Back to Journals » Drug Design, Development and Therapy » Volume 15
Exploration of the Effect and Mechanism of Fructus Lycii, Rehmanniae Radix Praeparata, and Paeonia lactiflora in the Treatment of AMD Based on Network Pharmacology and in vitro Experimental Verification
Authors Chen X, Zuo J, Hu T, Shi X , Zhu Y, Wu H, Xia Y, Shi W, Wei W
Received 10 March 2021
Accepted for publication 25 May 2021
Published 28 June 2021 Volume 2021:15 Pages 2831—2842
DOI https://doi.org/10.2147/DDDT.S310481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Xi Chen,1– 3,* Jing Zuo,2,* Tianming Hu,2,* Xiaoqing Shi,1– 3 Yujie Zhu,1– 3 Hao Wu,2 Ying Xia,2 Wei Shi,2 Wei Wei1,2
1First College of Clinical Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China; 2Department of Ophthalmology, The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China; 3Key Laboratory for Metabolic Diseases in Chinese Medicine, First College of Clinical Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Wei; Wei Shi
Department of Ophthalmology, The Affiliated Hospital of Nanjing University of Chinese Medicine, No. 155 Hanzhong Road, Qinhuai District, Nanjing, Jiangsu, 210029, People’s Republic of China
Tel +86 25-86617141
Email [email protected]; [email protected]
Purpose: The aim of this study was to observe the mechanism of Fructus Lycii (FL), Rehmanniae Radix Praeparata (RRP) and Paeonia lactiflora (PL) in treating age-related macular degeneration (AMD) based on network pharmacology and biological experiments.
Methods: Bioactive compounds, potential targets of FL, RRP and PL, and genes related to AMD, were acquired from public databases. Functional and pathway enrichment analyses of the core targets were conducted by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Subsequently, the finding was further verified with cell experiments. The MTT assay and flow cytometric analysis were used to assess cell viability and apoptosis. The production of reactive oxygen species (ROS) was analyzed by DCFH-DA staining; the activity of antioxidant enzymes was chemically measured with assay kits. The expression of key proteins was evaluated by Western blot analysis.
Results: Fifty-nine active compounds, 182 potential targets, and 2536 AMD-related human genes were identified. A total of 103 key targets of the three herbs on AMD were identified by protein–protein interaction (PPI) analysis. The abovementioned targets were correlated with nuclear receptor activity, oxidative stress, and apoptosis pathways according to the GO and KEGG analyses. MTT assay and flow cytometry demonstrated that pretreatment of ARPE-19 cells with the three herbs significantly increased cell viability and decreased apoptosis induced by H2O2. The three herbs might reduce the intracellular ROS levels and increase the SOD and CAT activities after H2O2. Furthermore, the three herbs significantly inhibited oxidative stress via increasing the expression of Nrf2, HO-1 and NQO1.
Conclusion: The combined results of network pharmacology and validation experiments showed that FL, RRP and PL reduce oxidative stress and apoptosis in RPE cells to exert its effect in the treatment of AMD.
Keywords: age-related macular degeneration, Fructus Lycii, Rehmanniae Radix Praeparata, Paeonia lactiflora, network pharmacology, oxidative stress
Introduction
Age-related macular degeneration (AMD) is the most common cause of permanent vision loss among elderly populations worldwide.1 AMD is classified into dry and wet forms. Repeated injection of substantial amounts of anti-vascular endothelial growth factor (VEGF) has become an effective therapy for patients with wet AMD.2 Currently, no therapies are available for dry AMD, and dry AMD accounts for approximately 80% of AMD cases.3 Dry AMD is characterized by changes in macular pigmentation and drusen in the early stage and by areas of geographic atrophy (GA) in the late stage.4 This gradual degeneration of retinal cells in GA patients may cause profound vision loss.5 Therefore, there is an urgent need for an effective and safe method for treating dry AMD.
Oxidative stress leads to RPE cell dysfunction or apoptosis, and it is an important factor in the pathology of AMD.6 In retinal pigment epithelium (RPE) cells, the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway plays a major role in oxidative stress regulation.7 Nrf2 is released from Keap1 and translocates into the nucleus, where it binds to AREs, thus activating the transcription of heme oxygenase (HO)-1, NAD(P)H: quinone oxidoreductase 1 (NQO-1), superoxide dismutase (SOD), catalase (CAT), and other antioxidant genes.8 Loss of Nrf2 leads to damage to RPE cells that resembles dry AMD.9 Therefore, activation of Nrf2 and its downstream target genes is one of the most pivotal mechanisms of defense against oxidative stress in AMD.
