Back to Journals » Risk Management and Healthcare Policy » Volume 17
Feedback Intervention for the Control of Glucocorticoid Prescription in Primary Care Institutions: A Cluster Randomized Cross-Over Controlled Trial in Southwest China
Authors Liu L, Wang L, Zhou H, Yang J, Wang W, Luo X, Chang Y
Received 12 October 2023
Accepted for publication 17 December 2023
Published 5 January 2024 Volume 2024:17 Pages 49—63
DOI https://doi.org/10.2147/RMHP.S441165
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Gulsum Kubra Kaya
Ling Liu,1,* Lei Wang,2,* Hanni Zhou,3,4 Junli Yang,3 Wenju Wang,1 Xiaobo Luo,1 Yue Chang3,4,*
1School of Public Health, the Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China; 2Primary Health Department of Guizhou Provincial Health Commission, Guiyang, Guizhou Province, People’s Republic of China; 3School of Medicine and Health Management, Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China; 4Center of Medicine Economics and Management Research, Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yue Chang; Hanni Zhou, School of Medicine and Health Management, Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China, Email [email protected]; [email protected]
Purpose: The purpose of this study is to assess the effect of glucocorticoid prescription feedback intervention in complex primary care institutions for regulating its inappropriate use.
Design, Setting and Interventions: A six-month cluster randomized cross-over controlled trial was conducted in primary care institutions. A total of 347 physicians from 69 participating institutions were randomly allocated to either group A or group B. Both groups were given feedback interventions or serve as control. The feedback intervention comprised two components: a real-time pop-up warning of inappropriate glucocorticoid prescriptions based on the Hospital Information System and a high-proportion prescription feedback intervention warning system.
Outcome Measures: The primary outcome measure was the 10-day inappropriate glucocorticoid prescription rate, while the 10-day glucocorticoid prescription rate served as secondary outcome measure.
Results: At baseline, the 10-day inappropriate glucocorticoid prescription rates were 66.63% and 66.57% in group A and group B, respectively, showing no significant difference (p = 0.140). Following the intervention, group A exhibited a significant reduction in 10-day inappropriate glucocorticoid prescription rate at the crossing point by 13.69% (p < 0.001). In contrast, group B, which served as the control group, experienced an increase of 5.93% (p = 0.037) at the same crossover point. After the crossover point, there was a decrease in 10-day inappropriate glucocorticoid prescription rate for both groups. Group B as the intervention group demonstrated a reduction of 28.22% compared to the crossing point (p < 0.001), whereas group A showed a decrease of 12.20% (p = 0.339). The characteristics of physicians did not significantly influence the inappropriate glucocorticoid prescription rate.
Conclusion: The real-time pop-up warning of inappropriate glucocorticoid prescriptions based on the Hospital Information System and high-proportion prescription feedback intervention warning system can effectively regulate the inappropriate glucocorticoid prescribing behavior of physicians.
Trial Registration: ISRCTN11747547.
Keywords: glucocorticoids, feedback intervention, primary care institutions, cluster randomized cross-over controlled trial
Introduction
Currently, the inappropriate use of glucocorticoids remains a significant issue in both developed and developing nations. In the UK, there has been a more than 30% increase in the prescription of oral glucocorticoids.1 Among 113 patients in India, an unreasonable proportion of 88.4% was found for glucocorticoid prescriptions.2 In France, the prescription rate of oral glucocorticoids exceeds 17%, with a majority being prescribed inappropriately.3 In the United States, over 11% of acute respiratory tract infections are deemed inappropriate for systemic glucocorticoid therapy.4 In addition, abuse and overuse of glucocorticoids are prevalent in China,5,6 particularly within primary care institutions.7,8 According to a research report, in 2012, glucocorticoid prescriptions constituted 63.5% of inappropriate prescriptions across 27 primary care institutions in Jiangxi Province.9 Our previous retrospective study found that 68.2% of the prescriptions for glucocorticoids were inappropriately used in 58 primary care institutions in Guizhou Province in 2020.10 The inappropriate use of these glucocorticoids can lead to adverse reactions,11–14 thus, it is crucial to identify an efficacious intervention to alleviate their inappropriate use.
Researchers have previously developed various interventions to regulate prescription inappropriate use, such as mail delivery intervention, web-based intervention, educational intervention, expert audit and feedback intervention.15–22 However, these methods may be perceived as mandatory and censored by physicians, potentially eliciting negative emotions and requiring long-term intervention by professionals. Therefore, it is imperative to identify an intervention that is widely accepted, cost-effective, and efficacious.
Based on our previous studies on the patterns of glucocorticoid prescriptions,9 high rates of antibiotic prescription feedback interventions and real-time antibiotic pop-up warning intervention,23,24 this study aimed to control the inappropriate use of glucocorticoid prescription and over-proportion while analyzing the outcomes and intervention methods. A non-mandatory, non-censored and individual feedback intervention was implemented to enhance the prescribing practice of glucocorticoids by general practitioners in primary care institutions.
Materials and Methods
This study adheres to the Consolidated Standards of Reporting Trials (CONSORT)25,26 and its extensions for cluster randomized trials27 and randomized crossover trials,28 as demonstrated in Additional File 1.
