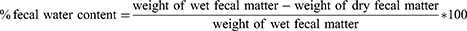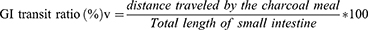Back to Journals » Journal of Experimental Pharmacology » Volume 16
Evaluations of the in vivo Laxative Effects of Aqueous Leaf and Stem Extracts of Artemisia Abyssinica in Mice
Received 21 December 2023
Accepted for publication 19 March 2024
Published 21 March 2024 Volume 2024:16 Pages 135—142
DOI https://doi.org/10.2147/JEP.S456029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Akeberegn Gorems Ayele, Jeylan Sinba Kawet
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Akeberegn Gorems Ayele, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Zambia Street, PO Box 9086, Addis Ababa, Ethiopia, Tel +251985408322, Email [email protected]
Background: People frequently complain of long-term constipation. The cost associated with using modern medications to treat constipation is significant, and the probability of encountering side effects is notably high. These limitations restrict their effectiveness in therapy, remain unresolved, and underscore the need for research on alternative therapeutic approaches. Plants of the genus Artemisia have been used to treat constipation. Therefore, the aim of this study was to evaluate the laxative effects of aqueous A. abyssinica leaf and stem extracts of Artemisia abyssinica in mice.
Methods: The laxative activity of A. abyssinica leaf and stem extracts was evaluated using three models: laxative activity, gastrointestinal motility, and gastrointestinal secretion. In this study, bisacodyl was used as a standard laxative and loperamide was used to induce constipation.
Results: In the laxative test, 200 (P< 0.05) and 400 mg/kg (p< 0.01) doses of plant extract significantly increased the percentage of fecal water content. Moreover, the highest dose of extract increased the frequency of defecation after 12 h (p< 0.05). In the remaining two models, the experimental plans also showed significantly higher gastrointestinal motility and noticeable accumulation of intestinal fluid.
Conclusion: The results of this study indicate that aqueous leaf and stem extracts of A. abyssinica have laxative effects.
Keywords: laxative, constipation, Artemisia abyssinica, castor oil, loperamide
A Letter to the Editor has been published for this article.
Background
Constipation is a persistent gastrointestinal (GI) condition that is indicated by fewer than three bowel movements per week. It can present clinically with a variety of symptoms, such as difficulty in defecating, irregular bowel movements, hard bowel, and sensation of incomplete defecation.1 If left untreated, constipation and its related conditions impose a significant burden on individuals, leading to increased morbidity and a consequential decline in quality of life.2
Since time immemorial, medicinal plants have played important roles in the development of potent therapeutic agents. The use of traditional medicine is gaining recognition owing to a variety of benefits and advantages: decrease in cost, easy accessibility, availability of these plants, particularly in rural areas, and fear of adverse effects associated with the use of modern medications.3 The use of conventional laxatives as a therapeutic intervention for treating and managing constipation is a common practice. However, their application is progressively limited, possibly due to issues like resistance, affordability, accessibility, or the occurrence of adverse effects.4 Consequently, a large portion of individuals afflicted by the condition in different parts of the world now turns primarily to traditional medicine as a supplementary support alongside conventional medicines.5
Various plant species are used to treat constipation. Aloe alepidea amatybica, ferox mill, aloe tenuior, rumex crispus, echinops kebericho mesfin are among the few used in Africa. The most commonly used plant parts are the leaves, but the roots, rhizomes, and bark have also been found to possess medicinal properties.6
The experimental plant, A. abyssinica is widely used as an insect repellent to treat headaches. In Saudi Arabia, decoctions of fresh whole plants is traditionally used to treat diabetes mellitus.7 The plant has also been used in folk medicine as an anthelmintic, antispasmodic, antirheumatic, and antibacterial agent. Antioxidant, antileishmanial, and antitrypanosomal activities of A. abyssinica essential oil have also been reported.8 However, there is no specific information about A. abyssinica regarding its use in constipation, and many species, such as Artemisia afra Jacq from this genus, are claimed to have a laxative effect.9 In Ethiopia, the aqueous leaves and stems of Artemisia afra Jacq, which are prepared using the decoction technique, are commonly used to treat constipation.10 From chemotaxonomic knowledge and molecular phylogenetic data, species from genera or families known to have an effect, such as the above-mentioned Artemisia afra Jacq, against certain disease conditions are believed to be associated with a certain bioactivity or therapeutic potential; thus, selection of species from these hot taxa would lead to higher success rates in drug discovery.11 Thus, this study attempted to validate the traditional use of A. abyssinica by evaluating its possible laxative effects using different models. The findings of this study will provide direction for the scientific community in terms of conducting advanced research on the molecular mechanisms and formulation of plant-source drugs by identifying the specific agents responsible for their effects.