Currently, common drug therapies for dry AMD such as vitamin C, vitamin E, β-carotene, and zinc oxide are used to reduce risk of progression to neovascular AMD.10 However, the supplementation of lutein and zeaxanthin or docosahexaenoic acid and eicosapentaenoic acid did not reduce the risk of progression to neovascular AMD.10 Our precious network meta-analysis indicated that traditional Chinese medicine (TCM) achieved higher visual acuity than vitamin C and vitamin E.11 In recent years, the use of TCM in the treatment of AMD has appeared to provide a solution and has attracted substantial attention.12 In clinical application, TCM offers the advantages of multicomponent, multipathway and multitarget synergies for the prevention and treatment of AMD. Mingmu Dihuang Pills (MMDHP), first named “Shenshi Yaohan” during the Ming dynasty, is a traditional prescription used for nourishing the kidney, calming the liver and improving eyesight. MMDHP is widely used in the treatment of various ophthalmic diseases, cerebrovascular diseases, and endocrine diseases, such as diabetes.13 Fructus Lycii (FL), Rehmanniae Radix Praeparata (RRP) and Paeonia lactiflora (PL) are more frequently used in the treatment of AMD and are mainly composed of MMDHP.14,15 However, the pharmacological activities and underlying mechanisms associated with these formulas are still largely unknown.
Network pharmacology, an emerging field of pharmacology, is based on network theory and systems biology.16 In the present work, network pharmacology, which combines drug-likeness evaluation, oral bioavailability prediction, multiple drug target prediction and other network pharmacology techniques, was used to attempt to answer these questions.17 Network pharmacology has been widely used to investigate the active ingredients and multitarget effects of TCMs in the context of several diseases.18,19 This approach provides a new research paradigm for translating TCM from experience-based medicine to an evidence-based medical system. In this study, we identified genes related to selected ingredients or AMD by using public databases, and the genes that were both related to ingredients and target genes in AMD were identified. Next, pathway enrichment analysis of these overlapping genes was carried out to explore the molecular mechanisms. In addition, an in vitro RPE cell model was established to verify the curative effects of these formulas on AMD as predicted by network pharmacology analysis. The results suggested that FL, RRP and PL may exert a therapeutic effect against AMD because they can regulate key factors in the oxidative stress pathway.
Materials and Methods
Data Preparation
Chemical Ingredient Database Construction
The three constituent herbs of MMDHP were retrieved from the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP, http://tcmspnw.com). The TCMs in the TCMSP were searched with keywords including “Fructus Lycii,” “Rehmanniae Radix Praeparata,” and “Paeonia lactiflora.” Oral bioavailability (OB) is an indicator of the percentage of orally administered drugs that are delivered into systemic circulation.20 Drug-likeness (DL) mainly indicates the qualitative properties of chemicals and refers to the similarity between a compound and a known drug in the component.21 The screening conditions were as follows: OB ≥ 30% and DL ≥ 0.18. We screened targets related to the active compounds using the TCMSP platform. Then, the UniProt database (https://www.uniprot.org/) was used to obtain the gene name and gene ID of the targets.
Disease-Associated Targets
Treatable diseases related to the potential targets were identified through GeneCards (https://www.genecards.org/), OMIM (https://www.omim.org/), Therapeutic Targets database (http://bidd.nus.edu.sg/BIDD-Databases/TTD/TTD.asp), DisGeNET database (http://disgenet.org/) and PharmGkb database (https://www.pharmgkb.org/). After removing duplicate genes, we obtained all the AMD targets. The genes that were both targets related to AMD and active compounds were identified as candidate targets.
Construction and Analysis of a Protein-Protein Interaction (PPI) Network
The component-target network was constructed using Cytoscape (https://cytoscape.org/, Version 3.7.2). Additionally, the components and targets were imported into the STRING biological database (https://string-db.org/) to explore proteins that interact with these targets. The selection parameters were set to “Homo sapiens” for species, and the confidence level was set at 0.9 for the minimum required interaction score. The TSV format of the updated results was downloaded and then visualized in Cytoscape.
Enrichment Analysis of GO and KEGG
GO and KEGG pathway enrichment analyses of the candidate targets were conducted using R3.6.2 software. We prepared a file of intersecting genes and then used the Bioconductor package to perform a GO enrichment analysis. The top 20 items of three analysis items, biological processes (BP), cellular components (CC), and molecular functions (MF), in the GO functional enrichment analysis were selected. The top 20 signaling pathway enrichment analyses were downloaded from the KEGG database (https://www.genome.jp/kegg/pathway.html, Last updated: March 10, 2020). The downloaded results were sorted using P values and count values.