Trial Design and Participants
A cluster randomized cross-over controlled trial was conducted in Guizhou Province from April 1 to October 10, 2022. According to the principle of randomization, study subjects were randomly allocated into two groups, A and B, during this trial. Based on pre-determined intervention sequences, the two groups will undergo alternating intervention and control periods. Both groups underwent intervention and control periods to effectively mitigate selection bias resulting from the choice of research subjects by either the intervention or control group.29 As illustrated in Figure 1, during the first phase of the study, group A underwent a behavior change intervention for a duration of three months, while group B served as the control without any intervention. As this was a crossover design study aimed at exhorting behavioral changes, no washout period was implemented. In the second phase, group B underwent an identical intervention for a duration of three months while group A was assigned to the control cohort. Physicians treating patients in the control arm did not receive any interventions and followed standard treatment protocols. During this time, all prescribing information from the physicians was recorded. The trial run for 6 months, with a crossing point on July 11.
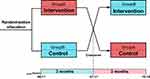 |
Figure 1 Schematic diagram depicting cross-intervention strategies. |
The study was carried out in the primary care institutions in Guizhou Province, a region of southwest China characterized by relatively low levels of medical expertise. As of 2021, the number of practicing (assistant) physicians per 1000 inhabitants residing in rural areas of Guizhou amounted to 2.32.30 The study encompassed 289 public primary care institutions in Guizhou Province, all utilizing a standardized Hospital Information System (HIS), with the inclusion criteria being as follows: (1) public primary care institutions with more than 3 general practitioners; (2) outpatient physicians who prescribe glucocorticoids every month while on duty; (3) physicians with a tenure of over 1 year in primary care institutions’ hospitals. Prior to the commencement of the trial, informed consent was obtained from all participating physicians (Additional File 2). A total of 347 physicians from 69 primary care institutions met the inclusion criteria.
The HIS system involved in this study was designed and developed by Guizhou Lianke Weixin Technology Co., LTD. (LWTC). The company accesses data from the data platform port of the Information Center Guizhou Provincial Health Commission (ICGPHC). Our project team has signed a tripartite cooperation intention agreement (Additional File 3) with ICGPHC and LWTC. In March 2021, we conducted an early feedback intervention in antibiotic prescription for infectious diseases in primary care institutions.24 The warning system utilized in this feedback intervention was also co-developed with LWTC. We endeavored to apply this early warning system in the glucocorticoid prescription control intervention study. Building upon that foundation, this study aims to further optimize the real-time early warning system and expand its research scope.
Interventions
In this study, a combined early warning and real-time system was employed to implement an intervention for the inappropriate use of glucocorticoids. Feedback intervention is a deliberate behavior aimed at enhancing individual performance by providing purposeful and conscious feedback on past behaviors and operations, thereby inducing internal motivation in individuals and influencing their perceptions.31 The two components of the feedback intervention included:
The first part was a real-time pop-up warning of inappropriate glucocorticoid prescriptions based on the HIS. In our prior research, a recommendation form was developed to evaluate the appropriate use of glucocorticoid prescriptions in primary care institutions (Additional File 4).10 The corresponding contents of the recommendation form were recorded in HIS by LWTC’s engineers as algorithm rules: (1) The initial step involves identifying the presence of glucocorticoids in a physician’s prescription based on the drug name. (2) Subsequently, the patient’s disease type is determined by examining the first three digits of their ICD-10 code (International Classification of Diseases, 10th Edition) or, if unavailable, by identifying their disease name. (3) Finally, the appropriateness of the prescription is assessed based on recommendations provided in a reference table. The real-time pop-up warning system automatically retrieves physician prescription data from the background and compares it with algorithmic rules to determine the appropriateness of glucocorticoid prescriptions in this diagnosis and treatment service. Whenever a physician prescribes inappropriate glucocorticoids in the HIS, a pop-up window will appear in the lower right corner of the screen to alert them. As illustrated in Figure 2, an example of a pop-up window is presented to indicate inappropriate use of glucocorticoids. The pop-up window not only displays the specific type of prescription being inappropriately employed by the physicians but also provides accurate medical guidance. Upon clicking the pop-up window, it will disappear. Otherwise, it will vanish automatically after five minutes. The system records the duration of each pop-up window viewed by physicians and ensures confidentiality. Physicians have the autonomy to either read or disregard this information. Based on our previous research,10 and with reference to the Chinese Clinical Application Guidelines for glucocorticoids32 and Chinese Ministry of Health Standards for Hospital Prescription Review and Management.33 Additionally, we consulted the articles by Liu et al34 and Yasir et al.35 In this study, inappropriate glucocorticoid prescriptions were categorized into two types: (1) Inappropriate indications, such as systemic use of glucocorticoid to relieve cold, fever, pain, etc. (2) Inappropriate selection, such as short-acting rather than long-acting systemic glucocorticoids should be selected.
 |
Figure 2 Examples of the pop-up windows indicating inappropriate use of glucocorticoids. |
The second part is a high-proportion prescription feedback intervention warning system. Based on the plug-in developed in previous studies on antibiotic prescription intervention,36 we replaced it with a new glucocorticoid prescription rate warning plug-in and asked LWTC engineers to implant it in the target HIS. Additionally, a more user-friendly interface was designed incorporating feedback from physicians in prior research. Physicians have the freedom to peruse feedback at their discretion. During the intervention period, when outpatient physicians in participating primary care institutions accessed the HIS, pop-ups and links would automatically appear with information updated every ten days. In our previous research,23,24 we discovered that conducting feedback three times per month provides sufficient intensity for busy physicians without feeling overly frequent. Upon clicking any of them, the information shown in Figure 3 popped up. The top left corner displays the top 5 prevalent diseases in glucocorticoid prescriptions by physicians every 10-day. The upper right corner provides information on the start and end times of each 10-day period, along with the total number of glucocorticoid prescriptions issued during that time frame. The lower left corner presents data on the frequency of different types of glucocorticoid prescriptions and the glucocorticoid prescription rates per 10 day. In the middle-right section, physicians’ rankings based on glucocorticoid prescription rate per 10-day within their primary care institutions are shown. Finally, in the lower right corner, contraindications for the most use of glucocorticoids are listed. The provided information is strictly confidential and accessible exclusively to the physician. If ESC is pressed by the physician, it disappears. All on-screen actions, including click-through rate and time spent reading messages, are automatically recorded.