Materials and Methods
Drugs and Chemicals
The following drugs and chemicals were used in the experiments conducted during the study period: loperamide (Medochemie Ltd., Limassol, Cyprus), bisacodyl (Remedica Ltd. Limassol, Cyprus), castor oil, and distilled water.
Plant Material
The experimental plant, A. abyssinica was collected from the Amhara region, North Shewa zone, Ensaro wereda, located approximately 130 km north of Addis Ababa. The plant material was packaged in plastic after collection to prevent physical changes in the leaves and stems during shipment. The plant was authenticated by a senior botanist, and a voucher specimen (JS001) was kept at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Extraction of the Plant Material
The leaves and stems of the plant were cleaned, rinsed with tap water, and dried in the shade. After drying, the mixture was crushed into coarse powder using a mortar and pestle. About 200 grams of plant leaves and stems, with a 1:1 ratio, were immersed in 0.8 liters of distilled water and subjected to decoction for roughly 20 minutes.12,13 The extract was allowed to cool. Thereafter, it was filtered twice using a muslin cloth. The filtrate was then concentrated in a hot oven at 37 C. The dried extract was weighed and transferred to vials for use.6 Finally, From 200 g of dried leaves, 42 g of brown coarse powder with a percentage yield of 21% was obtained.
Experimental Animals
Healthy Swiss albino mice (body weight: 25–35 g; age: 6–8 weeks) were used in this study. All animals were obtained from the animal house of the School of Pharmacy, College of Health Sciences, Addis Ababa University. Mice were maintained under standard conditions and fed a standard pellet diet and water ad libitum. They were then acclimatized to the environment for one week before the commencement of the experiment. All animals were handled in accordance with internationally accepted guidelines.14
Acute Oral Toxicity Study
The acute toxicity of the plant extracts was evaluated according to Organization for Economic Co-operation and Development (OECD) guideline 425. Five female mice (6–8 weeks old; average weight: 32 g) were used in this study. All mice were fasted for 4 h before and 2 h after administration of the extract. First, the study was performed in a single mouse at a dose of 2000 mg/kg. As no death of the first mouse was observed within 24 h, the same dose of the extract was administered to the remaining four mice. The animals were observed continuously for 4 h at 30-min intervals during the first 24 h. General signs and symptoms of toxicity, such as unusual skin and tremors, fur color, convulsions, salivation, diarrhea, coma, and mortality, were observed. Observations were continued for 14 days.15
Induction of Constipation
Loperamide was administered orally for six days at a dose of 5 mg/kg to induce constipation. Throughout the course of treatment, all animals had regular access to food and water.16,17
Grouping and Dosing of Animals
Mice treated and constipated with loperamide were divided into five groups to determine the laxative effects and results of the GI motility test. In both models, Groups I and II received 0.25 mg/kg of bisacodyl (BIS0.25) and 10 mL/kg of distilled water, respectively, as the positive and negative controls. The test groups (Groups III–V) were administered different doses of the Artemisia abyssinica aqueous extract (AAAE). Accordingly, Group III received 100 mg/kg (AAAE100), Group IV received 200 mg/kg (AAAE200), and Group V received 400 mg/kg (AAAE400).
