Back to Journals » Nanotechnology, Science and Applications » Volume 17
Factors Affecting the Synthesis of Bovine Serum Albumin Nanoparticles Using the Desolvation Method
Authors Tanjung YP, Dewi MK , Gatera VA , Barliana MI , Joni IM, Chaerunisaa AY
Received 16 October 2023
Accepted for publication 17 January 2024
Published 31 January 2024 Volume 2024:17 Pages 21—40
DOI https://doi.org/10.2147/NSA.S441324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Kattesh Katti
Yenni Puspita Tanjung,1,2 Mayang Kusuma Dewi,1 Vesara Ardhe Gatera,3,4 Melisa Intan Barliana,3,5 I Made Joni,6,7 Anis Yohana Chaerunisaa1
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 2Bumi Siliwangi Academy of Pharmacy, Bandung, West Java, Indonesia; 3Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia; 4Department of Pharmacy and Health Sciences, Universiti Kuala Lumpur – Royal College of Medicine Perak, Ipoh, Perak, Malaysia; 5Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 6Department of Physics, Faculty of Mathematics and Natural Science, Universitas Padjadjaran, Bandung, Indonesia; 7Functional Nano Powder University Center of Excellence (FiNder U CoE), Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Anis Yohana Chaerunisaa, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang Km 21, Jatinangor, 45363, Indonesia, Email [email protected]
Abstract: Currently, protein-based nanoparticles are in high demand as drug delivery systems due to their exceptional qualities, including nontoxicity, nonantigenicity, and biodegradability. Other qualities include high nutritional value, abundance of renewable resources, excellent drug binding capacity, greater stability during storage and in vivo, as well as ease of upgrading during manufacture. Examples of protein suitable for this purpose include ovalbumin (OVA) derived from egg white, human serum albumin (HSA), and bovine serum albumin (BSA). To create albumin nanoparticles, six different processes have been investigated in depth and are frequently used in drug delivery systems. These included desolvation, thermal gelation, emulsification, NAB technology, self-assembly, and nanospray drying. Several experimental conditions in the synthesis of albumin nanoparticles can affect the physicochemical characterization. Therefore, this study aimed to provide an overview of various experimental conditions capable of affecting the physicochemical characteristics of BSA nanoparticles formed using the desolvation method. By considering the variation in optimal experimental conditions, a delivery system of BSA nanoparticles with the best physicochemical characterization results could be developed.
Keywords: bovine serum albumin, BSA, desolvation, experimental conditions, physicochemical characteristics
Introduction
In recent decades, nanotechnology, particularly, the use of nanoparticles is reported to have transformed the therapeutic process, leading to more potent medicines, diminished adverse effects, and intelligent therapeutics capable of targeting disease areas.1,2 Nanoparticles, defined as solid or liquid colloidal particles with a size ranging from 10 to 1000 nm offer various benefits, including the administration route and the enhancement of the therapeutic impact. Therefore, this nanotechnology is more advanced and extensively explored by various studies.3 Nanoparticles can be constructed from synthetic or natural macromolecules and must be biocompatible or preferably biodegradable.4
Due to their exceptional qualities, including nontoxicity, nonantigenicity, biodegradability, high nutritional value, abundant renewable sources, extraordinary drug binding capacity, greater stability during storage and in vivo, alongside ease of scaling up during manufacture, protein-based nanoparticles have attracted significant interest as drug delivery devices.5 The main objective of designing protein-based nanoparticles as a drug delivery system is to regulate the particle size, surface area, and characteristics. This ensures that nanoparticles system carries the necessary amount of drug and releases the active substance to achieve part-specific action and the desired pharmacological activity.6 Since 2008, numerous studies have focused on the use of protein as drug delivery in the form of nanoparticles.1 One example of protein suitable for this purpose is albumin,4 which has unique features making it an ideal choice as a drug transporter. For instance, given the abundance in the body, injecting excessive albumin would have lower side effects compared to other carriers.7 Transporting therapeutic drugs with albumin not only reduces costs but also decreases toxicity.8 The bond between albumin and hydrophobic substances is reversible, facilitating drug transport in the body and the release to the cell surface.9 Albumin has many functional groups, which result in a high capacity for binding drugs. Furthermore, the amino acid sequence and albumin structure allow the conjugation of drugs to nanoparticles through different mechanisms such as electrostatic power with negatively charged drugs (Ganciclovir), positively charged drugs, amphipathic compounds (Doxorubicin) and hydrophobic drugs (Paclitaxel).10
The macromolecular carrier known as albumin is biodegradable, non-toxic, and degraded in vivo to yield innocuous, non-immunogenic, easily purifiable, and water-soluble products. Given these properties, albumin is an excellent choice for nanoparticles formulations.11 The high water solubility and easy purification process make albumin a good candidate for the manufacture of nanoparticles or as a versatile drug carrier that can be easily delivered by injection. Through covalent bonding, the medicine and other molecules adhere to nanoparticles by the amine and carboxyl groups.12 Moreover, albumin is one of the most attractive materials for the development of novel medications, as evidenced by its successful use in the clinical nanomedicine application of Abraxane.13 Ovalbumin (OVA), a protein generated from egg white, human serum albumin (HSA), and bovine serum albumin (BSA) are three different forms of albumin proteins that have been isolated and used for various biomedical applications.9
Nanoparticles can be created using two methods namely top-down and bottom-up methods. The top-down method entails milling, etching, photolithography, or ball milling to reduce a material with a large particle size into nanoparticles. The process normally takes a long time and produces large particles. On the other hand, the term “bottom-up method” describes the creation of nanoparticles one atom at a time, one molecule at a time, or one cluster at a time. The formation and growth of nanoparticles occur due to a chemical reaction that takes place under carefully regulated conditions in a liquid or gas phase. Albumin nanoparticles are prepared through bottom-up methods such as the desolvation, thermal gelation, emulsification, NAB technology, self-assembly, and nanospray drying.12
All these methods have been widely studied and the desolvation method is the most widely used in the synthesis of BSA nanoparticles.14,15 The results of nanoparticles size, polydispersity index, zeta potential, and encapsulation efficiency (EE) in the desolvation method are influenced by several variables. These include variations in the pH and the amount of BSA present, as well as the rate at which the desolvating agent is added.6
This study aimed to provide an overview about the types of albumin and the method of synthesizing nanoparticles as a drug delivery system, with a focus on the desolvation method. In addition, factors influencing the synthesis of BSA nanoparticles by the desolvation method were identified.
Materials and Methods
All electronic databases were examined for this review, including PubMed, Scopus, and Google Scholar. The articles analyzed were those relevant to the production of BSA nanoparticles by the desolvation method and published in the last 10 years. The search was carried out using the keywords “BSA nanoparticles with the desolvation method”, “synthesis of BSA nanoparticles desolvation method”, and “production of BSA nanoparticles desolvation method”. The exclusion criteria applied were review articles, non-BSA studies, synthesis without any optimization, optimization with software, and non-desolvation method. The flowchart of the literature search flow is presented in Figure 1.