 |
Figure 3 Prescription of glucocorticoids and ranking information of primary care outpatient physicians. |
Data Collection and Management
With the assistance of LWTC engineers, a download program was utilized to extract data pertaining to glucocorticoid prescriptions, as well as total prescription data and patient information from in primary care physicians participating in this study. The medical records of glucocorticoids comprise patient ID, personal basic information, existing disease, disease code of ICD-10, and types of glucocorticoids. The demographic information of physicians was obtained from the personnel section of primary care institutions. The relationship between physicians and patients can be established through coding to generate a database of medical service information. For the purpose of this study, systemic glucocorticoids were analyzed while excluding prescriptions for topical glucocorticoids such as nasal inhalation and skin creams.
Outcomes
Based on our previous research,23,24 a frequency of 10-day was determined to be appropriate. The primary outcome measure was the 10-day inappropriate glucocorticoid prescription rate (IGPR), calculated as the number of inappropriate glucocorticoid prescriptions written in the 10-day period divided by the total number of glucocorticoid prescriptions. The 10-day glucocorticoid prescription rate (GPR) was a secondary outcome measure, which was the number of glucocorticoid prescriptions written in a 10-day period divided by the total number of prescriptions. The characteristics of physicians (gender, age, education, title, working years) and prescription volume were analyzed as covariates.
Sample Size
This study involved the sample size calculation of the number of general outpatient physicians in primary care institutions. The dependent variable (IGPR) was continuous variables, so two independent means (two-tailed test) are used to calculate the sample size. Based on the results of a previous intervention study,24 the incidence rates of inappropriate prescriptions were recorded at 65% and 60%, respectively, while maintaining a standard deviation of 15% within each group. It is anticipated that there will be a reduction of 5% in inappropriate glucocorticoid prescriptions following the intervention. The ratio of sample size between the two groups was 1.00, the type I error (α) was 0.05, and the typeII error (β) was 0.20. Therefore, the intervention group and control group need at least 142 observation objects in each group. Considering the problem of drop-out, the sample size is set as 160 physicians in each group, and a total of 320 physicians.
Randomization and Blinding
Using a table of random numbers, the information technology staff at LWTC randomly selected 69 primary care institutions out of 100 that met the inclusion criteria. A total of 347 eligible outpatient physicians participated in the intervention trial. Figure 4 illustrates the study’s flow chart. The physicians were divided into two groups through randomization, and due to their clear awareness of participation in the intervention, blinding was not necessary.
 |
Figure 4 The flow chart of this study. |
Statistical Methods
Following normality test, Student’s t test and rank-sum test were used to compare the IGPR and GPR between groups A and B. Or whether there are differences before and after the same group of interventions. Intervention effects were measured by comparing the IGPR and the GPR at baseline, crossover and at the end of the trial. Dynamic changes in the number of glucocorticoid prescriptions were also used to measure intervention effectiveness. The multilevel model was employed to investigate the factors that influence the effectiveness of the intervention. Specifically, a two-level model was constructed with repeated measurement points as level 1 and physicians as level 2. The multilevel model separates the random variation among physicians from the random errors in the measurements while also allowing for further adjustment of complex error structures at the physician level. All data in this study were analyzed using R version 4.2.1.
Trial Registration
The trial was registered with the ISRCTN Registry on December 2, 2021, and assigned the registration identifier ISRCTN11747547 in accordance with established protocols for clinical trial registration (https://doi.org/10.1186/ISRCTN11747547).
Results
The study recruited 347 physicians from 69 primary care institutions for a 180-day (6-month) intervention trial. A total of 169 physicians from 34 primary care institutions were randomly assigned to group A and received the intervention for a duration of 90 days, followed by a subsequent 90-day period designated as the control group. In contrast, group B comprised 178 physicians from 35 primary care institutions who served as a control group during the first phase and subsequently intervened in the second phase. A total of 35,572 glucocorticoid prescriptions were included in the analysis. The baseline characteristics of the participating physicians are presented in Table 1. No statistically significant difference was observed between the two groups of physicians with regard to gender, age group, education, title and working years. The two were deemed to be commensurate.
 |
Table 1 Baseline Characteristic of the Physicians [n (%) or M (P25, P75)] |
Figure 5 depicts the IGPR over a period of 10 days for both groups, A and B. The graph illustrates a general decrease in IGPR for both groups from the baseline to the conclusion of the trial. In the first phase of the study, following intervention in group A, there was a significant reduction of 13.69% (p < 0.001) in IGPR compared to baseline, whereas group B experienced an increase of 5.93% (p = 0.037). In this phase, group A demonstrated a consistent downward trend during the initial 30-day period. However, by day 30 of the intervention, there was a slight increase in IGPR that did not exceed baseline levels and persisted until day 80. On the other hand, group B exhibited a slight decrease in IGPR during the first 30 days of this phase but displayed an overall upward trend from day 30 of intervention onwards and surpassed baseline levels. We observed a consistent pattern in both groups during this phase, wherein IGPR levels beginning to increase on day 30 and subsequently declining by day 80. In the second phase (after the crossover point), group B was subjected to intervention, while group A served as the control. The IGPR in group B decreased by 28.22% (p < 0.001), and in group A, it decreased by 12.20% (p = 0.339) from the crossover point onwards. At this phase, both groups demonstrated a significant decline in IGPR. However, by day 150 of the intervention, there was a slight increase in IGPR for both groups that did not surpass the crossover point. At the crossover point, there was a statistically significant difference between the two groups (p < 0.001). We observed distinct differences in the IGPR trends between the two groups following the implementation of the same intervention. Furthermore, when both groups were subjected to control, divergent IGPR trends were also evident.