For the third model (GI secretion test in normal mice), 25 albino mice were fasted for 18 h and divided into 5 groups, each containing 5 mice. Group I (normal control) was treated with distilled water, whereas group II (positive control) was administered castor oil (0.5 mL per animal.18 The crude extract was administered at doses of 100, 200, and 400 mg/kg to Groups III, IV, and V (test group).
Laxative Activity Test in Loperamide Constipated Mice
Prior to receiving the fifth dose, constipated mice were fasted for 18 h. Subsequently, mice were housed in separate cages. Fecal pellets or wet feces from each mouse were collected at two-hour intervals over a span of 12 h. The feces were then dried at room temperature for 24 h before being reweighed to assess the fecal water content. Formula (formula-1) was used to compute the percentage of fecal water content, as follows.17
Gastrointestinal Motility Test in Loperamide Constipated Mice
Gastric motility tests was performed in loperamide-treated mice, as previously described. The mice underwent a 12-h fast before receiving the fifth dose based on grouping. After a lapse of 40 minutes, each mouse received an oral dose of 0.3 mL of a freshly prepared 5% aqueous suspension of charcoal meal. The abdomen was opened and the small intestine from the pylorus to the cecum was eviscerated. The distance covered by charcoal in the small intestine from the pylorus to the cecum was measured, and the ratio of distance traveled by the charcoal meal to the length of the small intestine was calculated using the following formula (formula-2).19
Gastrointestinal Secretion Test in Normal Mice
After animals had been given distilled water, castor oil or different doses of plant extract based on the grouping, they were killed an hour after treatment, and each mouse’s small intestine was removed from the pylorus to the cecum and weighed right away. The contents were milked and weighed. The weight difference was recorded as GI secretion/content.20
Data Analysis
Statistical analyses were performed using SPSS version 25. Statistical differences between groups were analysed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Results were expressed as mean ± standard error of the mean (SEM), and P-values of less than 0.05 were considered statistically significant.
Results
Acute Toxicity
The acute toxicity test results revealed that the plant extract was safe when administered orally at a dose of 2000 mg/kg. After 24 h, the mice were found to tolerate the administered dose, and there were no significant changes in behavior, such as motor activity, alertness, restlessness, diarrhea, and convulsions within 24 h since administration. Moreover, there was no mortality within 14 days of observation, and the lethal dose 50 (LD50) was assumed to be greater than 2000 mg/kg.
Laxative Effects of the Aqueous Leaf and Stem Extracts of Artemisia Abyssinica in Loperamide-Induced Constipated Mice
Compared to the negative control, the higher doses of plant extract 400 mg/kg (p<0.05) tended to produce an apparent increase in the frequency of defecation over 12 h. However, these effects were not observed at the lower and middle doses. Moreover, the extract significantly increased the fecal water content at doses of 200 mg/kg (p<0.05) and 400 mg/kg (p<0.01). Similarly, the standard drug produced a significant frequency of stooling and an increased percentage of fecal water content (p<0.01) (Table 1). Nevertheless, significant different was not noticed while different doses of plant extract was compared to each other and with the standard drug.
 |
Table 1 Laxative Effects of the Aqueous Leaf and Stem Extracts of Artemisia Abyssinica in Loperamide Induced Constipated Mice |
Effects of Aqueous Leaf and Stem Extracts of Artemisia Abyssinica on Gastrointestinal Transit in Loperamide-Induced Constipated Mice
The effect of A. abyssinica aqueous extract on gastrointestinal motility was evaluated by examining its effect on charcoal meal transit in the small intestine of mice. The extract significantly increased motility except the lower dose compared to negative control groups. While the gastrointestinal transit ratio was only 29.0±4.40 in the negative control group, the recorded value was slightly higher in the lowest dose of plant extract and became more apparent in the middle (72.0±2.43, AAAE200) and higher (79.0±1.06, AAAE400) doses (Table 2).