 |
Figure 1 Flowchart of the Methodology Research. |
Type of Albumin
Albumin is easily obtained commercially with the main source being animal, vegetable, or human. BSA, HSA, and egg white (OVA) are a few examples of albumin frequently used. These three types of albumin have been widely adopted both in different studies and for the food industry.16 BSA is commonly used in studies due to its low price and easy purification process.17 HSA is frequently used to avoid losses from animal serum albumin, one of which is bovine spongiform encephalopathy.18 Furthermore, the ability of OVA to create foam and gel networks has led to its widespread use in the food industry.11 BSA and HSA are homologous in structure, conformation, and properties, sharing approximately 76% sequential identity, and the major difference lies in tryptophan (Trp) residue location. In HSA, only one Trp exists, located at position 214, while BSA has a Trp-212 at subdomain IIA, with an additional Trp-134 exposed in the solvent at subdomain IB.19
In general, albumin structure contains three homologous alpha-helical domains I, II, and III. Each domain comprises two subdomains A and B, which consist of four and six alpha helices, respectively. Additional important binding sites include the free thiol located at the cysteine-34 amino acid residue and Sudlow sites I and II, which bind a variety of hydrophobic drugs. The unique affinity and nature of each of these binding sites are of interest for the production of albumin-binding drugs.20 Preformed albumin treatment and in situ binding are the two common binding methods. Drugs categorized as in situ binders have the ability to bind circulating (endogenous) albumin after being ingested. This in situ binding facilitates the transport, blood circulation, and medication dissolution of hydrophobic substances. Exogenous albumin is associated with the medicine in prepared formulations before ingestion by the patient.21
Ovalbumin (OVA)
The dietary protein OVA, commonly known as egg albumin, is frequently used in the creation of food matrices,22 and is the major globular protein in chicken egg whites.12 OVA has an isoelectric point (PI) of 4.8 and a molecular weight of ~47 kDa. Furthermore, it is a 385-amino acid monomeric phosphoglycoprotein with four free sulfhydryl groups and an internal disulfide bond.11 Due to its affordability and availability in comparison to other proteins, capacity to form emulsions, gels, and foams, as well as sensitivity to pH and temperature, OVA is used in drug delivery systems.9
Human Serum Albumin (HSA)
HSA is derived from human serum and represents a heart-shaped globular protein monomer.22 With a molecular weight of ~67 kDa and a half-life of 14 days in the body, HSA is a blood protein that serves as a carrier for fatty acids, thyroid hormones, steroids, endogenous ligands, metabolites, and medications. Several other features include maintaining extracellular fluid volume,12 stable in the pH range of 4–9, and heat resistance up to 60°C for 10 hours.23 HSA is a single polypeptide of 585 amino acids produced in the liver. It has a high cysteine and a low tryptophan concentration.8 Due to the benefits, such as high drug delivery capacity, targeting ability, strong stability, good biocompatibility, long half-life, and simple purification procedure, HSA has received significant attention recently as a drug carrier.24,25
Bovine Serum Albumin (BSA)
BSA is derived from bovine serum as a globular protein with a molecular weight of ~66.43 kDa and an isoelectric point of 4.7.26 The water-soluble monomeric protein BSA has 583 amino acid residues, 17 disulfide bonds, 9 loops created by bridges, one cysteine, and 8 pairs of disulfide bonds.22 BSA is the most widely used type of protein in the pharmaceutical field,8 specifically as a protein model to examine how proteins accumulate and fibrillate. One of the most crucial drug delivery systems used to treat illnesses, including cancer, is made from amyloid nanofibers generated from BSA.1,27 Furthermore, this natural biomacromolecule has been extensively applied as a drug delivery system,28 gene drugs, antibodies, and others.29 The advantages compared to other carrier materials include biodegradability, non-toxicity, and non-immunogenicity.30 BSA also has dual targeting capabilities for tumor tissue, binding to albumin gp60 receptor on the surface of vascular endothelial cells and interacting with secreted proteins acidic and rich in cysteine (SPARC) overexpressed on the surface of a variety of tumor cells. This efficient binding to albumin promotes the aggregation of nanoparticles loaded with drugs in the stroma of tumor cells (Figure 2).31,32
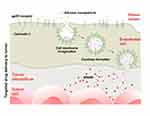 |
Figure 2 Schematic Diagram of the Mechanism of Albumin Nanoparticles Targeting Tumor Tissue. |
The three types of albumin (OVA, HSA, and BSA) have differences in terms of molecular weight, length (amino acid), organism, half-life, and main functions. The different characteristics are presented in Table 1 and the 3D structure is depicted in Figure 3.33,34
 |
Table 1 Basic Information on the Three Types of Albumin |
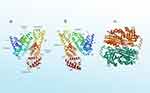 |
Figure 3 The 3D Structure of Albumins: (a) Human Serum Albumin (b) Bovine Serum Albumin (c) Ovalbumin. |
Method for Synthesizing Albumin Nanoparticles
Albumin nanoparticles synthesis method can be divided into two, namely physical and chemical.8 The desolvation, emulsification, and self-assembly are included in the chemical method,1 while the physical method consists of thermal gelation, nanoparticles albumin-bound (NAB) technology, and nanospray drying.40 A total of six methods for synthesizing albumin nanoparticles have been extensively explored and used in drug delivery systems.5 A schematic representation of the various processes is shown in Figure 4.
 |
Figure 4 Various Process for Synthesis Albumin Nanoparticle. |
The Desolvation
The desolvation method is the initial method in the synthesis of albumin nanoparticles.41 This method entails the gradual addition of water-soluble organic solvents such as ethanol to dilute albumin solution with constant stirring. This leads to a reduction in the solubility of albumin, resulting in phase-based separation.35 However, albumin particles are still not totally stable at this point and may return to the aqueous phase,12 showing the need to add a crosslinker to increase the rigidity of nanoparticles formed. Crosslinking on desolvated particles is a critical step in the creation of albumin nanoparticles as it affects particle durability, biodegradability, and drug release from the carrier system.35 The addition of glutaraldehyde as a crosslinker causes condensation of the amino group on the lysine residue and the arginine group on the guanidin albumin side chain through a condensation process with the aldehyde group.12 The flow of preparing albumin nanoparticles using the desolvation method is illustrated in Figure 5.
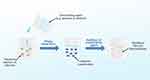 |
Figure 5 Desolvation Process for Synthesis of Albumin Nanoparticles. |
Albumin nanoparticles continue to maintain their shape and physical state even when subjected to several centrifugation and reconstitution cycles. These processes aim to remove ethanol and glutaraldehyde.41 The interaction between BSA, crosslinker, and drug is illustrated in Figure 6.