 |
Figure 5 Comparison of IGPR over time between the two groups. |
The temporal trend of GPR is illustrated in Figure 6. In the first phase, group A demonstrated a decrease of 0.11% (p = 0.581) in GPR compared to baseline, while group B demonstrated an increase of 1.33% (p < 0.001). During this phase, group A’s GPR displayed a consistent downward trend for the before 40 days. However, by day 50 of the intervention, there was a slight upturn observed. Conversely, group B’s GPR demonstrated an overall ascending trajectory throughout this phase. In addition, both groups exhibited a gradual increase in GPR from day 40 onwards, followed by a subsequent decline on day 80. In the second phase, group B exhibited a decrease in GPR by 2.01% (p < 0.001), while group A showed a reduction of 0.35% (p = 0.198). Both groups demonstrated a declining trend in GPR during this phase, with the difference between them being statistically significant at the crossover point (p = 0.008). Meanwhile, we observed a distinct divergence in the trajectory of GPR change between the intervention and control groups. Specifically, as an intervention group, group A exhibited fluctuations in GPR, while group B demonstrated a stable trend. Conversely, as a control group, both groups experienced fluctuations but group B displayed a clear upward trend in GPR.
 |
Figure 6 Comparison of GPR over time between the two groups. |
Figure 7 present the dynamics of the number of glucocorticoid prescriptions in groups A and B and compare the number of prescriptions with the IGPR. Specifically, Fig 7-A and Fig 7-B respectively depict the situations of group A and group B. These two figures indicate a comparable pattern between fluctuations in IGPR and variations in the number of glucocorticoid prescriptions. As the number of prescriptions decreased, the proportion of inappropriate glucocorticoid prescriptions relative to appropriate ones also decreased, resulting in a decline in IGPR. In the first phase, there was a gradual decrease in the number of glucocorticoid prescriptions within group A during the first 30 days of intervention, which corresponded with a reduction in IGPR. However, on day 50, an increase in prescription volume led to a subsequent rise in IGPR. The number of prescriptions in group B exhibited an upward trend at this phase, accompanied by a corresponding increase in the IGPR. During the second phase, there was a general decline in glucocorticoid prescriptions for participants in group B, which corresponded with a decrease in IGPR as well. Group A also observed a similar trend during this period. Simultaneously, we observed a minor peak in the IGPR during the second phase, specifically on day 160 for group A and day 170 for group B. However, it is worth noting that the overall prescription volume remained low.
The results of a multilevel model analysis on the factors influencing the intervention effect are presented in Table 2. ICC (intra-class correlation) is 0.17 obtained from fitting the null model suggests a high level of clustering in the data, indicating that the hierarchical structure cannot be disregarded. This implies that there are variations in IGPR among different physicians. The results indicate that IGPR is influenced not only by the presence or absence of an intervention (p < 0.001) but also by the order in which intervention is administered across different groups (p < 0.001). In this study, group A received the intervention first and then served as the control group, while group B underwent the opposite sequence. Furthermore, there was significant variability observed at various time points of measurement (p < 0.001). The intercept coefficient is 0.61, indicating that the baseline’s average predicted IGPR is 61%. Physicians in the control group exhibited higher IGPR than those in the intervention group (95% CI: 0.01–0.05). Moreover, physicians in group B who underwent control followed by intervention exhibited higher IGPR compared to those in group A who underwent intervention followed by control (95% CI: 0.04–0.11). The pertinent physician characteristics did not exert a significant impact on the IGPR. The findings also suggest that gender (p = 0.016) may be the sole factor influencing GPR.
 |
Table 2 Multilevel Model Was Employed to Investigate the Factors That Influence the Effectiveness of Intervention |
Discussion
We devised, implemented, and assessed two feedback interventions aimed at addressing the issue of inappropriate glucocorticoid prescribing by physicians. Our findings indicate that both groups exhibited an overall downward trend in IGPR during the trial period. The trend of IGPR was observed to be in line with the number of glucocorticoid prescriptions. The results of the multilevel model indicate that the application of feedback intervention and the order in which interventions are implemented have significant effects on IGPR, with variations observed at different measurement points. However, there was no significant change in GPR following the intervention.
Our previous investigation into prescription patterns revealed a widespread inappropriate use of glucocorticoids in primary care institutions throughout China. The persistent lack of effective regulatory mechanisms for glucocorticoid usage in Guizhou Province and the entire country is concerning.10 Therefore, this study applied the main interventions that were previously used in our trials on antibiotic prescribing to control the inappropriate prescription of glucocorticoids.23,24 Previous studies have focused on changes in the rate or quantity of prescriptions to regulate appropriate prescription use.15,36–39 Similarly, our 2018 trial on antibiotic prescribing interventions also emphasized changes in prescribing rates.23 The subsequent expanded trial conducted in 2021 considered both prescribing and inappropriate rates.24 In this study, we employed the IGPR as the primary outcome measure to assess the impact of the intervention. Additionally, we considered the GPR as a secondary outcome measure. In this manner, the issue of glucocorticoid prescription can be more comprehensively and effectively managed.
