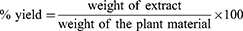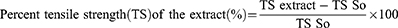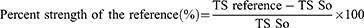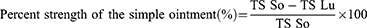Back to Journals » Journal of Experimental Pharmacology » Volume 16
Evaluation of Wound Healing Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Root of Verbascum Sinaiticum Benth. (Scrophulariaceae) in Swiss Albino Mice
Authors Essa AM , Getahun KA , Wubneh ZB
Received 12 January 2024
Accepted for publication 20 March 2024
Published 26 March 2024 Volume 2024:16 Pages 143—158
DOI https://doi.org/10.2147/JEP.S454096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Aziza M Essa,1,* Kefyalew A Getahun,2,* Zewdu Birhanu Wubneh2,*
1Department of Pharmacology, College of Medicine and Health Science, Wolkite University, SNNPR, Wolkite, Ethiopia; 2Department of Pharmacology, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
*These authors contributed equally to this work
Correspondence: Aziza M Essa, Tel +251920776345, Email [email protected]
Background: The roots of Verbascum sinaiticum have been used traditionally for the management of wound in different regions of Ethiopia. Despite the presence of several claims and in vitro studies regarding its role in wound healing, no scientific studies have been conducted so far. Therefore, this study aims to scientifically evaluate the wound healing activities of the crude extract and solvent fractions of the roots of Verbascum sinaiticum in Swiss albino mice.
Methods: The dried root powder of Verbascum sinaiticum was extracted using 80% methanol by maceration technique. This was then fractionated with chloroform, ethyl acetate, and water. These extracts were formulated as ointment at 5% and 10% concentration by using simple base. Acute dermal toxicity was performed on mice. The wound healing potential was evaluated using excision, incision, and burn wound models.
Results: In excision wound, 10% and 5% of crude extract ointment provided a significant (P< 0.001) percentage of contraction starting from day 4 and day 6 onwards respectively. Moreover, the rate of epithelialization was significantly (P< 0.001) improved in 10% crude extract. In burn wound, 10% and 5% crude extract showed significant (P< 0.001) wound contraction starting from day 4 and 8 onwards respectively. In both excision and burn wounds, a moderate concentration of fibroblast proliferation and collagen deposition was observed on the 10% crude extract. The 5% and 10% aqueous and ethyl acetate fractions produced a significant (P< 0.001) percentage of wound contraction and shortening of epithelialization at different time points compared to simple ointment.
Conclusion: The results of this study demonstrated that the 80% methanolic crude extract, aqueous and ethyl acetate fractions of Verbascum sinaiticum root have wound healing potential which assimilates its traditional use.
Keywords: wound healing, crude extract, solvent fraction, Verbascum sinaiticum
Background
A wound is defined as a disruption of anatomical, cellular, or functional integrity of tissue in the presence or absence of microbial infection.1 It can be classified based on the type of injury (burn, incised, and crushed), timing (acute, early, and late), and depth (superficial, dermal, and full-thickness).2 Wounds impose a huge clinical and financial burden to healthcare systems around the globe by imposing a significant effect in reducing the quality of life in those who are challenging with the wound.3 Worldwide, the amount of money required for wound care has been increasing, which makes institutions to acquire a more relevant and consistent ways to improve wound assessment.4 Approximately 2% of the world population who are admitted to the hospital has chronic wounds5,6. According to the study done in 2017, at Gondar university hospital, 25% emergency visits were due to injury.7 Another studies which assessed the epidemiology of burn injuries from 2000–2018 reported that burn injuries in Ethiopia accounts for about 1.5% to 9%.8
Medicinal plants are important in the management of different type of diseases which affect humans. Their parts have been utilized in different techniques to get their active constituent.9
Verbascum sinaiticum is one species of Verbascum which is popularly known in Ethiopia by the name kutitina (Amharic), ye aheya joro (amharic), Gurra Harree (Afan Oromo), and Tirnake (Tigrigna) (Figure 1).10
 |
Figure 1 The photograph of Verbascum sinaiticum January 2021, Maraki campus, University of Gondar, Gondar, Amhara regional state, Ethiopia. |
For wound healing purposes, in debark district, North Gondar, Ethiopia, the roots of V. sinaiticum are crushed, powdered, mixed with butter, and creamed on the affected part10 and in dera district, South Gondar zone, Amhara region, Ethiopia, the dry root is ground and applied on the wound.11 It is also used to treat the wound in Ensaro District, North Shewa Zone, Amhara Regional State, Ethiopia.12 The leaves are also used for fire burn and external wounds.13
Scientifically investigated in vitro studies have reported that methanol extract of V. sinaiticum root shows outstanding activity against S. aureus and S. epidermides11. Another in vitro study on V. sinaiticum reported its antioxidant activity.14
Rationale of the Study
Wound has been still a challenge for medical care system and it imposes a great problem with in the society by causing morbidity and mortality and its cost of care has been increasing. Current treatment strategies are unable to heal the wound completely and the emergence of drug resistance strains as well as the limited tolerability of the systemic and topical drugs and their cost bends the focus of the treatment of wound in to traditional medicine or medicinal plants. Verbascum sinaiticum, one of the known plant in Ethiopia, its root has been claimed by traditional healers for the management of wound. Different in-vitro studies showed that the plant has a good antimicrobial activity, and the presence of different chemical components. Therefore, based on its use in folk medicine for wound healing and its invitro antibacterial activities and secondary metabolites, it is reasonable to conduct an invivo evaluation of its healing potential to provide scientific basis for its use in traditional medicine as a healing agent. There is also a need of scientific validation, standardization and safety evaluation to protect consumers from overdose as well from low therapeutic dose. The results of this study may provide baseline information for future studies intended at identification, isolation, purification and efficacy determination of the active compound for synthesis of lead compound with wound healing activities.