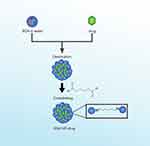 |
Figure 6 Synthesis of Albumin Nanoparticles. |
Emulsification
The emulsification method is the most established and effective method for producing albumin nanoparticles.12 This method entails emulsifying a hydrophilic protein solution in a non-aqueous media, such as oil.42 Two alternative emulsification processes, including high-energy and low-energy procedures, can be used to synthesize these emulsions. Specifically designed mechanical equipment, such as high-pressure homogenizers and ultrasonic generators, are used in the high-energy emulsification process. Due to the high efficiency of the device, emulsification only requires a small portion of the mechanical energy generated. The application of such particular mechanical devices will result in nanometric emulsions, with the formation of droplets, initial deformation, macrometric disruption, and the adsorption of surfactants at the interfaces to ensure steric stability.5 Meanwhile, the creation of emulsion droplets in the nanometric range is achieved by the low-energy emulsification process, which transfers the intrinsic physicochemical characteristics of the surfactants, co-surfactants, and excipients in the formulation using the stored chemical energy of the system.43
Albumin nanoparticles formed by the emulsification method can be stabilized in two ways, namely chemically or physically.12 Thermal heating for 10 minutes at a temperature of 175–180 °C is used to carry out the physical stabilization procedure. The viscosity of the oil is then reduced by adding ethyl ether to this combination after cooling, facilitating centrifugation.5 This thermal method only applies to drugs that are thermostable.35 The chemical stabilization process is carried out by adding crosslinkers such as glutaraldehyde, formaldehyde, or diacid chloride.31
Self-Assembly
The self-assembly method is a new method in the synthesis of albumin nanoparticles.44 This method entails eliminating the primary amine group on the protein surface or enhancing the hydrophobicity of albumin by breaking the disulfide bonds. By introducing lipophilic drugs into the process, albumin nanoparticles can be formed through the self-assembly of molecules.12 During the process, hydrophobic drugs or reducing agents are incorporated into the core to gradually accumulate hydrophobically modified albumin.41 Commonly used reducing agents include β-mercaptoethanol, dithiothreitol, and cysteine. Several stages in the self-assembly method are similar to those in the desolvation. Albumin nanoparticles formed from this self-assembly method can better maintain protein function, resulting in active targeting properties.36
Thermal Gelation
Albumin is degraded in the presence of heat in a method called thermal gelation, which leads to the aggregation of particles under optimum environmental conditions. This procedure, which is inexpensive and simple,45 entails thermally mediated albumin opening and is induced by heating the solution at a temperature of 75–80 °C.46 Interactions between hydrophobic proteins occur through electrostatic, hydrogen bond, and disulfide-sulfhydryl exchanges,11 resulting in a stable three-dimensional protein molecular network. The addition of a denaturing agent, such as urea (CO(NH2)2) accelerates the denaturation of albumin.35 Therefore, the addition of 5 M urea in BSA solution can fasten the rate of gelation.45 In general, thermal gelation is one of the methods used to avoid the potential for toxicity caused by the addition of organic solvents in the desolvation.46
Nanoparticle Albumin-Bound (NAB) Technology
Currently, the NAB Technology method has been widely used, specifically in the development of anticancer drug delivery systems following the approval of the Nab-Paclitaxel (AbraxaneTM) product by the US Food and Drug Administration.5 This method is excellent for encapsulating hydrophobic anticancer drugs in a protein matrix for effective delivery.12 NAB drug is produced by mixing an organic solution of chloroform/acetone+acetone with an aqueous albumin solution, which includes a hydrophobic drug with low rotation, resulting in a crude emulsion. Subsequently, a high-pressure homogenization is used to produce nanoemulsion. Rotary evaporation under vacuum is used to remove the organic solvent initially present in the colloid. After the production, nanoparticles are centrifuged or filtered (using a syringe filter), and the supernatant is lyophilized to produce nanoparticles powder.47,48
Nanospray Drying
The pharmaceutical industry uses a method known as “nanospray drying” to convert liquid materials into powder nanoparticles.5 This method has several benefits due to the narrow size distribution, high EE, and long shelf life of albumin nanoparticles produced. Additionally, this method may be applied at low temperatures and depends less on the solubility of albumin and drugs, minimizing the risk of degradation.49 The spray drying method comprises four stages, namely atomization of the material, drying through gases, forming, and collecting particles.31 This process is accomplished by means of a liquid sample sprayed into a chamber where heated nitrogen and carbon dioxide gases flow toward the spray from a nozzle. To stabilize the polymer particles, albumin liquid sample is added with surfactant. The electrodes at the bottom of the chamber are used to collect nanoparticles. The sprayed droplets are electrostatically charged due to these electrodes, causing movement toward the bottom of the chamber. This process is a fast and cost-effective way to produce small-scale protein particles.6
This review article focuses on the method of synthesizing albumin nanoparticles using the desolvation method, which has several advantages such as a simple preparation process, rapid reaction, and no need for surfactants. This method is suitable for the encapsulation of various hydrophobic drugs, and the preparation of albumin nanoparticles. By using the desolvation method, nanoparticles can be stored for a long time after freeze-drying. Moreover, the resuspended nanoparticles can be further treated for adsorbing drugs or covalently modifying targeted ligands on the particle surface.31 The desolvation method is widely used in making albumin nanoparticles because the emulsification method has shortcomings including the difficulty of removing organic solvents and the need for surfactants as stabilizers in the emulsion system.5 Meanwhile, albumin nanoparticles derived using the Nab-Technology method have less stable colloidal stability and tend to separate into bound drug-albumin, albumin, and particles in systemic administration.35 Thermal gelation and nano-spray drying methods are not suitable for hydrophobic drugs,5 while the self-assembly method can cause potential toxicity due to the reducing agent used.35 In recent years, nanoparticles prepared by the desolvation method have been widely studied in antitumor applications. For example, doxorubicin-loaded HSA nanoparticles prepared using the method showed good antitumor activity.50 Ziaaddini et al constructed a type of BSA nanoparticles that enhanced the efficacy and reduced cytotoxicity of anticancer drugs.51
Factors Affecting the Synthesis of BSA-NP Using the Desolvation Method
This review article focused on using polymeric BSA as a carrier, a choice well-received in the pharmaceutical industry and extensively used in fundamental investigations.4 Nanoparticles based on BSA can be prepared by two methods, namely precipitation and emulsification.52 The principal components of nanoparticles are dissolved in a solvent, and nanoprecipitation is based on the degradation of the solvent quality. Nanoprecipitation can be divided into several submethods, including non-solvent precipitation, desolvation, coacervation, salting, and albumin-bound technology, depending on how the solvent quality is reduced. In essence, this entails dissolving proteins in water while ethanol acts as a poor solvent. Combining these stages leads to saturation and development of a protein nucleus.53 The free protein units condense around the core, creating nanoparticles. This method yields small particles with an unimodal distribution, and three phases make up the non-solvent-based deposition process namely supersaturation production, nucleation, and growth. Each phase is influenced by one or more experimental factors, and the deposition step in the process rate and formulation behavior impact the physicochemical characteristics of nanoparticles.54 Therefore, this review article discussed several experimental conditions affecting the physicochemical characterization of BSA nanoparticles synthesized using the desolvation method (Table 1).
Effect of BSA Concentration and Drugs: BSA Ratio
The classical nucleation theory can be used to explain why the concentration of BSA in the initial solution is a major variable in the formation of nanoparticles.55 About eight studies have been conducted to optimize variations in BSA concentrations to obtain the best characterization results for particle size, isoelectric point (IP), and zeta potential. According to Table 2, there are six studies conducted by Tazhbayev et al56 Fatemeh et al57 Wilson et al58 Aljabali et al59 Diaz-Saldivar et al60 and Noorani et al61 the greater the concentration of BSA, the larger the particle size. BSA concentration affects the physicochemical characteristics of nanoparticles. This was supported by a report from Galisteo-González and Molina-Bolivar stating that increasing the concentration of BSA from 1.2% to 10% induced a rise in particle size from 75 to 135 nm.2 Furthermore, as stated by Noorani et al, high concentrations of BSA increased the possibility of coagulation through electrostatic and hydrophobic interactions.61
 |
Table 2 Characterization of BSA Nanoparticles (NP) by Size, Polydispersity Index (PDI), Zeta Potential, and %EE of Encapsulation Efficiency in Different Experimental Conditions |
Similar results were obtained by Fatemeh et al, stating that increasing the concentration of BSA could lead to a rise in particle size and polydispersity index. Nucleation theory helps explain the significance of BSA initial concentration in the synthesis of nanoparticles.57 According to this theory, when the concentration of BSA increases, the viscosity rises and the frequency of protein transfer between water and the desolvating agent decreases, resulting in a slower nucleation rate and increased particle sizes.55 A high BSA concentration also results in a greater degree of saturation, leading to smaller and more numerous nuclei. Concurrently, this high level of saturation accelerates agglomeration through collisions between larger particles to produce larger nanoparticles.62
The drug: ratio in the preparation of nanoparticles is also important because it affects particle formation, stability, and loading capacity.55 In principle, the effect of this ratio on the physicochemical characteristics of nanoparticles drugs is the same as the impact of BSA concentration.68 Based on the literature study (Table 2), six studies discussed optimization by varying the ratio of drug: BSA. According to Abd Ellah et al.69 Jenita, Wilson, and Chocalingam,70 Spada et al.71 Jenita,72 and Syed and Sailaja73 the particle size and polydispersity index value increase with the amount of BSA used. This is in accordance with previous studies also stating that the higher the concentration of BSA, the larger the particle size and the greater the polydispersity index. This relationship can be explained through the classical nucleation theory.65 On the other hand, several studies have reported that increasing BSA concentrations only had a slight influence on the size of BSA nanoparticles.55 Radwan SES et al stated that an increase in BSA concentration from 2.5% to 10% resulted in a decrease in particle size from 260 nm to 100.5 nm.74 Yang et al, also found that the greater the amount of BSA used, the smaller the particle size.68
The changes in particle size with increased BSA concentrations may be attributed to several factors such as solution pH, and salt concentration. Moreover, the volume and speed of adding desolvating agents affect the protein precipitation process, causing changes in the environment and altering its conformation.4
Effect of pH in BSA Solution
The pH is the most influential factor in controlling the size of the resulting nanoparticles. As reported by Burns and Zydney, the surface charge had a strong influence on protein adsorption.75 Based on the literature review in Table 2, five studies conducted by Fatemeh et al.57 Bronze-Uhle et al.76 Hemlata, Gupta, and Tejavath,77 Jenita et al65 and Tarhini et al4 discussed the influence of BSA solution pH on the physicochemical characterization of the formed nanoparticles.