Experimental Verification
Cell Culture and Treatment
The ARPE-19 cell line (Nanjing Kebai Biotech Co., Ltd., Nanjing, China) was cultured at 37°C and 5% CO2 in DMEM/F12 supplemented with 10% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Grand Island, NY, USA), 100 units/mL penicillin and 100 μg/mL streptomycin (Beyotime, Nanjing, China). According to the experimental design, the cells were incubated with FL, RRP and PL (1:1:1) freeze-dried powder (10 μg/mL, 50 μg/mL, 100 μg/mL, 500 μg/mL, or 1000 μg/mL) for 24 h and with 200 μM H2O2 for 24 h.
Proliferation Assay and Cell Apoptosis
To examine the effect of the freeze-dried powders on the proliferation of cells undergoing oxidative stress induced by H2O2, cells were plated in 96-well plates at a density of 5×103 cells per well. The viability of the ARPE-19 cells was determined using an MTT reduction assay. Apoptotic cells were analyzed using an Annexin V/PI Apoptosis Detection kit (BD Company, New York, NY, USA) following the manufacturer’s instructions.
ROS Measurement
ROS generation was evaluated by 2′,7′-dichlorofluorescein-diacetate (DCFH-DA, Sigma-Aldrich, MO, USA) staining. After incubating the cells with 10 μM DCFH-DA for 30 min at 37°C, the results were visualized. The mean fluorescence intensity of each group was analyzed using a fluorescence microscope, and this value represented the intracellular ROS level.
SOD and CAT Measurement
The activities of superoxide dismutase (SOD) and catalase (CAT) were determined by using commercial assay kits (Jiancheng, Nanjing, China) according to the instructions. The activities of SOD and CAT were calculated and are expressed as U/mg protein.
Western Blot Analysis
Protein concentrations were measured by the BCA protein assay (Thermo Fisher Scientific, Grand Island, NY, USA). The nuclear extraction procedure was performed according to the manufacturer’s instructions (Beyotime, Nanjing, China). All the samples were separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). After blocking, the membranes were incubated overnight with primary antibodies, including anti-β-actin (sc-47778), anti-Nrf2 (ab137550), anti-NQO1 (ab34173) and anti-HO-1 (ab52947), at 4°C. The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (ComWin, Beijing, China) at room temperature for 1.5 h. Protein expression was measured by using a chemiluminescence kit (Millipore, Plano, TX, USA) and analyzed by using ImageJ.
Statistical Analysis
The data are expressed as the mean ± SD and analyzed using one-way ANOVA (followed by Tukey’s or LSD multiple comparison post hoc test) among multiple groups. The SPSS 20.0 software package was used for all the statistical analyses. P-values lower than 0.05 were considered statistically significant.
Results
Compound Determination and Target Prediction
According to the active ingredient screening thresholds of OB ≥ 30% and DL ≥ 0.18, 59 active compounds were identified in the TCMSP databases (Supplementary Table 1). After screening the TCMSP databases, 182 targets related to 15 active ingredients in three herbs were associated with AMD. As a result, after removing 44 unrelated active ingredients, 15 active ingredients were finally selected. The gene names and gene IDs were obtained from the UniProt database.
Target Prediction and Analysis
A total of 2536 human genes associated with AMD were identified in the GeneCards, OMIM, Therapeutic Targets, DisGeNET, and PharmGkb databases. After combining the AMD-related targets and active compound targets, 103 overlapping targets were recognized as common genes. As shown in Figure 1, the compound-disease target network analysis showed 118 nodes and 192 edges in total. The yellow rectangle represents the active ingredients, and the blue rectangle represents the targets of the drugs. These results indicate that the active ingredients of the three herbs can act on different targets in the network and regulate the protein-interaction network.