Materials and Method
Chemical and Drugs
Absolute methanol (Follium Pharmaceuticals, Ethiopia), Ethyl acetate (Sisco research laboratories Pvt. Ltd, India), n-Hexane (Loba Chemie Pvt. Ltd. India), diazepam injection (Gland pharm limited, India), normal saline 0.9% (IV infusion BP Medsol pharmaceuticals), ketamine hydrochloride injection (Neon Laboratories Limited, India). All materials employed were of analytical quality.
Plant Material
V. sinaiticum roots were collected from the Maraki campus, University of Gondar, in Gondar town, Amhara, Ethiopia. The plant material was identified and authenticated by Mr. Abiyu Enyew (botanist at the University of Gondar), and a specimen with voucher number 0002AME/2021 was deposited in the herbarium for future reference.
Extraction and Fractionation
Extraction Powdered roots of V. sinaiticum (1kg) were weighed and soaked with 80% methanol (1:6, w/v) at room temperature in Erlenmeyer conical flask covered with aluminum foil.15,16 It was then maintained for three days, accompanied by periodic stirring and shaking. After three days, it was filtered through Whatman filter paper (No-1) using pressurized suction filtration. The remaining residue was further macerated with methanol for three days (twice, for a total of six days) to obtain a higher yield. The extract solution was evaporated using a rotary evaporator set at 40°C to remove methanol. Finally, the concentrated aqueous solution was dried by using lyophilizer to remove the aqueous solution. The percentage yield was calculated and stored in a refrigerator at +4°C until use for the formulation of ointments and solvent fractionation.16,17
Fractionation 80% methanolic crude extract of V. sinaiticum roots (85 g) was subjected to different solvents (chloroform, ethyl acetate, and water). The powdered form of the crude extract was suspended in distilled water and shaken to obtain a completely mixed solvent mixture. The mixture was transferred to a separatory funnel and 510 mL of chloroform was added. The mixture was left for some time after shaking until it formed two separate phases, according to their density. The chloroform layer was collected in a separate flask and the procedure was repeated twice by adding an equal amount of chloroform. The aqueous fraction was then fractionated with ethyl acetate (510 mL) three times using a procedure similar to that used for chloroform. Finally, the aqueous residue was obtained. The chloroform and ethyl acetate filtrates were dried at 40°C in an oven and the aqueous fraction was frozen in the refrigerator overnight and dried using a lyophilizer. The percentage yield of each dried fractions was calculated using the formula mentioned in the previous section and stored in a refrigerator at 4°C until it is utilized for ointment formulation as 5% and 10% concentration.18,19
Ointment Preparation
Ointment were prepared based on the formula and proportions stated in the British Pharmacopoeia (Table 1).20
 |
Table 1 Formula Used for the Preparation of Simple and Medication Ointment |
The simple ointment base (100mg) which served as a negative control was prepared by placing hard paraffin in a beaker and melting it in a water bath. The remaining components were added in decreasing order based on their melting points, with cetostearyl alcohol being added first, followed by wool fat and white soft paraffin. The mixture of the ingredients was stirred to obtain a homogenous mixture.
Crude extracts of 5% and 10% ointments were prepared by mixing 5 g and 10 g of crude extract with 95 g and 90 g of simple ointment, respectively. Whereas, solvent fractions (chloroform, ethyl acetate, and aqueous) were prepared by mixing 1.5 gm (5%) and 3 gm (10%) of each fraction with 28.5 gm and 27 gm of simple ointment respectively. As a negative control, 100 mg of simple ointment without an active ingredient was prepared and nitrofurazone 0.2% was used as a positive control.
Preliminary Phytochemical Screening
The crude extract and solvent fractions (chloroform, ethyl acetate, and water) of the roots of V. sinaiticum were screened for the presence of alkaloids, anthraquinones, flavonoids, glycosides, phenols, saponins, steroids, tannins, and terpenoid according to standard procedures.21
Acute Dermal Toxicity
Dermal toxicity tests were performed according to OECD guidelines 402.22 Adult female mice (20–30 gm, 8–12 weeks) were obtained from animal housing facilities of the Department of Pharmacology, University of Gondar. Six female mice were assigned in two groups, treatment and control.23 Initially, a preliminary study involved subjecting one mouse to assess the initial dosage. This involved the application of 2000 mg/kg of a ten percent formulation of the hydromethanolic crude extract uniformly over a shaved area, which was then covered with surgical gauze and a nonocclusive bandage for 24 hours. The mouse was individually caged during this exposure period. After 24 hours, any remaining test substance was washed away with water, and no signs of irritation or mortality were observed during this time frame. Subsequently, the remaining two mice from each group were subjected to testing the next day with a limited test dose of 2000 mg/kg of a 10% ointment formulation of the extract. These mice were observed for 24 hours post-application. Observations included immediate scrutiny for any adverse skin reactions after dosing, continuous monitoring for the first half-hour, periodic checks during the initial 24 hours with particular attention in the first four hours, and daily observations for a total of 14 days.22,24.
