and antioxidant activity of caffeine fast melt tablets. Res J Pharm Technol. 2018;11(7):3131–3138. doi:10.5958/0974-360X.2018.00575.9
44. Moqbel HA, ElMeshad AN, El-Nabarawi MA. A pharmaceutical study on chlorzoxazone orodispersible tablets: formulation, in-vitro and in-vivo evaluation. Drug Deliv. 2016;23(8):2998–3007. doi:10.3109/10717544.2016.1138340
45. Tayel SA, El Nabarawi MA, Amin MM, AbouGhaly MHH. Comparative study between different ready-made orally disintegrating platforms for the formulation of sumatriptan succinate sublingual tablets. AAPS PharmSciTech. 2017;18(2):410–423. doi:10.1208/s12249-016-0517-z
46. Khairnar DA, Anantwar SP, Chaudhari CS, Shelke PA. Superdisintegrants: an emerging paradigm in orodispersible tablets. Int J Biopharm. 2014;5(2):119–128.
47. Naguib MJ, Makhlouf AIA. Scalable flibanserin nanocrystal-based novel sublingual platform for female hypoactive sexual desire disorder: engineering, optimization adopting the desirability function approach and in vivo pharmacokinetic study. Drug Deliv. 2021;28(1):1301–1311. doi:10.1080/10717544.2021.1938755
48. Dobetti L. Fast-melting tablets: developments and technologies. Pharm Technol Eur. 2000;12(9):32–42.
49. Fouad SA, Malaak FA, El-Nabarawi MA, Zeid KA. Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: in-vitro optimization/in-vivo evaluation. PLoS One. 2020;15(1212):e0244646. doi:10.1371/journal.pone.0244646
50. Jacob S, Shirwaikar A, Joseph A, Srinivasan K. Novel co-processed excipients of mannitol and microcrystalline cellulose for preparing fast dissolving tablets of glipizide. Indian J Pharm Sci. 2007;69(5):633–639. doi:10.4103/0250-474x.38467
51. Brniak W, Maślak E, Jachowicz R. Orodispersible films and tablets with prednisolone microparticles. Eur J Pharm Sci. 2015;75:81–90. doi:10.1016/j.ejps.2015.04.006
52. Chang SI, Yen DC, Chou CC, Wu HC, Lee HP. Applying six Sigma to the management and improvement of production planning procedure’s performance. Total Qual Manag Bus Excell. 2012;23(3–4):291–308. doi:10.1080/14783363.2012.65738743
53. Erbiyik H, Saru M. Six sigma implementations in supply chain: an application for an automotive subsidiary industry in bursa in turkey. Procedia - Soc Behav Sci. 2015;195:2556–2565. doi:10.1016/j.sbspro.2015.06.447
54. Gawas SM, Dev A, Deshmukh G, Rathod S. 65 ©Pharmaceutical and biological evaluations current approaches in buccal drug delivery system. Pharm Biol Eval. 2016;3(2):165–177.
55. Bhusnure O, Nandgave A, Gholve SB, Thonte SS, Shinde CA, Shinde N. Formulation and evaluation of fast dissolving tablet on montelukast sodium by using QbD approach. Indo Am J Pharm Sci. 2015;5:1092.
56. Gunda RK, Kumar J, Jayakumari S, Vijayalakshmi A, Satyanarayana V. Formulation development and evaluation of risperidone fast dissolving tablets. J Pharm Res. 2016;2016:10.
57. Khan N, Aslam M, Ahmad L, Jun CH. A control chart for gamma distributed variables using repetitive sampling scheme. Pakistan J Stat Oper Res. 2017;13(1):47–61. doi:10.18187/pjsor.v13i1.1390
58. Giner-Bosch V, Tran KP, Castagliola P, Khoo MBC. An EWMA control chart for the multivariate coefficient of variation. Qual Reliab Eng Int. 2019;35(6):1515–1541. doi:10.1002/qre.2459
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