Jenita et al, underscored the importance of pH in the desolvation process, attributing changes in chemical characteristics and molecular structure of proteins to pH variations. This study showed that the particle size of BSA-Tenofovir nanoparticles decreased with increasing pH values. BSA solution with a pH of 8 produced a small particle size because the aggregation between molecules was hindered by electrostatic repulsion.65
In a study by Bronze-Uhle et al where salicylic acid was used as the active ingredient, modifying the pH of the solution significantly affected the net charge on the protein surface. This is because proteins are made up of amino acids and hydrogen, which interact with ions in the solution. The isoelectric point (IP) of BSA is 4.9, and at this pH, the protein has no surface charge. At pH 4.9, the electrostatic repulsion is lower, leading to the rapid formation of amorphous aggregates through non-specific interactions, specifically hydrophobic.76 Positive charges can be produced at pH levels beyond BSA IP value by protonation of main amino groups, particularly lysine. Meanwhile, negative charges can be produced by deprotonation of carboxylic acid groups (glutamate and aspartate).78 By increasing electrostatic repulsion and decreasing hydrophobic interactions, the resultant charge across the protein surface reduces aggregation and enhances structural rearrangement of the protein. Additionally, it significantly changes how pharmacological molecules interact or bind with one another. The hydrophobic interactions are dominated by the negative charge and electrostatic forces at pH levels above the IP value.79
The results obtained by Hemlata, Gupta, and Tejavath are similar to those of Bronze-Uhle et al in which the pH of the solution directly changed the particle size. At different pH values of the solution, the average particle size and zeta potential of BSA nanoparticles from Cucumis prophetarum fruit extract differ based on the principle of charge conductivity to ensure formulation stability. Furthermore, the particle diameter decreased as the pH of the solution approached the IP value of BSA. This implies that electrostatic contact is one of the critical aspects influencing the synthesis of BSA nanoparticles.77 At a solution pH close to the IP value, a lower EE is produced because the charge on the protein surface inhibits the trapping of the active substance into nanoparticles products.2
Fatemeh et al and Tarhini et al conducted a study to determine the effect of pH in BSA solution on the physical characterization of nanoparticles without the addition of drug compounds. As showed by Tarhini et al, the zeta potential value of BSA nanoparticles was significantly impacted by variations in pH. The zeta potential value was positive at pH 3, while at 5 to 11, the value was negative.4 The properties of the protein, which modify its conformation at various pH values, can be used to explain the outcomes of these fluctuating surface charges. Additionally, protein loading may be affected by pH, which potentially alters the formation profile and particle properties.80 Fatemeh et al stated that the different pH of BSA solution significantly influenced the polydispersity index (PDI) value and the size of nanoparticles. The results showed that a pH value of more than 9 produced more uniform nanoparticles and smaller particle sizes through the desolvation process. The interaction between the amino acid hydrogens and the various ions in the protein solution affects the net surface charge of protein. This was further supported by Tarhini et al and Bronze-Uhle et al stating that the value of the isoelectric point (IP) could affect the solubility of the protein. Amorphous aggregates are formed by the non-specific interactions between protein molecules around the isoelectric point of the protein, where there are no electrostatic repulsions.57 The high pH of the solution enhanced the solubility and surface charge of the protein, producing particles with a small size. When the pH of a solution is high, surface protein molecules have a negative charge and precipitation tends to occur. The secondary structure is impacted by this pH level, causing a rise in beta-sheet production. Smaller particles are produced through hydrophobic interactions, such as thiol-disulfide. Furthermore, the pH value, a critical factor in the cross-linking process, determines the degree of protonation for the -amino group. By increasing the amount of protonated amino groups and decreasing pH, the possibility of cross-linking will reduce. At high pH, the amino group deprotonates, release ing a free amino that can connect with another group.80
Effect of Drug Concentration
The drug concentration in the initial solution plays a crucial role in the particle formation process,54 and this impact is associated with two mechanisms. A higher concentration of dissolved drugs leads to the formation of more and smaller nuclei, increasing the degree of saturation. However, concurrently, the growth of the nuclei is enhanced due to an elevated frequency of particle collisions and a shortened diffusion length. The increase in drug concentration also raises viscosity, hindering drug diffusion between the solvent and the anti-solvent. This results in inconsistent saturation, a slower nucleation rate, and greater particle agglomeration, leading to bigger particles.66,81 Furthermore, the increased viscosity reduces the number of collisions and mass transfer due to diffusion.64
Based on the literature review in Table 2, three studies conducted by Olaitan and Chaw,67 Wilson et al30 and Aljabali et al59 discussed the effect of drug concentration on characteristics of BSA nanoparticles formed. Olaitan and Chaw obtained the best characterization results at a Sulfasalazine concentration of 1%, Aljabali et al at a Piceatannol concentration of 0.125%, and Wilson et al at a 5-fluorouracil concentration of 0.25%. Previous studies by Olaitan and Chaw and Aljabali et al showed that the higher the drug concentration, the larger the particle size. Therefore, a low drug concentration was considered the optimum formula for further testing. As stated by Aljabali et al, the particle size and zeta potential value increase with rising drug concentration. This was attributed to the higher viscosity and less dispersion of the organic into the aqueous phase.63,82 On the other hand, Wilson et al, found that the formula with the highest drug concentration was considered optimal. This was based on the particle size required for tumor cell targeting (less than 400 nm). In this context, the higher the drug concentration, the greater the resulting drug loading.30
Effect of Solvent and Non-Solvent Ratio
In the desolvation method, the addition of a desolvating agent (non-solvent), such as ethanol or acetone, reduces the hydration level and solubility of albumin in water (solvent), leading to the formation of nanoparticles.83 The parameter of solvent and non-solvent ratio has been shown to dramatically affect the size and zeta potential of the particles formed. Based on the literature review in Table 2, two studies conducted by Tarhini et al, and Santos-Rebelo et al examined the effect of solvent and non-solvent ratio on characteristics of nanoparticles formed. Visual examination of the samples in the study by Tarhini et al clearly showed that characteristics of nanoparticles synthesized at different solvent and non-solvent ratios varied. When the ratio was adjusted to 1:2, the color (or opacity) of the samples changed from a faded, transparent white to a brighter, opaque white. Furthermore, when the ratio was increased to (2:3 and 1:1), the turbidity gradually returned before disappearing completely. The increased opacity shows an increase in particle size.4
The interfacial turbulence produced during solvent transfer is the foundation for the theory of nanoprecipitation. Due to the continuous mixing of the two solvents, the additional non-solvent disperses widely, enhancing the nucleation of protein nanoparticles when the proteins disperse beyond the thermodynamic solubility limitations. In other words, the amount of non-solvent injected affects the number of nanoparticles formed. Inadequate non-solvent in the mixture prevents precipitation, resulting in poor particles with a broad size distribution.84,85