Grouping and Dosing of Experimental Animals
For the excision wound model, four groups each containing six mice were used. Group I mice were treated with a simple ointment, which served as a negative control. Group II and III were treated with 5% and 10% 80% methanol crude extract ointments, respectively. Group IV was treated with 0.2% (w/w) nitrofurazone, which served as the positive control. After observing the effectiveness of the crude extract, the different solvent fractions formulated as ointments were applied as follows. For the solvent fraction, a circular excision wound model and eight groups of mice (six mice in each group) were used. Group I mice were treated with a simple ointment, which served as a negative control. Group II was treated with 0.2% w/w nitrofurazone, which served as the positive control. Groups III and IV were treated with 5% w/w and 10% w/w of the ethyl acetate fraction, respectively, Groups V and VI were treated with 5% w/w and 10% w/w chloroform fractions, respectively, and Groups VII and VIII were treated with 5% w/w and 10% w/w aqueous fractions, respectively.25
For the burn model, grouping was similar to that of excision wound model, whereas for the incision wound model, an additional fifth group was incorporated, which was left untreated group.26
Wound Healing Models
Excision Wound Model
To create an excision wound, ketamine (50 mg/kg) and diazepam (5 mg/kg) were used to anesthetize mice.27 After cleaning with 70% ethanol, the dorsolateral flank was shaved using a surgical blade. A full-thickness circular excision wound measuring approximately 300 mm2 was created using toothed forceps, a scalpel, and scissors. The wound was then left undressed in an open environment. The day on which the wound was created was considered day 0. After 24 h, the standard drug, extract, and simple ointment were applied topically once daily, as described in the grouping section.28,29
Measurement of Wound Contraction
The rate of wound contraction was measured using transparent polythene paper and a 1 mm2 graph sheet on days 0, 2, 4, 6, 8, 10, 12, 14, 16 18 and 20th post wounding. The wound healing activities of the crude and solvent fractions were calculated using the wound size (300mm2) as 100%, as follows:
Where n is the number of days when measurements were taken ie 2nd,4th, 6th, 8th, 10th, etc. till full wound healin.30
Epithelialization Time Measurement
The number of days required for the scar to fall off from the dead tissue, excluding leaving a raw wound, was taken as the epithelialization period.30
Histopathological Examination
After completion of the experiment, skin specimens were collected from each group of excision and burn wound models. Samples were fixed in 10% buffered formalin, processed, blocked with paraffin, sectioned into 5 µm sections, and stained with hematoxylin and eosin. The sections were examined and recorded by a blinded pathologist and recorded.31.
Incision Wound Model
In the incision wound model, the mice were anesthetized using the same method as that used for excision, and the dorsal fur of each mouse was shaved. Three cm long and 2 mm depth of incision wound was made using a sterile blade. The wounds were closed with interrupted sutures, 1 cm apart, using a silk no. 00 round and curved needle (no. 11). The wound was left open (Figure 2a).32 After 24 hours of after wound creation, animals were treated as described in the grouping section. The sutures were removed on day 9 and the tensile strength of the healed wound was measured on day 10 using a continuous and constant water flow technique (Figure 2b and c). The anesthetized mice were placed on an operating table Two forceps were suspended on each side of the edge of the skin, one suspended on the metal rod on the operation table, and the other suspended in a polyethylene bag through a string run over to a pulley. Water was slowly added to the bag until the wound broke. Then the amount of water in grams was recorded and taken as the breaking strength of the wound.33,34
 |
Figure 2 Photograph of incision wound. (a) On the day of wound creation; (b) water flow technique; (c) measuring breaking strength. |
Where So= simple ointment, LU = left untreated28
Burn Wound Model
A partial-thickness burn wound was created by pouring hot molten beeswax at 80°C °C into a metal cylinder with 300 mm2 circular openings placed on the shaven area of the mice until the wax solidified. Subsequently, the metal cylinder with wax adhering to the skin was removed. After 24 h, the cells were treated as described in the dosing section. The wound was monitored every two days, and the percentage of wound contraction, epithelialization period, and histopathological analysis were performed.35
Statistical Analysis
All the results are expressed as the mean ± SEM for each group. The differences between groups were statistically analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s tests using SPSS version 26 software, and results were considered significant if P< 0.05.
Results
The Yield of the Extract
The yield of the crude extract was 11%, followed by the ethyl acetate (18.75%), chloroform (12.5%) and aqueous solutions (68.8%).
Acute Dermal Toxicity
During the 24 hour follow-up, dermal toxicity, such as inflammation, swelling, and redness was not observed. The extract did not show any signs of toxicity or mortality during case side observation of 14 days.