The study revealed that the feedback interventions implemented to modify prescribing behavior among primary care physicians were efficacious in reducing IGPR. At baseline, both groups exhibited similar IGPR of 66.63% and 66.57%, respectively, which was consistent with our previous research.10 The results depicted in Figure 5 demonstrates that intervention led to a significant reduction in IGPR for both group A and B, decreasing by 25.89% and 22.29%, respectively, from baseline to trial end. Prescription feedback interventions are a cost-effective and timely approach that has demonstrated positive outcomes across various medical domains.40 The feedback intervention applied to the physician is presented automatically, ensuring privacy and granting initiative to the physician. It has been observed that an intervention which does not impede workflow or infringe upon freedom of choice is appealing to clinicians’ sense of pride in their performance.41 The “real-time pop-up warning of inappropriate glucocorticoid prescriptions based on the HIS” in the feedback intervention is designed to serve as a timely alert system for physicians regarding potential issues associated with glucocorticoid prescriptions and provide repeated feedback over time. This approach is increasingly recognized as effective in improving the quality of feedback.42 Another measure in the feedback intervention, the high-proportion prescription feedback intervention warning system contained statistical information regarding the diagnosis and use of glucocorticoids, as well as contraindications and precautions for their administration. Additionally, a ranking of glucocorticoid prescribing rates is included, which is essentially a peer comparison. Peer comparison is an effective and low-cost tool for changing physician behavior at the health system level.38 A cluster randomized clinical trial conducted in the United States, which utilized email communication to clinicians also demonstrated positive results.43 Similarly, a randomized controlled trial aimed at addressing excessive prescription of antibiotics and injectable medicines in middle-income countries substantiated the efficacy of peer comparisons.36
Furthermore, as depicted in Figure 5, despite observing a downward trend in the IGPR of both groups A and B during the intervention period, it is worth noting that this trend did not exhibit a continuous decline but rather displayed some fluctuations. These fluctuations may be attributed to behavior change being a gradual and phased process.44 There is a growing interest in utilizing behavioral science to identify novel social cognitions or devices that can facilitate clinical decision-making.45 Behaviorology regards behavior as a product of reinforcement, often originating from specific situations and forming conditioned reflexes through repeated repetition.46 This emphasizes that behavior change is not sudden but requires continuous reinforcement. While physicians’ inappropriate prescribing behavior can be significantly improved through intervention, consistent reinforcement is necessary to establish proper prescribing habits in the long term.
In addition, Figure 5 illustrates that group A, serving as the control group in the second phase, also demonstrated a declining trend in IGPR potentially attributed to carry-over effects. We have also observed carry-over effects in our two previous crossover trials on antibiotic prescription.23,24 The cross-over design is a frequently utilized approach for comparing the effects of two treatments in clinical trials.29 In our study, we adopted a crossover design to observe the dynamic impact of an intervention applied to physicians on their inappropriate prescribing behavior. It is commonly assumed that the physicians included in our studies have an equal probability of being assigned to either the intervention or control group. Thus, allowing for the possibility of all physicians initially receiving the intervention and subsequently acting as part of a control group. Thereby mitigating any potential impact on trial results due to different intervention sequence among groups. However, physicians who were initially assigned to the intervention group and then switched to the control group may have been influenced by intervention sequence, resulting in a sustained reduction in the IGPR. Therefore, the sequence effect essentially represents a carry-over effect. The multilevel model analysis results provide evidence for the carry-over effects. As anticipated, the overall variation in trial outcomes among physicians was contingent upon the sequence of interventions resulting from different groups. Due to the difference in grouping, physicians assigned to group A, who received the intervention first, exhibited a lower IGPR on average compared to those in group B. The intervention had an impact on the outcome of the second phase for group A as a control. The sustained decrease in group A’s IGPR during the second phase is likely attributed to the carry-over effect of the intervention implemented during the first phase. Moreover, the multilevel model revealed that physician characteristics did not significantly impact changes in IGPR, except for feedback interventions and sequence and measurement points. This indicates that our interventions are highly feasible for implementation.
The intervention did not yield a significant impact on GPR change in this study, as illustrated in Figure 6. These negative findings contrast with the results obtained from our previous study.24 At the end of the trial, there was only a marginal reduction of 0.46% and 0.44% in GPR compared to baseline, which could be attributed to the relatively low prescription rate of glucocorticoids as opposed to antibiotics or similar drugs. When prescription rates are inherently low, any strategy aimed at reducing their use becomes more challenging than in other cases.42
In Figure 6, we have also observed that these fluctuations in GPR during first phase may have been influenced by the prevalence of influenza during the same period. Possibly attributed to an upsurge in influenza-like illness (ILI) cases during influenza epidemics. Physicians’ prescribing practices may be affected by a general rise in consultations for infectious pathologies during periods of increased circulation of respiratory infections.47 ILI is characterized by an acute onset of fever, cough, and a range of other possible symptoms such as headache, myalgia, nasal congestion, fatigue, chills, and sore throat.48 The manifestation of respiratory infection symptoms may lead to an increased frequency of glucocorticoid prescription by physicians. In our previous study,10 we observed that primary care physicians frequently prescribed glucocorticoids for respiratory diseases. A cross-sectional study conducted at outpatient departments in referral hospitals in Ethiopia reported that 63.50% of glucocorticoids were utilized for the treatment of respiratory diseases.49 However, it is important to note that evidence-based clinical practice guidelines and recommendations from the Centers for Disease Control and Prevention suggest that glucocorticoids are not effective for acute respiratory infections.50 According to the 19th Week 2022 Influenza Weekly report released by the Chinese National Influenza Center,51 the positivity rate of influenza virus detection in the southern provinces exhibited a slight increase during the 30th to 40th day when our intervention was implemented. On the 50th to 60th day of the intervention, the positivity rate for influenza virus detection continued to increase in southern provinces, with some areas entering their summer peak period. The percentage of ILI reported by sentinel hospitals in these provinces was higher than that of the previous week and the corresponding period from 2019 to 2021.52 During this period, we observed an increase in GPR levels among both the intervention group A and control group B. After the 80th day of the intervention, there was a decrease in the percentage of ILI observed in specific regions, indicating a decline from the previous week.53 Simultaneously, both group A and group B demonstrated a reduction in their GPR at this point. Therefore, both groups exhibited an increase in GPR at day 40 of the intervention followed by a subsequent decrease at day 80. It is noteworthy that although influenza may have affected GPR in both groups during first phase, group A, which received the intervention, exhibited a lower GPR than baseline at the crossover point. In contrast, group B demonstrated an increasing trend in GPR from baseline when no intervention was administered. The reason for this difference is that only group A received the intervention while group B did not. Additionally, it has been observed that the impact of influenza can result in divergent trends in GPR when implementing the same intervention across different groups or when both groups serve as controls.