Similar to particle size, the value of the polydispersity index decreased when the solvent and non-solvent ratio was increased from (1:3) to (1:2). This shows that the higher the ratio (up to 1:2), the lower the polydispersity index value. However, when the ratio was raised to more than 1:2, the polydispersity index value increased and the measurement became inaccurate. A similar conclusion was reached by Stainmesse et al who produced poly - ɛ - caprolactone nanoparticles using the nanoprecipitation method. When the solvent and non-solvent ratio was equal to or higher than 1, identifying the particle size became impossible.86
Concerning the zeta potential, the yield decreased with increasing solvent and non-solvent ratio from 1/3, 2/5 to 1/2, then increased to reach a maximum at S/NS of 1/1. According to a previous study by Jahanshasi et al, samples can be very polydisperse, leading to incorrect results at high solvent and non-solvent ratios. It is also well known that colloidal nanoparticles show good stability when the absolute zeta potential is high.87 As stated by Pal et al, a zeta potential value of less than −30 mV or more than +30 mV was considered stable, meaning that the particles would not flocculate during the storage time.88
Effect of Desolvating Agent Type
The type of the desolvation agent has been clearly proven to affect the particle size and distribution of BSA nanoparticles formed. Due to the poor solubility in organic solvents such as acetone and ethanol, BSA can facilitate nucleation and deposition, leading to the spontaneous aggregation of nanoparticles.89 Based on the literature review in Table 2, three studies conducted by, Fatemeh et al, Sailaja, Santos-Rebelo et al, and Krishna Sailaja and Nandini examined the effect of desolvating agent types on characteristics of the formed albumin nanoparticles. Fatemeh et al reported that the ethanol desolvating agent produced more nanoparticles characteristics compared to acetone.57 Furthermore, the advantage of ethanol compared to acetone is that the characterization of the formed nanoparticles including particle size, PDI, and zeta potential is more stable even though there are variations in temperature during the manufacturing process. The use of ethanol desolvating agent also produces smaller nanoparticles sizes compared to acetone due to the difference in polarity of the two desolvating agents. The dielectric constants of acetone and ethanol are 21 and 24.5 respectively.65
Sailaja90 also showed that ethanol is the best desolvation agent because it produces nanoparticles with smaller sizes and better entrapment efficiency compared to acetone and sodium sulphate. This study identified two important characteristics of the desolvation agents contributing to the production of large nanoparticles. These include the ability to form hydrogen bonds and low polarity which potentially generates additional hydrophobic interactions.91 Two types of the desolvation agents were used in this study namely polar-protic (ethanol and sodium sulfate) and polar-aprotic (acetone). In this context, ethanol can act as a hydrogen donor and acceptor, producing nanoparticles better than acetone.90
Santos-Rebelo et al92 and Krishna Sailaja and Nandini93 showed that acetone desolvation agent produced nanoparticles with better characterization compared to ethanol, hexane, and DMSO. Acetone is an ICH Class 3 solvent, which is less toxic and poses less risk to human health at acceptable limits in pharmaceuticals.92 These results were consistent with an earlier study by Arroyo-Maya et al, who produced uniform, smaller, and spherical α-lactalbumin nanoparticles using acetone as the optimum desolvation agent. The differences in the results of these studies could be influenced by variations in nanoparticle preparations, protein configurations, and different measurement methods.94
Effect of Crosslinker Type
The type of crosslinker added to BSA nanoparticles manufacturing process plays a crucial role in affecting the results of particle size, zeta potential, and PDI. However, the different types of crosslinkers used do not have a significant effect on entrapment efficiency (EE). The surface charge of nanoparticles is modified by the crosslinker. Additionally, this feature affects the electrostatic potential and colloidal stability of albumin nanoparticles in solution.76 The literature review showed that three studies conducted by Fatemeh et al.57 Niknejad and Mahmoudzadeh,95 and Santos-Rebelo et al92 examined the effect of the crosslinker type used on the characterization results of BSA nanoparticles (Table 2).
Fatemeh et al, and Niknejad and Mahmoudzadeh found that glutaraldehyde was the best type of crosslinker, producing BSA nanoparticles with low size and PDI characteristics. Furthermore, Fatemeh et al reported that different types of crosslinkers had varying effects on particle size and PDI nanoparticles, but did not significantly impact efficiency. Compared to other crosslinkers, glutaraldehyde, sorbitol, and glucose produced more stable BSA nanoparticles as showed by the measurement results of the zeta potential value. Glutaraldehyde, in particular, formed a structure encapsulating the drug molecules within BSA nanoparticles.57 Varying amounts of glutaraldehyde significantly changed the surface charge of the resulting nanoparticles but did not alter the size. This phenomenon was attributed to the constant quantity of glutaraldehyde associated with free surface amino groups of proteins during nanoparticles production. Glutaraldehyde interacts with all free amine groups in albumin. According to a study, protein cross-linking occurs when two carbonyl groups attack the amino groups of lysine and arginine residues in proteins, forming Schiff bases in solution. When the pH is alkaline, Schiff base is very stable, but when the pH is acidic, it is unstable.76
Other chemical cross-linking agents, such as glucose, have comparable abilities to glutaraldehyde.96,97 A study conducted by Santos-Rebelo et al reported glucose as the best type of crosslinker. Niknejad and Mahmoudzadeh also confirmed glucose as a reliable type of crosslinker in the manufacture of BSA nanoparticles. This was supported by the particle size and PDI characterization results, which were not significantly different compared to those of glutaraldehyde. Physical cross-linking can be achieved in several ways, including drying, heating, or UV irradiation. Among these methods, UV irradiation has proven to be a better and more potent way to crosslink materials.98,99 According to Niknejad and Mahmoudzadeh, UV exposure changed the electrical characteristics of albumin nanoparticles, with significantly different zeta potential values compared to that of the glutaraldehyde group. The results also showed that UV-crosslinked nanoparticles were more toxic than glutaraldehyde. This could be attributed to the fact that the core of aromatic residues such as tyrosine and phenylalanine contains free radicals produced by UV exposure.99 Furthermore, when glucose + UV was used as the crosslinker, the resulting particle size, PDI, and zeta potential were not significantly different from those of glutaraldehyde. The addition of glucose caused an increase in the matrix strength and stability. This was attributed to UV-generated free radicals, which could speed up crosslinking with glucose by forming reactive linear glucose molecules. In other words, immediately UV radiation produced harmful free radicals, linearly cross-linked complexes were formed with free glucose molecules. Since each substance (in free form) is poisonous to cells, using UV and glucose as cross-linkers “simultaneously” may explain the reduction in cellular toxicity. Furthermore, crosslinked glucose+UV nanoparticles were found to be less harmful to cells compared to those crosslinked with glutaraldehyde. This suggests that glucose+UV is a better option for cross-linking albumin nanoparticles than glutaraldehyde.95
Conclusion
In conclusion, the desolvation method was identified as the most frequently used method for the synthesis of BSA nanoparticles. In the synthesis process, several experimental conditions may affect the physicochemical characteristics of the formed nanoparticles. These included BSA concentration and drugs: BSA ratio, pH of BSA solution, drug concentration, solvent: non-solvent ratio, as well as type of desolvating agent, and crosslinker. The best experimental conditions from previous studies could be used as a reference in the process of synthesizing BSA nanoparticles using the desolvation method. However, other influencing parameters need to be further studied to optimize the production of nanoparticles with the best physicochemical characteristics.