Phytochemical Test
Preliminary phytochemical screening of the crude and solvent fractions results is presented in Table 2.
 |
Table 2 Preliminary Phytochemical Screening of the Crude Extract and Solvent Fractions of the Root of V. Sinaiticum |
Effect of Verbascum Sinaiticum Root on Wound Healing
Excision Wound Model
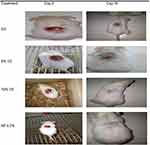
The results obtained from the excision wound model revealed that the 5% and 10% crude extracts showed a significant wound healing effect compared to the simple ointment (Table 3, Figure 3).
 |
Table 3 The Effect of the Root Verbascum Sinaiticum Crude Extract on Excision Wound Area (mm2) in Swiss Albino Mice |
No significant difference was observed in the percentage of wound contraction until day 4 between the simple ointment-treated groups and groups treated with 5% VS, 10% VS, and the standard drug (NF 0.2%). From day 4 to day 18, the 10% crude extract-treated group showed significant (P<0.001) wound contraction compared to the simple ointment-treated group. In comparison with the 5% crude extract-treated group, the 10% vs NF 0.2% treated groups appeared to have statistically significant (P<0.05) percentages of wound contraction from day 10 to day 18 and the maximum percentage of wound contraction (98.2% and 100%) in the 10% crude extract-treated group was observed on day 16 and day 18 respectively. However, no significant difference was observed in wound contraction between the 10% VS and NF 0.2% treated groups. In contrast, the 5% VS- treated groups showed a significant percentage of contraction (P<0.001) starting from day 6 onwards in comparison with that of simple ointment-treated groups and the maximum percentage of wound contraction (98.7%) among the 5% VS-treated groups was observed on day 18.
In terms of shortening of the epithelialization period, 5% VS (16.2 days), 10% VS (15.33 day), and NF 0.2% (15 days)-treated groups showed a significant (P<0.001) effect compared to the simple ointment (20.20 day)-treated groups in improving the rate of epithelialization.
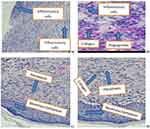
Histopathological Analysis
The 10% crude extract formulation-and 0.2% nitrofurazone-treated mice showed moderate fibroblast concentration, collagen deposition, and the absence of inflammatory cells (Figure 4c and d). Likewise, mice treated with the 5% crude extract showed low concentrations of inflammatory cells, moderate fibroblast concentrations, and neovascularization (Figure 4b). The simple ointment-treated groups showed moderate inflammatory cells, an absence of fibroblast proliferation, and collagen deposition (Figure 4a).
Incision Wound Model
The effects of V. sinaiticum root extract on the incision wound are shown in Table 4 and Figure 2. The mean tensile strength of the groups treated with 10% VS and NF 0.2% was found to be significant (P<0.001) in increasing the breaking strength by 60.5% and 62.5%, respectively, compared to the untreated and simple ointment-treated groups. In addition, significant effects (P<0.05) on breaking strength were observed in groups treated with 10% VS and NF 0.2%, when compared to the 5% VS, treated group. In contrast, groups treated with 5% crude extract showed a significant (P<0.001, P<0.01) increase in breaking strength compared to the untreated and simple ointment treated groups, respectively.
 |
Table 4 Effect of Crude Extract of the Root of Verbascum Sinaiticum Extract on Tensile Strength in an Incision Wound Model |
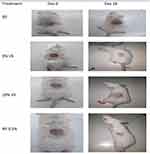
Burn Wound Model
The root of Verbascum sinaiticum root extract showed a significant effect on wound contraction and the period of epithelialization on alternate days of burn wound treatment (Table 5 and Figure 5). Groups treated with 10% VS and NF 0.2% showed a significant (P<0.001) percentage of wound contraction starting from day 4 onwards compared to the simple ointment treated group. Compared to the 5% crude extract-treated group, the 10% crude extract-and 0.2% nitrofurazone-treated groups showed significant (P< 0.01) wound contraction from days 12 to 18. In contrast, groups treated with 5% ointment showed a significant (P<0.001) effect from day 8 onwards on wound contraction compared to groups treated with simple ointment.
 |
Table 5 Effect of the Crude Extract of the Root of Verbascum Sinaiticum Extract on Burn Wound Model in Swiss Albino Mice |
At the time of shortening of the epithelialization period, the 5% VS, 10% VS, and NF 0.2% treated groups showed comparable and minimal days compared to the simple ointment-treated groups. Significant effects on the rate of epithelialization were observed in groups treated with 5% VS (15.7 days, P<0.001), 10% VS (14.2 days, P<0.001), and NF 0.2% (14 days, P<0.001) compared to the simple ointment treatment group.
Histopathological Analysis
Skin specimens from the burn model mice were taken on the 20th day of treatment. The highest dose of the extract (10%) and nitrofurazone (0.2%) resulted in a higher concentration of fibroblasts, collagen deposition, and a lower concentration of inflammatory cells. Mice treated with 5% crude extract also showed lower concentrations of inflammatory cells, moderate fibroblasts, neovascularization, and collagen deposition. In contrast, mice treated with a simple ointment showed a high concentration of inflammatory cells, low concentration of fibroblast proliferation, and an absence of collagen deposition.