PPI Network Analysis
We constructed a PPI network by importing the gene IDs of the common genes to the STRING database and used Cytoscape to visualize the PPI network. The results included a total of 96 nodes and 353 edges. These target proteins also play key roles in the whole interaction network and are important target proteins. As shown in Figure 2, most of the oxidative stress-related targets and apoptosis-related targets, such as NFE2L2 (Nrf2), NQO1, SOD, CAT, BAX, BCL2, BCL2L1, CASP3, CASP8 and CASP9, were interrelated.
 |
Figure 2 Important targets in the PPI network. |
GO Biological Function Annotation and KEGG Pathway Enrichment Analysis
Filtering was performed with a P value <0.05 as the threshold value. The BP were mainly involved in response to lipopolysaccharide, response to molecule of bacterial origin, reactive oxygen species metabolic process, response to metal ion, response to reactive oxygen species, response to oxidative stress, response to antibiotic, cellular response to oxidative stress, response to oxygen levels and regulation of apoptotic signaling pathway and so on. The CC were mainly involved in membrane raft, membrane microdomain, membrane region, vesicle lumen, caveola, secretory granule lumen, RNA polymerase II transcription factor complex, cytoplasmic vesicle lumen, transcription factor complex, nuclear transcription factor complex and so on. The MF were mainly involved in cytokine receptor binding, cytokine activity, nuclear receptor activity, transcription factor activity and oxidoreductase activity. The details are shown in Figure 3. Furthermore, the enriched KEGG pathways were involved in cancer, oxidative stress, apoptosis, and inflammation. As shown in Figure 3, pathways, including the AGE-RAGE signaling pathway in diabetic complications, IL-17 signaling pathway, HIF-1 signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, Cellular senescence and MAPK signaling pathway, were screened.
Experimental Outcomes
FL, RRP and PL Protect H2O2 Treated-RPE Cells
The effects of FL, RRP and PL on the viability of H2O2-treated ARPE-19 cells were detected by using the MTT assay. As shown in Figure 4A, the viability of ARPE-19 cells injured with H2O2 was significantly decreased compared with that of the control cells. Compared with H2O2, the freeze-dried powders of the three herbs (50 and 100 μg/mL) notably improved proliferation after 24 h. Therefore, we chose 10, 50 and 100 μg/mL for the subsequent experiments.
To determine whether pretreatment with the three herbs reduced ARPE-19 cell apoptosis, apoptosis was examined by flow cytometry. As shown in Figure 4B and C, H2O2-induced oxidative stress significantly increased the numbers of apoptotic cells, while significant enhancements in cell viability were observed after treatment with the three herbs at concentrations of 10–100 μg/mL for 24 h.
We also observed the changes in cell morphology after treatments with the three herbs and H2O2. As shown in Figure 4D, after exposure to 200 μM H2O2 for 24 h, RPE cells were severely damaged, and many cells were shrunken, round, detached from the surrounding cells, and dying. The three herbs significantly inhibited the H2O2-induced RPE cell damage.
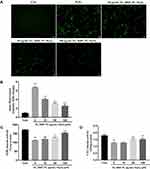
FL, RRP and PL Reduced the Intracellular ROS Levels but Increased the SOD and CAT Activities in H2O2-Treated RPE Cells
Intracellular ROS contents reflect intracellular oxidative conditions. We determined the ROS level by DCFH-DA staining and measured the mean fluorescence intensity in the cells. As shown in Figure 5A and B, the fluorescence intensity in the H2O2 group was higher than that in the three herb and control groups, indicating that intracellular ROS production was significantly promoted by H2O2 but was inhibited by pretreatment with the three herbs.
To elucidate the mechanism by which FL, RRP and PL enhanced the resistance of RPE cells to oxidative stress, we examined the effects of these herbs on the activities of the antioxidant enzymes SOD and CAT. As shown in Figure 5C and D, the activities of the antioxidant enzymes in the H2O2 group were lower than those in the three herb and control groups, indicating that the activities of SOD and CAT were significantly decreased by H2O2 but were increased by pretreatment with the three herbs.
Effect of FL, RRP and PL on Nrf2 Expression in H2O2-Treated RPE Cells
Based on the network pharmacology analysis, we tested whether FL, RRP and PL could promote Nrf2 translocation into the nucleus and activate Nrf2 signaling. The Nrf2 pathway was analyzed by Western blot. As shown in Figure 6, treatment with 200 μM H2O2 alone for 24 h significantly increased the nuclear Nrf2 protein level (P < 0.01). HO-1 and NQO1 are the downstream molecules of the Nrf2 signaling pathway and produced a significant expression in the H2O2 group than in the control group (P < 0.01). Furthermore, compared with H2O2 alone, pretreatment with the three herbs significantly promoted Nrf2 protein translocation into the nucleus in H2O2-treated RPE cells (P < 0.01). However, on increasing the concentrations of three herbs to 100 μg/mL, there were significant increases in HO-1 and NQO1 proteins (P < 0.05 and P < 0.01). The results demonstrate that FL, RRP and PL may protect RPE cells undergoing oxidative stress by increasing the expression of Nrf2, HO-1 and NQO1.