Abbreviation
AC, Acetone; AS, Ascorbic acid; BSA, Bovine serum albumin; BSA-NP: Bovine serum albumin-nanoparticles; CO(NH2)2: Carbonyl diamide; CA, Citric acid; DMSO, Dimethyl sulfoxide; EE, Encapsulation efficiency; ET, Ethanol; gp60, Glycoprotein 60; GLU, Glucose; GT, Glutaraldehyde; HAS, Human serum albumin; HX, Hexane; ICH, International Council for Harmonisation; IP, Isoelectric point; IP, Isopropanol; kDa, Kilodalton; OVA, Ovalbumin; NAB, Nanoparticle Albumin Bound; nm, Nanometer; pH, Potential of hydrogen; PDI, Polydispersity index; SOR, Sorbitol; SPARC: Secretory cysteine-rich acid protein; SS, Sodium sulphate; TA, Tannic acid; US, United States; UV, Ultraviolet.
Acknowledgments
The authors are grateful to FiNder U CoE.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Kouchakzadeh H, Safavi MS, Shojaosadati SA. Efficient Delivery of Therapeutic Agents by Using Targeted Albumin Nanoparticles.
2. Galisteo-González F, Molina-Bolívar JA. Systematic study on the preparation of BSA nanoparticles. Colloids Surf B Biointerfaces. 2014;123:286–292. doi:10.1016/j.colsurfb.2014.09.028
3. Dewi MK, Chaerunisaa AY, Muhaimin M, Joni IM. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials. 2022;12(22):1–19. doi:10.3390/nano12224073
4. Tarhini M, Benlyamani I, Hamdani S, et al. Protein-based nanoparticle preparation via nanoprecipitation method. Materials (Basel). 2018;11(3):1–18. doi:10.3390/ma11030394
5. Loureiro A, Azoia G, Gomes A, Cavaco-Paulo A. Albumin-Based Nanodevices as Drug Carriers. Curr Pharm Des. 2016;22(10):1371–1390. doi:10.2174/1381612822666160125114900
6. Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):1–28. doi:10.3390/pharmaceutics12070604
7. Sethi A, Sher M, Akram MR, et al. Albumin as a drug delivery and diagnostic tool and its market approved products. Acta Pol Pharm - Drug Res. 2013;70(4):597–600.
8. Karami E, Behdani M, Kazemi-Lomedasht F. Albumin nanoparticles as nanocarriers for drug delivery: focusing on antibody and nanobody delivery and albumin-based drugs. J Drug Deliv Sci Technol. 2020;55:101471. doi:10.1016/j.jddst.2019.101471
9. Karimi M, Bahrami S, Ravari SB, et al. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Delivery. 2017;13(11):1609–1623. doi:10.1080/17425247.2016.1193149
10. Tirkey B, Bhushan B, Uday Kumar S, Gopinath P. Prodrug encapsulated albumin nanoparticles as an alternative approach to manifest anti-proliferative effects of suicide gene therapy. Mater Sci Eng C. 2017;73:507–515. doi:10.1016/j.msec.2016.12.108
11. Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–182. doi:10.1016/j.jconrel.2011.07.031
12. Srivastava A, Prajapati A. Albumin and functionalized albumin nanoparticles: production strategies, characterization, and target indications. Asian Biomed. 2020;14(6):217–242. doi:10.1515/abm-2020-0032
13. An FF, Zhang XH. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics. 2017;7(15):3667–3689. doi:10.7150/thno.19365
14. Solanki R, Patel K, Patel S. Bovine Serum Albumin Nanoparticles for the Efficient Delivery of Berberine: preparation, Characterization and In vitro biological studies. Colloids Surf a Physicochem Eng Aspects. 2021;608:125501. doi:10.1016/j.colsurfa.2020.125501
15. Solanki R, Saini M, Mochi J, Pappachan A, Patel S. Synthesis, characterization, in-silico and in-vitro anticancer studies of Plumbagin encapsulated albumin nanoparticles for breast cancer treatment. J Drug Deliv Sci Technol. 2023;84:104501. doi:10.1016/j.jddst.2023.104501
16. Hornok V. Serum albumin nanoparticles: problems and prospects. Polymers. 2021;13(21):1–11. doi:10.3390/polym13213759
17. de Camargo LEA, Brustolin Ludwig D, Tominaga TT, et al. Bovine serum albumin nanoparticles improve the antitumour activity of curcumin in a murine melanoma model. J Microencapsul. 2018;35(5):467–474. doi:10.1080/02652048.2018.1526340
18. Matloubi Z, Hassan Z. HSA-curcumin nanoparticles: a promising substitution for Curcumin as a Cancer chemoprevention and therapy. DARU J Pharm Sci. 2020;28(1):209–219. doi:10.1007/s40199-020-00331-2
19. Bolaños K, Kogan MJ, Araya E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int J Nanomed. 2019;14:6387–6406. doi:10.2147/IJN.S210992
20. Van de Sande L, Cosyns S, Willaert W, Ceelen W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: a review. Drug Deliv. 2020;27(1):40–53. doi:10.1080/10717544.2019.1704945
21. Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. doi:10.1016/j.addr.2018.07.011
22. Kudarha RR, Sawant KK. Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater Sci Eng C. 2017;81:607–626. doi:10.1016/j.msec.2017.08.004
23. Zsila F. Subdomain IB is the third major drug binding region of human serum albumin: toward the three-sites model. Mol Pharm. 2013;10(5):1668–1682. doi:10.1021/mp400027q
24. Patel K, Jain P, Rajput PK, et al. Human serum albumin-based propulsive Piperlongumine-loaded nanoparticles: formulation development, characterization and anti-cancer study. Colloids Surf a Physicochem Eng Aspects. 2022;652:129738. doi:10.1016/j.colsurfa.2022.129738
25. Tao Yu H, Wang Qi R, Sheng W, Zhen Y. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int J Biol Macromol. 2021;187:24–34. doi:10.1016/j.ijbiomac.2021.07.080
26. Raoufinia R, Mota A, Keyhanvar N, Safari F, Shamekhi S, Abdolalizadeh J. Overview of albumin and its purification methods. Adv Pharm Bull. 2016;6(4):495–507. doi:10.15171/apb.2016.063
27. MacPhee CE, Woolfson DN. Engineered and designed peptide-based fibrous biomaterials. Curr Opin Solid State Mater Sci. 2004;8(2):141–149. doi:10.1016/j.cossms.2004.01.010
28. Solanki R, Rostamabadi H, Patel S, Jafari SM. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: a critical review. Int J Biol Macromol. 2021;193(PA):528–540. doi:10.1016/j.ijbiomac.2021.10.040
29. Bhushan B, Khanadeev V, Khlebtsov B, Khlebtsov N, Gopinath P. Impact of albumin based approaches in nanomedicine: imaging, targeting and drug delivery. Adv Colloid Interface Sci. 2017;246:13–39. doi:10.1016/j.cis.2017.06.012
30. Wilson B, Ambika TV, Dharmesh Kumar Patel R, Jenita JL, Priyadarshini SRB. Nanoparticles based on albumin: preparation, characterization and the use for 5-flurouracil delivery. Int J Biol Macromol. 2012;51(5):874–878. doi:10.1016/j.ijbiomac.2012.07.014