Meanwhile, as depicted in Figure 7, the comparison between the number of glucocorticoid prescriptions and the IGPR revealed a similar trend. As more prescriptions were written for glucocorticoids, there was a higher proportion of inappropriate use leading to an increase in IGPR. There may have been a shift in prescribing patterns between days 30 and 80 as a result of the impact of influenza. We observed an increase in IGPR in groups A and B on day 30 of the intervention and then a decrease on day 80, as shown in Figure 5. Similarly, resulting in divergent trends of IGPR changes when confronted with identical interventions. Although influenza may have impacted IGPR at this phase, the intervention we implemented on group A showed visible effects. In the first phase, when group A received the intervention, there was an overall downward trend observed in IGPR with a reduction of 13.69% in the crossover point from baseline, despite some fluctuations throughout this period. On the other hand, as the control group, group B demonstrated an overall upward trend in IGPR. The IGPR at crossover for group B increased by 5.93% from baseline.
In Figure 7, we also observed a small IGPR peak in group A on day 160 and group B on day 170. However, the total glucocorticoid prescription volume decreased during this period. This decline can be attributed to the impact of the COVID-19 pandemic on our trial region. The majority of patients exhibited symptoms related to respiratory infections, leading physicians to prescribe glucocorticoid accordingly.10 Nevertheless, due to adjustments in epidemic prevention policies, there has been a rapid increase in demand for mild cold drugs.54 Consequently, individuals are opting for nearby physical or online pharmacies to purchase medication instead of visiting hospitals. As a result, overall glucocorticoid prescription numbers have declined.
The study has several limitations. Firstly, the implementation of the two parts of the intervention trial in this study, namely the real-time pop-up warning of inappropriate glucocorticoid prescriptions based on the HIS and the high-proportion prescription feedback intervention warning system, occurred concurrently. Therefore, assessing its individual impact becomes unfeasible. Future studies could adopt a multi-group intervention approach to evaluate each effect separately. Secondly, the duration of intervention effects remains uncertain at this time. Therefore, it is considered to periodically reassess the efficacy of the intervention in future evaluations. Thirdly, it remains uncertain whether glucocorticoid interventions have an impact on the prescribing behavior of other medications and if such changes in prescribing patterns could affect patients.
Conclusion
Our non-mandatory, non-censored and individual feedback interventions have proven to be effective in controlling inappropriate glucocorticoid prescribing behavior among physicians. They effectively remind physicians of deviations in prescription behavior and provide humanized suggestions that greatly improve doctor participation and intervention effectiveness. These interventions are not influenced by factors such as gender, age, education, title or workload. However, interventions do not demonstrate efficacy in reducing GPR when the initial rate of prescribing is already low.
Abbreviations
HIS, Hospital Information System; LWTC, Lianke Weixin Technology Co., LTD.; ICD-10, International Classification of Diseases, 10thEdition; IGPR, inappropriate glucocorticoid prescription rate; GPR, glucocorticoid prescription rate; IGP, Inappropriate glucocorticoid prescriptions; AGP, Appropriate glucocorticoid prescriptions; Ref, Reference group.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available upon reasonable request from the corresponding author.
Ethics Approval and Informed Consent
The trial was approved by the Human Trial Ethics Committee of Guizhou Medical University (Certificate No.: 2021 (249)) in October 28, 2021). Informed consent was obtained from all participating physicians prior to the commencement of the trial.
Consent for Publication
The informed consent was signed by both us and the physicians participating in the trial.
Acknowledgments
We express our gratitude to all participating institutions for their invaluable information and assistance throughout the research process. The authors also extend their appreciation to every member of the survey team who diligently collected the data. Additionally, we would also like to acknowledge Edward McNeil, from Prince of Songkla University, Thailand, for his insightful feedback on improving this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study received support from the Natural Science Foundation of Guizhou Province, “Feedback intervention model of Gradient Boosting Decision Tree (GBDT) technology on glucocorticoid prescription control in primary care institutions” (Guizhou Science and Technology Foundation-ZK [2021] General 499), as well as the Medical Economics and Management Research Center of Guizhou Medical University (GMUMEM2022-A05). We would like to acknowledge the invaluable contributions of our funders who generously supported this research endeavor. Specifically, they facilitated travel expenses during data collection and provided expert guidance on study design, technical aspects, and data analysis.
Disclosure
The authors declare no conflicts of interest.