Discussion
TCMs treat diseases by regulating the stability of the entire body. A variety of herbs contain many ingredients, and they inevitably affect multiple targets and multiple approaches. Network pharmacology can analyze the interaction between drugs and diseases and provide a well-characterized approach for exploring the pharmacological mechanisms underlying the effects of TCMs. In this study, we conducted network pharmacology and in vitro experiments to scientifically and systematically elucidate the material basis and mechanism underlying the effects FL, RRP and PL in the treatment of AMD.
Fructus Lycii (FL) has been shown to exhibit multiple biological activities, including antioxidant,22 anti-inflammatory,23 antiapoptotic,24 and neuroprotective activities.25 The bioactive components of FL are carotenoids, polysaccharides and flavonoids, which exhibit strong antioxidant activities. Pretreatment with FL extract protects human RPE cells against the acute oxidative stress induced by exposure to H2O2.26 The antioxidant properties of FL may be related to endogenous ROS, apoptosis-related genes and deoxyribonucleic acid (DNA) damage.24 Rehmanniae Radix Praeparata (RRP) plays multiple protective roles in age-related diseases, including diabetes mellitus,27 neuronal disorders,28 and inflammation.29 The bioactive components of RRP are iridoid glycosides, nucleosides, phenethyl alcohol glycosides, and sugars.30 FL and RRP have protective effects on retinal function and structure in AMD model mice, and the effect of their combined treatment is more obvious.31 Paeonia lactiflora (PL) has been shown to exhibit multiple biological activities, including pain reduction, anti-inflammatory, immunomodulatory, antitumor, antioxidant and immune regulatory activities.32 The bioactive components of PL are volatile oil, monoterpene glycoside, flavonoids, triterpenoid, tannins and polysaccharides. Paeoniflorin, a monoterpene glucoside isolated from PL, reduces Nox1/ROS-associated oxidative stress, mitochondrial dysfunction and endoplasmic reticulum (ER) stress in ARPE-19 cells.15 According to the component-target network, quercetin, 7-O-methylluteolin-6-C-beta-glucoside, 6-fluoroindole-7-dehydrocholesterol, cyanin, 14b-pregnane, glycitein, atropine, stigmasterol, ethyl linolenate, mandenol, sitosterol alpha1, (+)-catechin, kaempferol, beta-sitosterol and paeoniflorin might participate in the main anti-AMD effects of these three herbs. These main active components are flavonoids, steroids, monoterpene glycosides, volatile oils and sugars.
Furthermore, our results suggest that NFE2L2, NQO1, SOD, CAT, BAX, BCL2, BCL2L1, CASP3, CASP8 and CASP9 are the key targets of the active compounds in quercetin, kaempferol, beta-sitosterol and paeoniflorin, and these targets play crucial roles in reducing RPE cell oxidative stress and apoptosis.33–35 Nrf2 is a transcription factor that targets and upregulates key antioxidant systems. In response to oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus, where it promotes the expression several antioxidant defense enzymes. These enzymes can quickly scavenge ROS, regulate cell proliferation and death, and maintain cellular redox homeostasis.36 When intracellular ROS production is elevated, the ROS interact with Bax and promote cytochrome c release into the cytoplasm.37 Cytochrome c combines with caspases-9 to activate the expression of downstream caspase-3, and eventually leads to apoptosis.38 Weng et al demonstrated that quercetin can improve cell viability, decrease the cellular apoptotic rate and exert protective effects via the activation of the Keap1/Nrf2/ARE pathway in H2O2-treated RPE cells.39 Xie et al show that paeoniflorin protected ARPE-19 cells from H2O2-induced cell apoptosis via decreased ROS production and caspase-3 activity.40
A total of 2531 GO terms and 166 pathways were identified by GO pathway and KEGG pathway enrichment analyses. The KEGG pathways through which the three herbs protect against AMD mainly included the HIF-1 signaling pathway, MAPK signaling pathway, TNF signaling pathway, hepatocellular carcinoma signaling pathway and apoptosis pathway.