31. Meng R, Zhu H, Wang Z, Hao S, Wang B. Preparation of Drug-Loaded Albumin Nanoparticles and Its Application in Cancer Therapy. J Nanomater. 2022;2022:1–12. doi:10.1155/2022/3052175
32. Solanki R, Patel S. Preparation, characterization and in vitro anticancer efficacy of biotin-conjugated, silibinin loaded bovine serum albumin nanoparticles. Food Biosci. 2023;56:103150. doi:10.1016/j.fbio.2023.103150
33. Huang BX, Kim HY, Dass C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J Am Soc Mass Spectrom. 2004;15(8):1237–1247. doi:10.1016/j.jasms.2004.05.004
34. Sengupta P, Sardar PS, Roy P, Dasgupta S, Bose A. Investigation on the interaction of Rutin with serum albumins: insights from spectroscopic and molecular docking techniques. J Photochem Photobiol B: Biol. 2018;183:101–110. doi:10.1016/j.jphotobiol.2018.04.019
35. Lamichhane S, Lee S. Albumin nanoscience: homing nanotechnology enabling targeted drug delivery and therapy. Arch Pharm Res. 2020;43(1):118–133. doi:10.1007/s12272-020-01204-7
36. Wang Y, Chen S, Yang X, Zhang S, Cui C. Preparation optimization of bovine serum albumin nanoparticles and its application for siRNA delivery. Drug Des Devel Ther. 2021;15:1531–1547. doi:10.2147/DDDT.S299479
37. Chang K, Liu J, Jiang W, Zhang R, Zhang T, Liu B. Ferulic acid-ovalbumin protein nanoparticles: structure and foaming behavior. Food Res Int. 2020;136:109311. doi:10.1016/j.foodres.2020.109311
38. Tazhbayev Y, Mukashev O, Burkeev M, Kreuter J. Hydroxyurea-loaded albumin nanoparticles: preparation, characterization, and in vitro studies. Pharmaceutics. 2019;11(8):8–16. doi:10.3390/pharmaceutics11080410
39. Bujacz A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr Sect D Biol Crystallogr. 2012;68(10):1278–1289. doi:10.1107/S0907444912027047
40. Battogtokh G, Kang JH, Ko YT. Long-circulating self-assembled cholesteryl albumin nanoparticles enhance tumor accumulation of hydrophobic anticancer drug. Eur J Pharm Biopharm. 2015;96:96–105. doi:10.1016/j.ejpb.2015.07.013
41. Lee ES, Youn YS. Albumin-based potential drugs: focus on half-life extension and nanoparticle preparation. J Pharm Investig. 2016;46(4):305–315. doi:10.1007/s40005-016-0250-3
42. Shimanovich U, Bernardes GJL, Knowles TPJ, Cavaco-Paulo A. Protein micro- and nano-capsules for biomedical applications. Chem Soc Rev. 2014;43(5):1361–1371. doi:10.1039/c3cs60376h
43. Anton N, Vandamme TF. The universality of low-energy nano-emulsification. Int J Pharm. 2009;377(1–2):142–147. doi:10.1016/j.ijpharm.2009.05.014
44. Safavi MS, Shojaosadati SA, Yang HG, et al. Reducing Agent-Free Synthesis of Curcumin-Loaded Albumin Nanoparticles by Self-Assembly at Room Temperature. Elsevier B.V; 2017. doi:10.1016/j.ijpharm.2017.06.087
45. Nnyigide OS, Oh Y, Song HY, Park EK, Choi SH, Hyun K. Effect of urea on heat-induced gelation of bovine serum albumin (BSA) studied by rheology and small angle neutron scattering (SANS). Korea Aust Rheol J. 2017;29(2):101–113. doi:10.1007/s13367-017-0012-4
46. Hassanin I, Elzoghby A. Albumin-based nanoparticles: a promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020. doi:10.20517/cdr.2020.68
47. Yu X, Di Y, Xie C, et al. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int J Nanomed. 2015;10(November):6825–6834. doi:10.2147/IJN.S93835
48. Thao LQ, Byeon HJ, Lee C, et al. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int J Pharm. 2016;497(1–2):268–276. doi:10.1016/j.ijpharm.2015.12.004
49. Arpagaus C. PLA/PLGA nanoparticles prepared by nano spray drying. J Pharm Investig. 2019;49(4):405–426. doi:10.1007/s40005-019-00441-3
50. Kimura K, Yamasaki K, Nishi K, Taguchi K, Otagiri M. Investigation of anti-tumor effect of doxorubicin-loaded human serum albumin nanoparticles prepared by a desolvation technique. Cancer Chemother Pharmacol. 2019;83:1113–1120. doi:10.1007/s00280-019-03832-3
51. Ziaaddini V, Saeidifar M, Eslami-Moghadam M, Saberi M, Mozafari M. Improvement of efficacy and decrement cytotoxicity of oxaliplatin anticancer drug using bovine serum albumin nanoparticles: synthesis, characterisation and release behaviour. IET Nanobiotechnol. 2020;14(1):105–111. doi:10.1049/iet-nbt.2019.0086
52. Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int J Pharm. 2017;522(1–2):172–197. doi:10.1016/j.ijpharm.2017.01.067
53. Elzoghby AO, Elgohary MM, Kamel NM. Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs.
54. Joye IJ, McClements DJ. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci Technol. 2013;34(2):109–123. doi:10.1016/j.tifs.2013.10.002
55. Rahimnejad M, Najafpour G, Bakeri G. Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloids Surf a Physicochem Eng Aspects. 2012;412:96–100. doi:10.1016/j.colsurfa.2012.07.022
56. Tazhbayev Y, Mukashev O, Burkeyev M, Lozinsky VI. Synthesis and comparative study of nanoparticles derived from bovine and human serum albumins. Polymers. 2020;12(6):1301. doi:10.3390/polym12061301
57. Amighi F, Emam-djomeh Z, Labbafi-Mazraeh-Shahi M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J Iran Chem Soc. 2020;17:1223–1235. doi:10.1007/s13738-019-01850-9
58. Wilson B, Paladugu L, Priyadarshini SRB, Jenita JJL. Development of albumin-based nanoparticles for the delivery of Abacavir. Int J Biol Macromol. 2015;81:763–767. doi:10.1016/j.ijbiomac.2015.09.015
59. Aljabali AAA, Bakshi HA, Hakkim FL, et al. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers. 2020;12(1):113. doi:10.3390/cancers12010113
60. Díaz-Saldívar P, Huidobro-Toro JP. ATP-loaded biomimetic nanoparticles as controlled release system for extracellular drugs in cancer applications. Int J Nanomed. 2019;14:2433–2447. doi:10.2147/IJN.S192925
61. Noorani L, Pourgholami MH, Liang M, Morris DL, Stenzel M. Albendazole loaded albumin nanoparticles for ovarian cancer therapy. Eur J Nanomedicine. 2014;6(4):227–236. doi:10.1515/ejnm-2014-0026
62. Kakran M, Sahoo NG, Li L, Judeh Z. Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. 2012;223:59–64. doi:10.1016/j.powtec.2011.08.021
63. Ganesh K, Archana D, Preeti K. Galactosylated albumin nanoparticles of simvastatin. Iran J Pharm Res. 2015;14(2):407–415.
64. Dalvi SV, Dave RN. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res. 2009;48(16):7581–7593. doi:10.1021/ie900248f
65. Jenita JJL, Vijaya C, Wilson B. Design and Characterization of Bovine Serum Albumin Nanocarriers For Tenofovir by Modified Desolvation Method. J Pharm Res. 2012;5(9)4663–4667.