References
1. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982–1990. doi:10.1093/rheumatology/ker017
2. Masih S, Cynthia Stephen S, Joy Armstrong L, Finny P. Use and misuse of glucocorticoids in the community of Raxaul Block, North Bihar. Trop Doct. 2015;45(2):68–72. doi:10.1177/0049475514567756
3. Bénard-Laribière A, Pariente A, Pambrun E, Bégaud B, Fardet L, Noize P. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open. 2017;7(7):e015905. doi:10.1136/bmjopen-2017-015905
4. Dvorin EL, Lamb MC, Monlezun DJ, Boese AC, Bazzano LA, Price-Haywood EG. High Frequency of Systemic Corticosteroid Use for Acute Respiratory Tract Illnesses in Ambulatory Settings. JAMA Intern Med. 2018;178(6):852–854. doi:10.1001/jamainternmed.2018.0103
5. Tang Y, Shang N, Du XL, Yuxuan Z, Dezhi W. Evaluation of Off-label Use of Oral Glucocorticoids in Outpatients. Acta Academiae Med Sinicae. 2015;37(04):430–434. doi:10.3881/j.issn.1000-503X.2015.04.011
6. Guodong C, Wenqing H, Nanxia X, et al. Survey on the use of glucocorticoids in severe community-acquired pneumonia in intensive care unit of forty-five hospitals in Zhejiang Province. Chine Critical Care Med. 2019;04:488–492.
7. Zhang J-X, Xiong S-J, Xu C, L-H L, Cheng Q, Xie J. Systemic administration of glucocorticoids in Guizhou province: a survey among 271 hospital outpatient pharmacies. Chin J Hospital Pharm. 2020;40(02):219–224.
8. Jiang Q, Yu BN, Ying G, et al. Outpatient prescription practices in rural township health centers in Sichuan Province, China. BMC Health Serv Res. 2012;12(1):324. doi:10.1186/1472-6963-12-324
9. Liu L-M, X-G Y, Huang M-Y, et al. Review on prescriptions of essential medicines in 27 township hospitals in Jiangxi Province. Herald Med. 2014;33(06):811–814.
10. Luo X, Yu S, Zeng Z, et al. Systemic glucocorticoid prescriptions pattern and factors of inappropriate use in primary care institutions of Southwest China. Front Public Health. 2022;10:952098. doi:10.3389/fpubh.2022.952098
11. Song HM. Rational use of glucocorticoids. Chin J Pediatrics. 2018;56(3):161–162.
12. Blodgett FM, Burgin L, Iezzoni D, Gribetz D, Talbot NB. Effects of prolonged cortisone therapy on the statural growth, skeleton maturation and metabolic status of children. N Engl J Med. 1956;254(14):636. doi:10.1056/NEJM195604052541402
13. Cheng X, Chen JL, Ma ZL, et al. Biophysic influence of dexamethasone exposure on embryonic vertebrate skeleton development. Toxicol Appl Pharmacol. 2014;281(1):19–29. doi:10.1016/j.taap.2014.09.014
14. Liu LZ. Clinical application of glucocorticoids. Curr Med. 2016;22(04):57–58.
15. Torrente F, Bustin J, Triskier F, et al. Effect of a Social Norm Email Feedback Program on the Unnecessary Prescription of Nimodipine in Ambulatory Care of Older Adults: a Randomized Clinical Trial. JAMA network open. 2020;3(12):e2027082. doi:10.1001/jamanetworkopen.2020.27082
16. Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomized controlled trial. Lancet. 2016;387(10029):1743–1752. doi:10.1016/S0140-6736(16)00215-4
17. Gude WT, van Engen-Verheul MM, van der Veer SN, et al. Effect of a web-based audit and feedback intervention with outreach visits on the clinical performance of multidisciplinary teams: a cluster-randomized trial in cardiac rehabilitation. Implement Sci. 2016;11(1):160. doi:10.1186/s13012-016-0516-1
18. Wan ES, Kantorowski A, Polak M, et al. Long-term effects of web-based pedometer-mediated intervention on COPD exacerbations. Respir Med. 2020;162:105878. doi:10.1016/j.rmed.2020.105878
19. Regev-Yochay G, Raz M, Dagan R, et al. Reduction in antibiotic use following a cluster randomized controlled multifaceted intervention: the Israeli judicious antibiotic prescription study. Clinl Infect Dis. 2011;53(1):33–41. doi:10.1093/cid/cir272
20. Little P, Stuart B, Francis N, et al.; GRACE consortium. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet. 2013;382(9899):1175–1182. doi:10.1016/S0140-6736(13)60994-0
21. Elouafkaoui P, Young L, Newlands R, et al.; Translation Research in a Dental Setting (TRiaDS) Research Methodology Group. An Audit and Feedback Intervention for Reducing Antibiotic Prescribing in General Dental Practice: the RAPiD Cluster Randomised Controlled Trial. PLoS Med. 2016;13(8):e1002115. doi:10.1371/journal.pmed.1002115
22. GolAli E, Sistanizad M, Salamzadeh J, Haghighi M, Solooki M. Antibiotic Prescribing Trends Before and After Implementation of an Audit and Feedback Program in Internal Ward of a Tertiary Hospital in Tehran. Iran J Pharm Res World. 2019;18(4):2136–2143. doi:10.22037/ijpr.2019.1100833
23. Chang Y, Sangthong R, McNeil EB, Tang L. Effect of a computer network-based feedback program on antibiotic prescription rates of primary care physicians: a cluster randomized crossover-controlled trial. J Infect Public Health. 2020;13(9):1297–1303. doi:10.1016/j.jiph.2020.05.027
24. Yang J, Cui Z, Liao X, et al. Effects of a feedback intervention on antibiotic prescription control in primary care institutions based on a Health Information System: a cluster randomized cross-over controlled trial. J Glob Antimicrob Resist. 2023;33:51–60. doi:10.1016/j.jgar.2023.02.006
25. Hopewell S, Clarke M, Moher D, et al.; CONSORT Group. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371(9609):281. doi:10.1016/S0140-6736(07)61835-2
26. Hopewell S, Clarke M, Moher D, et al.; CONSORT Group. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi:10.1371/journal.pmed.0050020
27. Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345(sep04 1):e5661. doi:10.1136/bmj.e5661
28. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. doi:10.1136/bmj.l4378
29. Zhan SY. Epidemiology.
30. National Bureau of Statistics. China Statistical Yearbook 2022. China Statistics Press. Available from: http://www.stats.gov.cn/sj/ndsj/2022/indexch.htm.
31. Avraham NK. The effects of feedback interventions on performance: a historical review, a meta-analysis and a preliminary feedback intervention theory. Psychological Bul-Letin. 1996;119.
32. Guidelines for clinical application of glucocorticoid hormones. Available from: https://www.gov.cn/gzdt/2011-02/24/content_1810219.htm.
33. Chinese Ministry of Health Standards for Hospital Prescription Review and Management. Available from: https://www.gov.cn/gzdt/2010-03/04/content_1547080.htm.
34. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. doi:10.1186/1710-1492-9-30
35. Asir M Y, Goyal A, Bansal P, Sonthalia S. Corticosteroid Adverse Effects. StatPearls Treasure Island. (FL): StatPearls Publishing Copyright ©; 2021.
36. Soleymani F, Rashidian A, Hosseini M, Dinarvand R, Kebriaeezade A, Abdollahi M. Effectiveness of audit and feedback in addressing over prescribing of antibiotics and injectable medicines in a middle-income country: an RCT. Daru. 2019;27(1):101–109. doi:10.1007/s40199-019-00248-5
37. Klop C, de Vries F, Vinks T, et al. Increase in prophylaxis of glucocorticoid-induced osteoporosis by pharmacist feedback: a randomised controlled trial. Osteoporos Int. 2014;25(1):385–392. doi:10.1007/s00198-013-2562-8
38. Aghlmandi S, Halbeisen FS, Saccilotto R, et al. Effect of Antibiotic Prescription Audit and Feedback on Antibiotic Prescribing in Primary Care: a Randomized Clinical Trial. JAMA Intern Med. 2023;183(3):213–220. doi:10.1001/jamainternmed.2022.6529
39. Glinz D, Mc Cord KA, Moffa G, et al. Antibiotic prescription monitoring and feedback in primary care in Switzerland: design and rationale of a nationwide pragmatic randomized controlled trial. Contemp Clin Trials Commun. 2021;21:100712. doi:10.1016/j.conctc.2021.100712
40. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;13(6):CD000259.
41. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345–2352. doi:10.1001/jama.2013.6287
42. Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized Prescription Feedback Using Routinely Collected Data to Reduce Antibiotic Use in Primary Care: a Randomized Clinical Trial. JAMA Intern Med. 2017;177(2):176–183. doi:10.1001/jamainternmed.2016.8040
43. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: a Randomized Clinical Trial. JAMA. 2016;315(6):562–570. doi:10.1001/jama.2016.0275
44. DiClemente CC, Prochaska JO. Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addict Behav. 1982;7(2):133–142. doi:10.1016/0306-4603(82)90038-7
45. Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298(20):2415–2417. doi:10.1001/jama.298.20.2415
46. Lu L. Social Medicine.
47. Ardillon A, Ramblière L, Kermorvant-Duchemin E, et al.; BIRDY study group. Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: a multicentric community-based cohort study. PLoS Med. 2023;20(6):e1004211. doi:10.1371/journal.pmed.1004211
48. Ramay BM, Jara J, Moreno MP, et al. Self-medication and ILI etiologies among individuals presenting at pharmacies with influenza-like illness: guatemala City, 2018 influenza season. BMC Public Health. 2022;22(1):1541. doi:10.1186/s12889-022-13962-8
49. Wondmkun YT, Ayele AG. Assessment of Prescription Pattern of Systemic Steroidal Drugs in the Outpatient Department of Menelik II Referral Hospital, Addis Ababa, Ethiopia, 2019. Patient Prefer Adherence. 2021;15:9–14. doi:10.2147/PPA.S285064
50. Sadeghirad B, Siemieniuk RAC, Brignardello-Petersen R, et al. Corticosteroids for treatment of sore throat: systematic review and meta-analysis of randomised trials. BMJ. 2017;358:j3887. doi:10.1136/bmj.j3887
51. Chinese National Influenza Center. The 700th edition of Chinese Influenza Surveillance Weekly for Week 19 in 2022. Available from: https://ivdc.chinacdc.cn/cnic/zyzx/lgzb/202205/t20220521_259298.htm.
52. Chinese National Influenza Center. The 703th edition of Chinese Influenza Surveillance Weekly for Week 22 in 2022. Available from: https://ivdc.chinacdc.cn/cnic/zyzx/lgzb/202206/t20220610_259628.htm.
53. Chinese National Influenza Center. The 708th edition of Chinese Influenza Surveillance Weekly for Week 27 in 2022. Available from: https://ivdc.chinacdc.cn/cnic/zyzx/lgzb/202207/t20220715_260343.htm.
54. Yuqing Y. The protocol and experience of COVID-19 prevention in Ruijin Hospital——information support. J Internal Med Concepts Pract. 2019;18(1):42.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