According to network pharmacology results, Nrf2 and antioxidant protein are common targets of AMD and three herbs. To verify the network pharmacology prediction results, in vitro cell experiments were carried out. Exogenous H2O2-induced oxidative injury is a simple and feasible cell model for studying RPE oxidative damage, and this model can effectively simulate the process of oxidative damage to the RPE in AMD.41 Therefore, we subsequently designed corresponding in vitro experiments to elucidate the prediction results of network pharmacology. The in vitro results showed that H2O2 treatment significantly inhibited the survival rate RPE cells and improved the apoptosis of RPE cells at the same time. As predicted by network pharmacology approach, pretreatment with the three herbs (10, 50, and 100 μg/mL) effectively inhibited H2O2-induced apoptosis and increased cell viability in RPE cells in a dose-dependent manner. Then, intracellular ROS generation has been reported to a potential mediator, which contributed to apoptosis in the progression of RPE cells42 and be associated with risk factors for AMD.43 We found that the three herbs preserve H2O2-induced RPE injury by inhibiting ROS and decreasing apoptosis. In RPE cells, the balance between ROS generation and antioxidant systems maintains cellular redox homeostasis. H2O2 increased the ROS level while FL, RRP and PL decreased it, which was consistent with the results of antioxidant enzymes. Low SOD and CAT levels denoted elevated oxidative stress, as noted in the H2O2-treated RPE cells. This study showed that the three herbs could increase the activities of CAT and SOD and maintain intracellular ROS homeostasis under H2O2-induced oxidative stress conditions.
Nrf2 is an important regulator of oxidative stress by the activation of antioxidant enzymes. We hypothesized that the specific mechanism underlying this protective effect may be related to the Nrf2/Keap1 signaling pathway. Saito et al indicated that Nrf2 activation on RPE cells could be a promising therapeutic approach for non-exudative AMD.44 Given the importance of Nrf2 in AMD, we assessed the major proteins of the Nrf2 pathway. The Western blot results showed that the activation by H2O2 promote Nrf2 nuclear translocation and subsequent antioxidative protein expression. We also confirmed that FL, RRP and PL exerted maximum protective effect at 100 μg/mL against H2O2-induced oxidative stress. When the concentration of three herbs was lower than 100 μg/mL, the expression levels of HO-1 and NQO1 were significantly unaltered in spite of Nrf2 levels was significantly increased. Unlike previous reports, the effects of three herbs were not dose-dependent, and high doses improved antioxidant enzymes HO-1 and NQO1 levels. We hypothesized that exogenous H2O2-induced oxidative injury induces Nrf2 activation and then the proper concentration of FL, RRP and PL is possibly altered. However, accurate quantification of Nrf2 activation levels is difficult. The mechanisms include the activation of the Nrf2 protein in the nucleus, suggesting that the three herbs may stimulate HO-1 and NQO1 expression by regulating Nrf2 signaling. These results indicated that FL, RRP and PL may be suitable for the treatment of AMD.
These results suggest that three herbs prevent H2O2-stimulated cell apoptosis in RPE cells, which may be strongly related to the antioxidative effects of FL, RRP and PL. According to the results of our study, network pharmacology is a scientific and feasible method for studying TCMs. However, other signaling pathways may also be involved in the anti-AMD effect of FL, RRP and PL, such as MAPK, TNF, HIF-1 signaling pathways. The detailed pharmacological mechanisms by which FL, RR and PL ameliorate AMD will be investigated in our future study.
Conclusion
In summary, our network pharmacological analysis and in vitro experiments provide a basis for the mechanisms underlying the protective effects of FL, RRP and PL against AMD. According to pathway enrichment analysis, the key mechanism underlying the effects of the three herbs in protecting against AMD may be related to oxidative stress, apoptosis and inflammation pathways. This study further confirmed that the three herbs can effectively enhance the activity and inhibit the apoptosis of RPE cells and exert key effects on the expression of Nrf2, HO-1, and NQO1.
Abbreviations
AMD, age-related macular degeneration; TCM, traditional Chinese medicine; MMDHP, Mingmu Dihuang Pills; FL, Fructus Lycii; RRP, Rehmanniae Radix Praeparata; PL, Paeonia lactiflora; OB, Oral bioavailability; DL, Drug-likeness; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPI, protein-protein interaction; ROS, reactive oxygen species; SOD, superoxide dismutase; CAT, catalase; CCK-8, cell counting kit-8; VEGF, vascular endothelial growth factor; GA, geographic atrophy; CC, cellular components; MF, molecular functions; BP, biological processes; RPE, retinal pigment epithelium; Nrf2, nuclear factor erythroid 2-related factor 2; ARE, antioxidant response element; HO-1, heme oxygenase; NQO1, NAD(P)H: quinone oxidoreductase 1.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding authors upon request.
Acknowledgments
The current work was supported by the National Natural Science Foundation of China (No. 81774370).
Disclosure
The authors declare that they have no competing interests.