66. Meer TA, Sawant KP, Amin PD. Liquid antisolvent precipitation process for solubility modulation of bicalutamide. Acta Pharm. 2011;61(4):435–445. doi:10.2478/v10007-011-0036-0
67. Olaitan V, Chaw CS. Desolvation conditions for production of sulfasalazine based albumin nanoparticles: physical properties. Pharm Front. 2019;1–15. doi:10.20900/pf20190006
68. Yang Z, Zhang N, Ma T, Liu L, Zhao L, Xie H. Engineered bovine serum albumin-based nanoparticles with pH-sensitivity for doxorubicin delivery and controlled release. Drug Deliv. 2020;27(1):1156–1164. doi:10.1080/10717544.2020.1797243
69. Abd Ellah NH, Ahmed EA, Abd-ellatief RB, Ali MF, Zahran AM, Hetta HF. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. Int J Nanomed. 2019;14:2383–2395. doi:10.2147/IJN.S196842
70. Jenita J, Wilson B, Chocalingam V. Albumin nanoparticles coated with polysorbate 80 as a novel drug carrier for the delivery of antiretroviral drug-Efavirenz. Int J Pharm Investig. 2014;4(3):142. doi:10.4103/2230-973x.138348
71. Spada A, Emami J, Sanaee F, et al. Albumin nanoparticles for the delivery of a novel inhibitor of β-tubulin polymerization. J Pharm Pharm Sci. 2021;24(5):344–362. doi:10.18433/jpps31877
72. Jenita JL. Development, evaluation and targeting of stavudine loaded serum albumin polymer based nanocarriers to HIV reservoirs. Asian J Pharm Clin Res. 2019;12(5):363–370. doi:10.22159/ajpcr.2019.v12i5.33005
73. Syed H, Sailaja AK. Preparation and characterization of nanoparticulate drug delivery system for naproxen sodium using various desolvating agents. Nano Biomed Eng. 2019;11(3):254–263. doi:10.5101/nbe.v11i3.p254-263
74. Radwan SES, El-Kamel A, Zaki EI, Burgalassi S, Zucchetti E, El-Moslemany RM. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int J Nanomed. 2021;16:4481–4494. doi:10.2147/IJN.S316564
75. Burns DB, Zydney AL. Effect of solution pH on protein transport through ultrafiltration membranes. Biotechnol Bioeng. 1999;64(1):27–37. doi:10.1002/(SICI)1097-0290(19990705)64:1<27::AID-BIT3>3.0.CO;2-E
76. Bronze-Uhle ES, Costa BC, Ximenes VF, Lisboa-Filho PN. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol Sci Appl. 2017;10:11–21. doi:10.2147/NSA.S117018
77. Hemlata Gupta S, Tejavath KK, Tejavath KK. ROS-mediated apoptosis induced by BSA nanospheres encapsulated with fruit extract of Cucumis prophetarum in various human cancer cell lines. ACS Omega. 2021;6(15):10383–10395. doi:10.1021/acsomega.1c00755
78. Eisele K, Gropeanu RA, Zehendner CM, et al. Fine-tuning DNA/albumin polyelectrolyte interactions to produce the efficient transfection agent cBSA-147. Biomaterials. 2010;31(33):8789–8801. doi:10.1016/j.biomaterials.2010.07.088
79. Rohiwal SS, Satvekar RK, Tiwari AP, Raut AV, Kumbhar SG, Pawar SH. Investigating the influence of effective parameters on molecular characteristics of bovine serum albumin nanoparticles. Appl Surf Sci. 2015;334:157–164. doi:10.1016/j.apsusc.2014.08.170
80. Langer K, Balthasar S, Vogel V, Dinauer N, Von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257(1–2):169–180. doi:10.1016/S0378-5173(03)00134-0
81. Kakran M, Sahoo NG, Li L, et al. Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int J Pharm. 2010;383(1–2):285–292. doi:10.1016/j.ijpharm.2009.09.030
82. Gebregeorgis A, Bhan C, Wilson O, Raghavan D. Characterization of Silver/Bovine Serum Albumin (Ag/BSA) nanoparticles structure: morphological, compositional, and interaction studies. J Colloid Interface Sci. 2013;389(1):31–41. doi:10.1016/j.jcis.2012.08.041
83. Sozer SC, Egesoy TO, Basol M, Cakan-Akdogan G, Akdogan Y. A simple desolvation method for production of cationic albumin nanoparticles with improved drug loading and cell uptake. J Drug Deliv Sci Technol. 2020;60:101931. doi:10.1016/j.jddst.2020.101931
84. Horn D, Rieger J. Organic nanoparticles in the aqueous phase - Theory, experiment, and use. Angew Chem Int Educ. 2001;40(23):4330–4361. doi:10.1002/1521-3773(20011203)40:23
85. Quintanar-Guerrero D, Allémann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm. 1998;24(12):1113–1128. doi:10.3109/03639049809108571
86. Stainmesse S, Orecchioni AM, Nakache E, Puisieux F, Fessi H. Formation and stabilization of a biodegradable polymeric colloidal suspension of nanoparticles. Colloid Polym Sci. 1995;273(5):505–511. doi:10.1007/BF00656896
87. Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7(25):4926–4934. doi:10.4314/ajb.v7i25.59701
88. Pal SL, Jana U, Manna PK, Mohanta GP, Manavalan R. Nanoparticle: an overview of preparation and characterization. J Appl Pharm Sci. 2011;1(6):228–234.
89. Sadeghi R, Moosavi-Movahedi AA, Emam-Jomeh Z, et al. The effect of different desolvating agents on BSA nanoparticle properties and encapsulation of curcumin. J Nanopart Res. 2014;16(9). doi:10.1007/s11051-014-2565-1
90. Sailaja AK. A comparative study of aspirin loaded bovine serum albumin nanoparticles prepared by desolvation technique using various desolvating agents. Nano Biomed Eng. 2017;9(2):143–151. doi:10.5101/nbe.v9i2.p143-15
91. Taheri A, Razavi SMA. Fabrication of cress seed gum nanoparticles, an anionic polysaccharide, using desolvation technique: an optimization study. Bionanoscience. 2015;5(2):104–116. doi:10.1007/s12668-015-0169-6
92. Santos-Rebelo A, Kumar P, Pillay V, et al. Development and mechanistic insight into the enhanced cytotoxic potential of parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers. 2019;11(11). doi:10.3390/cancers11111733
93. Krishna Sailaja A, Nandini M. Effect of various desolvating agents in the formulation of naproxen loaded BSA nanoparticles. J Bionanoscience. 2017;11(6):497–503. doi:10.1166/jbns.2017.1472
94. Arroyo-Maya IJ, Hernández-Sánchez H, Jiménez-Cruz E, Camarillo-Cadena M, Hernández-Arana A. α-Lactalbumin nanoparticles prepared by desolvation and cross-linking: structure and stability of the assembled protein. Biophys Chem. 2014;193–194:27–34. doi:10.1016/J.BPC.2014.07.003
95. Niknejad H, Mahmoudzadeh R. Comparison of different crosslinking methods for preparation of docetaxel-loaded albumin nanoparticles. Iran J Pharm Res. 2015;14(2):385–394.
96. Kim JY, Ahn JH, Song SB, et al. Enhancement of the Strength and Stability of Ultraviolet-Irradiated Acellular Dermal Matrix by Adding Glucose. Key Eng Mater. 2007;342–343:337–340. doi:10.4028/www.scientific.net/kem.342-343.337
97. Ohan MP, Dunn MG. Glucose stabilizes collagen sterilized with gamma irradiation. J Biomed Mater Res - Part A. 2003;67(4):1188–1195. doi:10.1002/jbm.a.20018
98. Suh H, Lee C. Biodegradable ceramic-collagen composite implanted in rabbit tibiae. ASAIO J. 1995;41(3):M652–M656. doi:10.1097/00002480-199507000-00091
99. Weadock KS, Miller EJ, Bellincampi LD, Zawadsky JP, Dunn MG. Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res. 1995;29(11):1373–1379. doi:10.1002/jbm.820291108
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