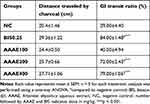 |
Table 2 Effects of Aqueous Leaf and Stem Extracts of Artemisia Abyssinica on Gastrointestinal Transit in Loperamide Induced Constipated Mice |
The standard drug (bisacodyl 0.25 mg/kg) and AAAE400 most significantly increased the distance traveled by the charcoal meal relative to the negative control group. However, no significant difference was observed when comparing various doses of the plant extract to each other and to the standard drug.
Effects of Aqueous Leaf and Stem Extracts of Artemisia Abyssinica on Gastrointestinal Secretion in Normal Mice
When compared to the normal control group, the crude extract significantly increased intestinal fluid accumulation at 400 mg/kg (1.61±0.44 g, P< 0.01) and 200 mg/kg (1.34 ± 0.70, P<0.05) (Table 3).
The standard drug, castor oil, induced the largest mean gastrointestinal fluid accumulation (1.73 ±0.35 g, P<0.001), which was significant when compared to the normal control group but the standard drug was failed to produce noticeable effect compared to the different doses of plant extract.
 |
Table 3 Effects of Aqueous Leaf and Stem Extracts of Artemisia Abyssinica on Gastrointestinal Secretion in Normal Mice |
Discussion
Constipation is a very common functional gastrointestinal illness that negatively impacts the quality of life of those who experience it.21 The basis of this research is that other herbs closely related to the experimental plant, such as Artemisia afra Jacq, are widely used for the management of constipation, and medicinal plants within the same phylogenetic groups possess the same or similar therapeutic effects.10,11 Based on this notion, we evaluated the laxative activity of A. abyssinica using three models. The effects of the plant extract on stool water content, defecation frequency, intestinal transit ratio, and intestinal fluid accumulation were assessed. These approaches are crucial to validate the laxative properties of various plants. The evaluations of different parameters in this study were aligned with established methods previously used in similar studies.22,23
The use of loperamide as a constipation-inducing agent is well documented. Loperamide is an opioid agonist and antidiarrheal agent that inhibits intestinal water secretion and colonic peristalsis.17 Previous studies have shown that oral and subcutaneous administration of 2–5 mg/kg body weight loperamide for 3–7 days can successfully induce constipation in mice.22,24
In the loperamide-induced constipated mouse experiment, the administration of oral doses of aqueous leaf and stem extracts of A. abyssinica produced significantly increased fecal water content at 200 mg/kg (p<0.05) and 400 mg/kg (p<0.001). Furthermore, the highest dose caused a considerable increase in defecation frequency over 12 h (p<0.05).
These results suggest the laxative activity of the plant extract, that is, the reversal of loperamide-induced constipation. The standard drug, bisacodyl, at the most significant level, also produced increments in wet fecal water content and stool frequency.
In the GI motility test, the plant extract caused a considerable increase in intestinal motility, as revealed by the GI transit ratio. The experimental plant exhibited a significant GI transit ratio at both the middle and highest doses (p<0.001), with a recorded increase in intestinal length travelled by charcoal compared to the negative control group. Promotion of intestinal motility reduced the residence of intestinal contents in the intestine, which may significantly decrease time for absorption of water and electrolytes from the small intestine by stimulating Cl− secretion and/or inhibiting Na+ absorption.25 This would increase the fecal moisture content and, in turn, increase intestinal motility, and hence, the laxative activity of the plant extract.