Wound Healing Effects of the Solvent Fraction of Verbascum Sinaiticum Root on Excision Wound
The effects of chloroform, ethyl acetate, and the aqueous solvent fraction of V. sinaiticum root on the area of the excision wound and the period of epithelialization were found to be significant at different p-values. However, the groups treated with both formulations of the chloroform fraction failed to show any significant effect compared to the simple ointment-treated group (Table 6). Groups treated with the ethyl acetate 5% fraction showed significant wound contraction (P<0.01) on day 6 and (P<0.001) from day 8 to day 18 as compared to the simple ointment treated group. In contrast, the 5% ethyl acetate-treated groups showed significant wound contraction (P<0.001) from day 8 onwards compared to the 5% and 10% chloroform-treated groups. The ethyl acetate 10% and aqueous 5% fraction-treated groups showed significant wound contraction (P<0.01) beginning on the 4th day and (P<0.001) on the 6th day onwards compared to the simple ointment-treated group. Both fraction-treated groups showed a higher percentage of wound contraction (P<0.01) on day 6 and (P<0.001) from day 8 onwards than the 5% and 10% chloroform fraction-treated groups.
 |
Table 6 Effect of Solvent Fractions of Verbascum Sinaiticum Root on Excision Wound Area in Swiss Albino Mice |
On the other hand, groups treated with aqueous 10% and nitrofurazone 0.2% showed a significant percentage of contraction (P<0.001) starting from the 4th day onwards when compared to the simple ointment, 5% chloroform, and 10% chloroform fraction-treated groups.
Shortening of the epithelialization period was observed at a significant level in groups treated with aqueous (5% VS and 10% VS) and ethyl acetate (5% VS and 10% VS) fractions of V. sinaiticum roots compared to groups treated with simple ointment (Figure 6). Groups treated with ethyl acetate (5% and 10%), aqueous (5% and 10%), and nitrofurazone 0.2% showed a significant effect (P<0.001) on the shortening of the epithelialization period compared to the simple ointment-treated group. Among these groups, mice treated with aqueous 10% and 0.2% nitrofurazone showed the shortest number of days. However, groups treated with 5% and 10% chloroform failed to show any significant effect on the number of days of shortening compared to the simple ointment-treated groups (Figure 6).
Discussion
Verbascum sinaiticum, a well-known plant in Ethiopia, is used by traditional healers for wound management. In different ethnobotanical studies, the powdered root of V. sinaiticum was topically applied to the affected area10–12 by mixing with butter.10 In this study, powdered roots were extracted. It was prepared in a simple ointment base that assimilated traditional use.36
No irritation or toxicity was observed during the dermal toxicity test, which confirmed that the methanolic crude extract of V. sinaiticum root is safe for use in topical preparation.
In a previous study on the roots of V. sinaiticum, different solvent fractions of the root extract showed the possible presence of secondary metabolites.37 Similarly, the current findings show that qualitative phytochemical screening of the crude extract and solvent fraction showed the possible presence of anthraquinone, alkaloids, flavonoids, phenols, tannins, saponins, and terpenoids.
Phenolic compounds and flavonoids are known to have antioxidant activity with considerable free radical scavenging activities.38,39 Tannins have a significant impact on the wound healing because of their antibacterial, antioxidant, and anti-inflammatory activities.40 Anthraquinone plays a role in wound healing by reducing inflammation and promoting tissue regeneration by enhancing the expression and signaling of growth factors.41 Therefore, secondary metabolites may partly contribute to the observed healing of wounds, alone or in combination, in the three models used in this study.
In the three models in this study, the 10% crude extract showed better wound healing activity than the 5% crude extract, which may be due to the concentration of the bioactive constituents, which may increase as the dose increases.
In the excision wound model, the wound healing effect of the extract in both formulations (5% w/w and 10% w/w) resulted in fast wound contraction and increased in the rate of epithelialization. This effect is probably similar to that of a previously studied plant in the same genus (V. speciosum) which showed fast wound contraction and resulted in shortening of the epithelialization period.42,43 The observed wound-healing effects of the crude extract in mice might be due to its antibacterial and antioxidant activities.
The crude extract of the root of V. sinaiticum and its solvent fractions in a previous study showed a significant inhibitory effect on S. aureus, S. epidermidis, K. pneumonia, and E. coli.11,37 These pathogens colonize wounds and delay healing by releasing toxins.44 The antibacterial activity is consistent with a previous study conducted on Verbascum thapsus methanolic extract, which possesses antibacterial activity against common pathogens mentioned above45 and the antioxidant activity is probably similar to the study performed in the same genus of three Verbascum species whose methanolic extracts showed good antioxidant activity.46
In addition, the healing effect of the crude extract on excision wounds might be due to an increase in fibroblast proliferation and collagen deposition, as evidenced by histopathology analysis.