References
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116. doi:10.1016/s2214-109x(13)70145-1
2. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi:10.1016/j.ophtha.2012.03.053
3. Nebbioso M, Lambiase A, Cerini A, Limoli PG, La Cava M, Greco A. Therapeutic approaches with intravitreal injections in geographic atrophy secondary to age-related macular degeneration: current drugs and potential molecules. Int J Mol Sci. 2019;20(7):1693. doi:10.3390/ijms20071693
4. Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: the beaver dam eye study. Ophthalmology. 2020;127(4):S122–s132. doi:10.1016/j.ophtha.2020.01.033
5. Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94(7):918–925. doi:10.1136/bjo.2009.165563
6. Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24(Pt B):286–298. doi:10.1016/j.arr.2015.09.002
7. Takayama K, Kaneko H, Kataoka K, et al. Nuclear factor (erythroid-derived)-related factor 2-associated retinal pigment epithelial cell protection under blue light-induced oxidative stress. Oxid Med Cell Longev. 2016;2016:8694641. doi:10.1155/2016/8694641
8. Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ. The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev. 2017;2017:9237263. doi:10.1155/2017/9237263
9. Felszeghy S, Viiri J, Paterno JJ, et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019;20:1–12. doi:10.1016/j.redox.2018.09.011
10. Broadhead GK, Grigg JR, Chang AA, McCluskey P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr Rev. 2015;73(7):448–462. doi:10.1093/nutrit/nuv005
11. Xi C, Dan Q, TianMing H, Wei W. Efficacy in the treatment of dry age-related macular degeneration by traditional Chinese medicine: a meta analysis. Lishizhen Med Materia Medica Res. 2019;30(09):2273–2276.
12. Fang Y, Liu X, Su J, Rehman K. Network pharmacology analysis of traditional Chinese medicine formula Shuang Di Shou Zhen tablets treating nonexudative age-related macular degeneration. Evid Based Complement Altern Med. 2021;2021:6657521. doi:10.1155/2021/6657521
13. Sai L, Dong L. Clinical application and discrimination of Qiju Dihuang Wan and Mingmu Dihuang Wan. China J Tradition Chin Med Pharm. 2013;28(07):2186–2188.
14. Xu X, Hang L, Huang B, Wei Y, Zheng S, Li W. Efficacy of ethanol extract of Fructus lycii and its constituents lutein/zeaxanthin in protecting retinal pigment epithelium cells against oxidative stress: in vivo and in vitro models of age-related macular degeneration. J Ophthalmol. 2013;2013:862806. doi:10.1155/2013/862806
15. Zhu X, Wang K, Zhou F, Zhu L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca(2+)/CaMKII-dependent activation of AMPK. Arch Pharm Res. 2018;41(10):1009–1018. doi:10.1007/s12272-018-1059-6
16. Yue SJ, Xin LT, Fan YC, et al. Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci Rep. 2017;7:40318. doi:10.1038/srep40318
17. Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145(1):1–10. doi:10.1016/j.jep.2012.09.051
18. Ge Q, Chen L, Tang M, et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur J Pharmacol. 2018;833:50–62. doi:10.1016/j.ejphar.2018.05.021
19. Zhai J, Song Z, Wang Y, et al. Zhixiong Capsule (ZXC), a traditional Chinese patent medicine, prevents atherosclerotic plaque formation in rabbit carotid artery and the related mechanism investigation based on network pharmacology and biological research. Phytomedicine. 2019;59:152776. doi:10.1016/j.phymed.2018.11.036
20. Liu H, Wang J, Zhou W, Wang Y, Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol. 2013;146(3):773–793. doi:10.1016/j.jep.2013.02.004
21. Walters WP, Murcko MA. Prediction of ‘drug-likeness’. Adv Drug Deliv Rev. 2002;54(3):255–271. doi:10.1016/s0169-409x(02)00003-0
22. Zhang XF, Chen J, Yang JL, Shi YP. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta. 2018;180:389–395. doi:10.1016/j.talanta.2017.12.078
23. Lam P, Cheung F, Tan HY, Wang N, Yuen MF, Feng Y. Hepatoprotective effects of Chinese medicinal herbs: a focus on anti-inflammatory and anti-oxidative activities. Int J Mol Sci. 2016;17(4):465. doi:10.3390/ijms17040465