The final model (GI secretion) was used to evaluate the effects of aqueous leaf and stem extracts of A. abyssinica in normal mice. The effects of the 200 mg/kg and 400 mg/kg extracts appeared to be significant in terms of increasing mean intestinal content and were comparable to those of castor oil. Castor oil was used as a standard drug in this model. In the current study, no significant differences were observed in any of the models when comparing different doses of the plant extract to each other and to the standard drug in terms of effect on GI secretion, fecal water content and intestinal motility
Among the various mechanisms proposed to elucidate the laxative effect of castor oil, one involves suppression of intestinal Na+/K+-ATPase activity. This results in a decrease in fluid absorption, which is achieved through the activation of adenylate cyclase or mucosal cyclic adenosine monophosphate-mediated active secretion coupled with the release of nitric oxide.25,26 Furthermore, induction of smooth muscle contraction using castor oil occurs through its derivative, ricinoleic acid. In the intestine, lipase breaks down castor oil into ricinoleic acid, which activates the EP3 and EP4 prostanoid receptors in smooth muscle cells. The activation of these receptors creates a transient calcium surge that leads to propulsion in the intestine.27 Therefore, the significant increase in mean weight of the intestinal content at 200 mg/kg and 400 mg/kg doses of plant extract might be similar to the effect of ricinoleic acid released from castor oil. Furthermore, laxative agents that enhance the secretion of water and electrolytes in the intestinal lumen promote the activation or expression of the cystic fibrosis transmembrane conductance regulator (CFTR) or aquaporin. As a result, the effect of aqueous extracts of A. abyssinica could potentially be linked to augmentation of CFTR.26 However, it is important to note that further research is needed to understand the specific mechanisms at work before reaching specific conclusions.
Overall, secondary metabolites may account for the laxative effects of the experimental plants. Preliminary phytochemical screening of the current experimental plant demonstrated that A. abyssinica contains alkaloids, polyphenols, tannins, flavonoids, and saponin essential oil triterpenes.28,29 Flavonoid derivatives such as apigenin and naringenin stimulate Cl− secretion. Moreover, saponins promote smooth muscle contraction, thereby increasing peristalsis.
Conclusion
The findings of the present study indicate that A. abyssinica possesses significant in vivo laxative activity. The extract increased the frequency of stool, weight, fecal water content, and intestinal fluid accumulation in mice. Although the specific molecular mechanism of action remains unknown, the general mechanism of action may involve increasing gastrointestinal motility, secretion, or both.
Abbreviations
Data Sharing Statement
The datasets used in this study are available from the corresponding author upon request.
Ethical Approval
The study protocol was approved by the Institutional Review Board of the School of Pharmacy, College of Health Sciences, Addis Ababa University (reference no. ERB/SOP/548/14/2023).
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was conducted under the auspices of Addis Ababa University. The role of the institution in this experiment was to provide laboratory animals, equipment, and the required chemicals.
Disclosure
The authors have no competing financial interests to declare.
References
1. McCrea GL, Miaskowski C, Stotts NA, Macera L, Varma MG. Pathophysiology of constipation in the older adult. World J Gastroenterol. 2008;14(17):2631–2638. doi:10.3748/wjg.14.2631
2. Sabiu S, Ashafa OTA. Toxicological implications and laxative potential of ethanol root extract of Morella serrata in loperamide-induced constipated Wistar rats. Pharm Biol. 2016;54(12):2901–2908. doi:10.1080/13880209.2016.1193885
3. Iizuka N, Hamamoto Y. Constipation and herbal medicine. Front Pharmacol. 2015;6:73. doi:10.3389/fphar.2015.00073
4. Ashafa A, Sunmonu T, Abass A, Ogbe A. Laxative potential of the ethanolic leaf extract of aloe vera (L.) Burm. f. in Wistar rats with loperamide-induced constipation. J Natural Pharmaceuticals. 2011;2(3):158–162. doi:10.4103/2229-5119.86268
5. Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 2006;20(9):717–724. doi:10.1002/ptr.1907
6. D’avigdor E, Wohlmuth H, Asfaw Z, Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):1–33.
7. Abad Martínez MJ, Del Olmo LM B, Apaza Ticona LN, Bermejo Benito P The artemisia l. genus: a review of bioactive essential oils; 2012.
8. Abad MJ, Bedoya LM, Apaza L, Bermejo P. The artemisia L. genus: a review of bioactive essential oils. Molecules. 2012;17(3):2542–2566. doi:10.3390/molecules17032542
9. Mulatu A Identification of artemisia afra for access and benefit sharing activities; 2016.
10. Wintola O, Afolayan A. Ethnobotanical survey of plants used for the treatment of constipation within Nkonkobe Municipality of South Africa. Afr J Biotechnol. 2010;9(45):7767–7770.