Furthermore, the wound healing activity of 80% methanolic crude extract was observed in the incision wound model by measuring the tensile strength. The tensile strength of the wound mainly relies on the synthesis of collagen and its maturation,47 and the greater the deposition of collagen, the stronger the wound becomes in resisting breaking.48 Thus, the observed effect on tensile strength might be due to the ability of the extract to increase the rate of synthesis and maturation of collagen.49 The increase in tensile strength may be similar to a study done on the same genus (seven Verbascum species) that revealed an increase in tensile strength, which might be associated with the presence of fibroblast proliferation and collagen deposition, as evidenced by histopathological analysis.50
In contrast, topical application of 5% crude extract and 10% crude extract ointment on burn wounds showed significant wound healing compared to simple ointments. Burn wounds are known to be associated with high inflammation, which allows matrix metalloproteinase (MMP) and other enzymes to destroy the extracellular matrix and render the site of the wound vulnerable to infection.51,52 This destruction as a result of inflammation can be prevented or destroyed by medicinal plants via reduction of excessive inflammation.53
According to a previous study done on Verbascum species, the species of Verbascum are known to possess anti-inflammatory activity, and it was indicated to be the principal mechanism for their wound healing activity54 which might indicate the wound healing effect of the root of V. sinaiticum is a result of its anti-inflammatory activity. An additional study conducted in the same genus (Verbascum pterocalycinum) also reported anti-inflammatory and antinociceptive activities55 which probably similar in effect with the root of V. sinaiticum.
The crude extract in the three models of the study with 5% and 10% concentration showed a decrease in the activity of wound healing as compared to the positive control, nitrofurazone 0.2%. The crude extract can exhibit significant variation in potency and may encompass inconsistent levels of bioactive compounds. The concentration of active compounds within the crude extract fall below a certain threshold, its wound healing activity might not match that of nitrofurazone.
The crude extract was further fractionated with different solvents, and according to the results of this study, the aqueous and ethyl acetate fractions showed significant wound healing activity compared with the simple ointment-treated groups.
In a previous study on the root of V. sinaiticum, it was reported that the highest yield was obtained in a polar solvent, and which implies that the roots of V. sinaiticum seem to be polar, which is consistent with the results of the current study.37
The difference in wound-healing activity among the solvent fractions might be due to their ability in inhibiting bacterial growth and the presence and effect of secondary metabolites. This idea was supported by two previously reported in vitro studies on the root of V. sinaiticum crude extract and the solvent fraction.11,37 According to those studies, the aqueous and chloroform fractions exhibit broad-spectrum antibacterial activity. The result is in line with the current finding that higher activity of the aqueous fraction of V. sinaiticum might be a result of broad-spectrum antibacterial activity.
Nevertheless, the chloroform fraction did not exhibit any notable impact on wound contraction or epithelialization duration compared to the simple ointment. This outcome could stem from the chloroform fraction’s inability to effectively release its active ingredient in vivo, and/or the insufficient concentration of secondary metabolites within the chloroform fraction, which may not provide adequate antibacterial and antioxidant activity in vivo.
When the fractions compared to crude extract excision wound model, the fractions showed a decrease wound healing effect. The reduced wound-healing effect observed in the fractions compared to the crude extract may be the crude extract may contain a combination of active compounds that work synergistically to promote wound healing. When the extract is fractionated, some of these synergistic interactions may be lost, leading to reduced activity of wound healing effect in the fractions. In addition, fractionation of the crude extract may result in the removal of certain bioactive compounds that are responsible for the wound-healing effect. These compounds may work in concert with others in the crude extract to produce the observed therapeutic effect.
In addition, the fractions of the crude extract showed a decrease in the wound healing effect than the standard drug, nitrofurazone 0.2%. Fractions may lack synergistic effects among the compounds present in the crude extract, which are necessary for optimal wound healing. Nitrofurazone, as a single compound, may possess synergistic properties that enhance its healing effect.
The crude extract and its fractions demonstrated wound healing effects across all three wound models. These effects are likely attributed to the antibacterial, anti-inflammatory, and antioxidant properties associated with the medicinal activity of the Verbascum genus, as supported by relevant studies.54,56,57
Limitations
Owing to the unavailability of operative machines that count the number of specific cells in the specimen, the concentration of cells in the specimen was subjectively ranked by a pathologist. This makes the results obtained from the histopathological analysis less accurate and tangible.
Conclusion
The root extracts of the 80% methanolic crude extract, aqueous fraction, and ethyl acetate fraction of V. sinaiticum showed significant wound healing activity in the models used in this study. However, the chloroform fraction of V. sinaiticum failed to show any significant wound healing activity. The observed wound healing effect might be associated with the presence of different phytochemical constituents in the extract. This justifies the use of V. sinaiticum root as a wound healing agent.
Data Sharing Statement
The corresponding author can provide the datasets utilized or examined in this study upon reasonable request.
Ethics Approval
The experiment was conducted in accordance with the guidelines for handling and care.58 The study commenced after ethical clearance was obtained from Department of Pharmacology, College of Medicine and Health Sciences, University of Gondar (protocol number SOP4/97/13). Experiments were carried out, and the data were compiled in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, National Research Council (2012).
Acknowledgments
The authors acknowledge the School of Pharmacy, University of Gondar, Ethiopia, for providing the experimental animals and all necessary laboratory facilities.