24. Neelam K, Dey S, Sim R, Lee J, Au Eong KG. Fructus lycii: a natural dietary supplement for amelioration of retinal diseases. Nutrients. 2021;13:1. doi:10.3390/nu13010246
25. Ye M, Moon J, Yang J, et al. The standardized Lycium chinense fruit extract protects against Alzheimer’s disease in 3xTg-AD mice. J Ethnopharmacol. 2015;172:85–90. doi:10.1016/j.jep.2015.06.026
26. Liu L, Lao W, Ji QS, Yang ZH, Yu GC, Zhong JX. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int J Ophthalmol. 2015;8(1):11–16. doi:10.3980/j.issn.2222-3959.2015.01.02
27. Gong W, Zhang N, Cheng G, et al. Rehmannia glutinosa libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int J Mol Sci. 2019;20(16):3964. doi:10.3390/ijms20163964
28. Yuan H, Ni X, Zheng M, Han X, Song Y, Yu M. Effect of catalpol on behavior and neurodevelopment in an ADHD rat model. Biomed Pharmacother. 2019;118:109033. doi:10.1016/j.biopha.2019.109033
29. Liu C, Ma R, Wang L, et al. Rehmanniae Radix in osteoporosis: a review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol. 2017;198:351–362. doi:10.1016/j.jep.2017.01.021
30. Ke D, Xiaoxia G, Feng W, PeiXi W, Mei QX. Research progress on quality and efficacy evaluation of Rehmanniae Radix Praeparata based on pharmacodynamic material basis. Chin Tradition Herbal Drugs. 2019;50(06):1477–1484.
31. XueChun W, Wei Z, YaTu G, YiBo G. Protective effects of rehmannia glutinosa,wolfberry and the mixed extracts on light-induced retinal injury of mice. Recent Adv Ophthalmol. 2021;41(01):12–17. doi:10.13389/j.cnki.rao.2021.0003
32. Parker S, May B, Zhang C, Zhang AL, Lu C, Xue CC. A pharmacological review of bioactive constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother Res. 2016;30(9):1445–1473. doi:10.1002/ptr.5653
33. Zhu Q, Liu M, He Y, Yang B. Quercetin protect cigarette smoke extracts induced inflammation and apoptosis in RPE cells. Artif Cells, Nanomed Biotechnol. 2019;47(1):2010–2015. doi:10.1080/21691401.2019.1608217
34. Moine E, Boukhallat M, Cia D, et al. New lipophenols prevent carbonyl and oxidative stresses involved in macular degeneration. Free Radic Biol Med. 2020. doi:10.1016/j.freeradbiomed.2020.10.316
35. Du W, An Y, He X, Zhang D, He W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid Med Cell Longev. 2018;2018:1610751. doi:10.1155/2018/1610751
36. Yuan Z, Du W, He X, Zhang D, He W. Tribulus terrestris ameliorates oxidative stress-induced ARPE-19 cell injury through the PI3K/Akt-Nrf2 signaling pathway. Oxid Med Cell Longev. 2020;2020:7962393. doi:10.1155/2020/7962393
37. Schonhoff CM, Gaston B, Mannick JB. Nitrosylation of cytochrome c during apoptosis. J Biol Chem. 2003;278(20):18265–18270. doi:10.1074/jbc.M212459200
38. Li P, Nijhawan D, Wang X. Mitochondrial activation of apoptosis. Cell. 2004;116(2):S57–59,52 p following S59. doi:10.1016/s0092-8674(04)00031-5
39. Weng S, Mao L, Gong Y, Sun T, Gu Q. Role of quercetin in protecting ARPE‑19 cells against H2O2‑induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Mol Med Rep. 2017;16(3):3461–3468. doi:10.3892/mmr.2017.6964
40. Xie W, Yu W, Zhou M, et al. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro. Mol Vis. 2011;17:3512–3522.
41. Du L, Chen J, Xing YQ. Eupatilin prevents H(2)O(2)-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed Pharmacother. 2017;85:136–140. doi:10.1016/j.biopha.2016.11.108
42. Justilien V, Pang JJ, Renganathan K, et al. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007;48(10):4407–4420. doi:10.1167/iovs.07-0432
43. Kaarniranta K, Uusitalo H, Blasiak J, et al. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog Retin Eye Res. 2020;79:100858. doi:10.1016/j.preteyeres.2020.100858
44. Saito Y, Kuse Y, Inoue Y, Nakamura S, Hara H, Shimazawa M. Transient acceleration of autophagic degradation by pharmacological Nrf2 activation is important for retinal pigment epithelium cell survival. Redox Biol. 2018;19:354–363. doi:10.1016/j.redox.2018.09.004
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.