11. Hao D-C, Xiao P-G. Pharmaceutical resource discovery from traditional medicinal plants: pharmacophylogeny and pharmacophylogenomics. Chinese Herbal Medicines. 2020;12(2):104–117. doi:10.1016/j.chmed.2020.03.002
12. Remington JP. Remington: The Science and Practice of Pharmacy. Lippincott Williams & Wilkins; 2006.
13. Evans WC. Trease and Evans Pharmacognosy. Edinburgh/New York: Saunders. Elsevier; 2009.
14. NRCotN A. Guide for the Care and Use of Laboratory Animals. Washington. DC: The National Academies Press; 2011.
15. Toxicity–Up AO. OECD guideline for testing of chemicals. Organ Economic Co-Operat Develop. 2001;1–13
16. Li C, Nie S-P, Zhu K-X, et al. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015;66(5):533–538. doi:10.3109/09637486.2015.1024204
17. Choi J-S, Kim JW, Kim K-Y, Lee J-K, Sohn JH, S-K K. Synergistic effect of fermented rice extracts on the probiotic and laxative properties of yoghurt in rats with loperamide-induced constipation. Evid Based Complement Alternat Med. 2014;2014:1–12. doi:10.1155/2014/878503
18. Seo JY, Kim SS, Kim HJ, Liu K-H, Lee H-Y, Kim J-S. Laxative effect of peanut sprout extract. nrp. 2013;7(4):262–266.
19. Guarize L, da Costa JC, Dutra LB, et al. Anti-inflammatory, laxative and intestinal motility effects of Senna macranthera leaves. Natural Product Research. 2012;26(4):331–343. doi:10.1080/14786411003754264
20. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J med virol. 2020;92(6):568–576. doi:10.1002/jmv.25748
21. Mearin F, Ciriza C, Mínguez M, et al. Clinical practice guideline: irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig. 2016;108(6):332–363. doi:10.17235/reed.2016.4389/2016
22. Ajayi CO, Funso-Babarimisa F, Elujoba AA. Laxative activities of Cassia sieberiana and Senna obtusifolia. Afr J Traditional, Complementary Altern Med. 2014;11(4):44–47. doi:10.4314/ajtcam.v11i4.7
23. Meite S, Bahi C, Yéo D, Datté JY, Djaman JA, N’guessan DJ. Laxative activities of mareya micrantha (benth.) müll. arg.(Euphorbiaceae) leaf aqueous extract in rats. BMC Complementary and Alternative Medicine. 2010;10:1–6. doi:10.1186/1472-6882-10-7
24. Beubler E, Juan H. Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by the rat colon. J Pharm Pharmacol. 1979;31(1):681–685. doi:10.1111/j.2042-7158.1979.tb13628.x
25. Deachapunya C, Poonyachoti S, Thongsaard W, Krishnamra N. Barakol extracted from Cassia siamea stimulates chloride secretion in rat colon. J Pharmacol Exp Ther. 2005;314(2):732–737. doi:10.1124/jpet.105.084210
26. Sayuk GS, Waldman SA, Brenner DM. Mechanisms of action of current pharmacologic options for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation. Am J Gastroenterol. 2022;117(4):S6–S13. doi:10.14309/ajg.0000000000001687
27. Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci. 2012;109(23):9179–9184.
28. Adugna M, Feyera T, Taddese W, Admasu P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Global J Pharmacology. 2014;8(3):460–468.
29. Achamo T, Zereffa EA, Murthy HA, Ramachandran VP, Balachandran R. Phyto-mediated synthesis of copper oxide nanoparticles using Artemisia abyssinica leaf extract and its antioxidant, antimicrobial and DNA binding activities. Green Chem Lett Rev. 2022;15(3):598–614. doi:10.1080/17518253.2022.2121620
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.