Author Contributions
All authors made a significant contribution to the work reported, in the conception, study design, execution, acquisition of data, analysis and interpretation, and also; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Firdous SM, Sautya D. Medicinal plants with wound healing potential. Bangladesh J Pharmacol. 2018;13(1):41–52. doi:10.3329/bjp.v13i1.32646
2. Percival NJ. Classification of wounds and their management. Surg. 2002;20(5):114–117. doi:10.1383/surg.20.5.114.14626
3. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17(3):790–803. doi:10.1111/iwj.13333
4. Sen CK. Human wounds and its burden: an updated compendium of estimates. Mary Ann Liebert. 2019;2019:39–48.
5. Powers JG, Higham C, Broussard K, et al. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol. 2016;74(4):607–625. doi:10.1016/j.jaad.2015.08.070
6. Gottrup F. A new concept of a multidisciplinary wound healing center and a national expert function of wound healing. Arch Surg. 2001;136(7):765–772. doi:10.1001/archsurg.136.7.765
7. Ayele TA, Zeleke BM, Tessema GA, et al. Magnitude and patterns of injuries among patients in Gondar University Hospital, northwest Ethiopia: an institutional-based study. Open Access Surg. 2017;10:25–31. doi:10.2147/OAS.S126043
8. Ogada EA, Gebreab AH, Potokar TS. Review of the epidemiology of burn injuries in Ethiopia; implications for study design and prevention. Burns Open. 2019;3(3):75–82. doi:10.1016/j.burnso.2019.05.002
9. Lamboro T, Mengistu M, Hordofa TG, Phytochemical screening, characterization of essential oil and antimicrobial activity of Schinus molle (Anacardiaceae) collected from eastern hararghe, Ethiopia. 2020.
10. Abebe E. Ethnobotanical Study on Medicinal Plants Used by Local Communities in Debark Wereda, North Gondar Zone, Amhara Regional State, Ethiopia. Addis Ababa University; 2011:1–139.
11. Mihertu B. Ethnobotanical study of medicinal plants and antibacterial test of selected medicinal plants in dera district, south Gondar zone, Amhara region. Ethiopia. 2021;2021:1.
12. Asfaw A. Ethnobotanical investigation on medicinal plants traditionally used against human ailments in ensaro district, north Shewa zone, Amhara regional state, Ethiopia; 2021.
13. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in kilte awulaelo district, Tigray region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):1–23. doi:10.1186/1746-4269-9-65
14. Gondal HY, Zamir R, Nisar M, et al. Verbascum sinaiticum: a rich source of antioxidant phenylethanoid glycosides. Nat Prod J. 2020;10(2):158–162. doi:10.2174/2210315509666190129160405
15. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharm Phytochem. 2014;2(5):1.
16. Kumar V, Khan AA, Nagarajan K. Animal models for the evaluation of wound healing activity. Int Bull Drug Res. 2013;3(5):93–107.
17. Bishu KG, Jenkins C, Yebyo HG, et al. Diabetes in Ethiopia: a systematic review of prevalence, risk factors, complications, and cost. Obesity Med. 2019;15:100132. doi:10.1016/j.obmed.2019.100132
18. Abubakar AR, Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci. 2020;12(1):1. doi:10.4103/jpbs.JPBS_175_19
19. Taddese SM. Wound healing activities of hydromethanolic crude extract and solvent fractions of bersama abyssinica leaves in mice. Evid Based Complement Alternat Med. 2021;2021:2021.
20. Pharmacopoeia B. Department of Health and Social Security Scottish Home and Health Department. Vol. 2. UK: Office of the British Pharmacopoeia Commission; 1988:713.
21. Cocan I. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp Ther Med. 2018;15(2):1863–1870.
22. OECD. Test No. 402. Acute Dermal Toxicity; 2017.
23. Albus U. Guide for the Care and Use of Laboratory Animals (8th Edn). London, England: SAGE Publications Sage UK; 2012.
24. Wilhelm K-P, Maibach HI. 33 OECD Guidelines for Testing of Chemicals. Dermatotoxicology; 2007:303.
25. Leake Gebremeskel LG. In vivo wound healing and anti-inflammatory activities of leaf latex of aloe megalacantha baker (Xanthorrhoeaceae); 2019.
26. Belachew TF. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of hagenia abyssinica (Bruce) jf gmel in mice. Evid Based Complement Alternat Med. 2020;2020:2020.
27. Ayal G, Belay A, Kahaliw W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med. 2019;25(1):100151. doi:10.1016/j.wndm.2019.100151
28. Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J.(Polygonaceae) in mice. BMC Complement Alter Med. 2015;15(1):1–10. doi:10.1186/s12906-015-0878-y
29. Thakur R. Practices in wound healing studies of plants. Evid Based Complement Alternat Med. 2011;2011:2011.
30. Arulprakash K, Murugan R, Ponrasu T, et al. Efficacy of ageratum conyzoides on tissue repair and collagen formation in rats. clinical and experimental dermatology. Experimental Dermatol. 2012;37(4):418–424. doi:10.1111/j.1365-2230.2011.04285.x
31. Beshir K, Shibeshi W, Ejigu A, et al. In vivo wound healing activity of 70% ethanol leaf extract of becium grandiflorum lam. (Lamiaceae) in mice. Ethiopian Pharm J. 2016;32(2):117–130. doi:10.4314/epj.v32i2.3
32. Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of acanthus polystachyus delile (Acanthaceae). Evid Based Complement Alternat Med. 2018;2018:1–9. doi:10.1155/2018/2047896
33. Kumar N, Prakash D, Kumar P, Wound healing activity of Solanum xanthocarpum schrad. & wendl. fruits. 2010.
34. Ghildiyal S, Gautam M, Joshi V, et al. Wound healing and antimicrobial activity of two classical formulations of laghupanchamula in rats. J Ayurveda Integr Med. 2015;6(4):241. doi:10.4103/0975-9476.157952
35. Fahimi S, Abdollahi M, Mortazavi SA, et al. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9):doi:10.5812/ircmj.19960
36. De Villiers M, Ointment bases. A practical guide to contemporary pharmacy practice, 2009: p. 277–290.
37. Yeabyo S. Antibacterial activity of root extracts of Verbascum sinaiticum against multidrug-resistant Enterobacteriaceae family gram-negative and two gram-positive bacteria. Drug Invention Today. 2018;10(8):1.
38. Barku VY. Wound Healing: Contributions from Plant Secondary Metabolite Antioxidants, in Wound Healing-Current Perspectives. IntechOpen; 2019.
39. Momtaz S, Wound healing activity of the flowers of Lilium candidum L. in burn wound model in rats. J MED PLANT. 2020;19(73):109–118.
40. Komakech R, Matsabisa MG, Kang Y. The wound healing potential of aspilia africana (pers.) C. D. adams (Asteraceae). Evid Based Complement Alternat Med. 2019;2019:1–12. doi:10.1155/2019/7957860
41. Tang T, Yin L, Yang J, et al. Emodin, an anthraquinone derivative from rheum officinale baill, enhances cutaneous wound healing in rats. Eur J Pharmacol. 2007;567(3):177–185. doi:10.1016/j.ejphar.2007.02.033
42. Kayır S, Demirci Y, Demirci S, et al. The in vivo effects of Verbascum speciosum on wound healing. S Afr J Bot. 2018;119:226–229. doi:10.1016/j.sajb.2018.09.013
43. Boakye YD, Agyare C, Ayande GP, et al. Assessment of wound-healing properties of medicinal plants: the case of Phyllanthus muellerianus. Front Pharmacol. 2018;9:945. doi:10.3389/fphar.2018.00945
44. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi:10.1097/00001432-200404000-00004
45. Panchal MA, Murti K, Lambole V. Pharmacological properties of Verbascum thapsus—A review. Int J Pharm Sci Rev Res. 2010;5(2):73–77.
46. Karamian R, Ghasemlou F. Total phenolic content, antioxidant and antibacterial activities of three Verbascum species from Iran. J med lan produc. 2013;2(1):43–51.
47. Bhaskar A, Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J Pharmacol. 2012;44(6):694. doi:10.4103/0253-7613.103252
48. Ahamed BMK, Krishna V, Malleshappa KH. In vivo wound healing activity of the methanolic extract and its isolated constituent, gulonic acid γ-lactone, obtained from Grewia tiliaefolia. Planta med. 2009;75(05):478–482. doi:10.1055/s-0029-1185315
49. Agyingi E, Maggelakis S, Ross D. The effect of bacteria on epidermal wound healing. Math Modell Nat Phenom. 2010;5(3):28–39. doi:10.1051/mmnp/20105303
50. Süntar I, Tatlı II, Küpeli Akkol E, et al. An ethnopharmacological study on Verbascum species: from conventional wound healing use to scientific verification. J Ethnopharmacol. 2010;132(2):408–413. doi:10.1016/j.jep.2010.08.004
51. Rangaraj A, Harding K, Leaper D. Role of collagen in wound management. Wounds Uk. 2011;7(2):54–63.
52. Said A, Wahid F, Bashir K, et al. Sauromatum guttatum extract promotes wound healing and tissue regeneration in a burn mouse model via up-regulation of growth factors. Pharm Biol. 2019;57(1):736–743. doi:10.1080/13880209.2019.1676266
53. Karunanidhi A. Allium stipitatum extract exhibits in vivo antibacterial activity against methicillin-resistant Staphylococcus aureus and accelerates burn wound healing in a full-thickness murine burn model. Evid Based Complement Alternat Med. 2017;2017:1.
54. Jamshidi-Kia F. Iranian species of Verbascum: a review of botany, phytochemistry, and pharmacological effects. Toxin Reviews. 2018:2018:1.
55. Kã¼peli E, Irem I, Zeliha S. Antinociceptive and anti-inflammatory effects of saponin and iridoid glycosides from Verbascum pterocalycinum var. mutense hub.-mor. Zeitschrift für Naturforschung C. 2014:2014:1.
56. Kahraman C, Akdemir ZS, Tatlı İ. Promising cytotoxic activity profile, biological activities and phytochemical screening of Verbascum L. species. Med Aromatic Plant Scii Biotechnol. 2012;6(2):63–75.
57. Ozay Y. Effects of methanolic extract of Verbascum inulifolium Hub.-Mor. on incisional and excisional skin wounds in diabetic and non-diabetic rats; 2019.
58. Council NR, Guide for the care and use of laboratory animals; 2010.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.