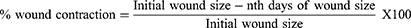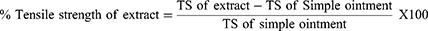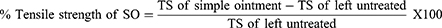Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of Wound Healing Activity of 80% Methanol Stem-Bark Extract and Solvent Fractions of Prunus africana (Hook.f.) Kalkman (Rosaceae) in Mice
Authors Hanbisa S, Tadesse WT , Abula T
Received 16 June 2023
Accepted for publication 29 August 2023
Published 7 September 2023 Volume 2023:15 Pages 349—365
DOI https://doi.org/10.2147/JEP.S426233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Roger Pinder
Sagni Hanbisa,1 Wondmagegn Tamiru Tadesse,2 Teferra Abula2
1Department of Pharmacy, Institute of Health Science, Wallaga University, Nekemte, Ethiopia; 2Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Wondmagegn Tamiru Tadesse, Tel +251 911637599, Email [email protected]; [email protected]
Purpose: Prunus africana is a well-known plant that is used in Ethiopian traditional medicine for the treatment of wounds and other ailments, although there is no scientific evidence to back up the claims of its wound-healing properties. Thus, the objective of this study is to evaluate the wound-healing potential of P. africana bark extract in mice.
Methods: The bark of the plant was extracted by successive maceration using 80% methanol and then fractionated with aqueous, n-butanol, and chloroform. The crude extract and solvent fractions were formulated as an ointment. Wound healing activity was evaluated using excision and incision wound models. Total phenol, flavonoid, and alkaloid contents of the crude extract, aqueous, and n- butanol fractions of the plant were determined.
Results: In both models, mice treated with 5% (w/w) and 10% (w/w) crude extract ointment exhibited a significant (p < 0.001) wound healing activity compared with control as evidenced by the increased rate of wound contraction and hydroxyproline content, the reduced epithelialization time, and the higher skin breaking strength. Mice treated with aqueous fraction ointment exhibited a high percentage of wound healing effect among all solvent fractions. The aqueous fraction consisted of higher phenolic (49.71 ± 0.73 mg/g) and flavonoid (39.58 ± 0.27 mg/g) content, while alkaloid (3.89 ± 0.55 mg/g) content was the lowest.
Conclusion: Prunus africana stem bark extract demonstrated wound healing activity in mice model which supports the acclaimed use by Ethiopian traditional medicine.
Keywords: wound healing, mice, P. africana, extract, fraction, incision, excision
Introduction
Wounds are the disruption of functional continuity and anatomical structure of cells and tissues at the sites of injury.1 According to their healing time, wounds are commonly classified into acute and chronic wounds.2 Acute wounds (such as traumatic and surgical wounds) involve normal inflammatory stages without underlying pathology, resulting in an expectable and organized tissue repair arrangement.3 Chronic wounds are, by definition, wounds that have failed to progress through the normal stages of healing and therefore enter a state of pathologic inflammation.4 Wound healing is a complex and protracted biological process involving coordinated interactions between diverse immunological and biological systems of tissue repair and remodeling.5
Globally, more than 400 plant species with wound-healing effects were reported. But, in Ethiopia, ethnobotanical studies conducted by different scholars show that more than 236 medicinal plants have been used to treat wounds and other illnesses by the traditional healthcare system.6 Among all, Prunus africana is one of the medical plants being used in the traditional health care system for the treatment of wounds and other diseases in Ethiopia.7
Prunus africana (Pygeum or African cherry) is an evergreen hardwood tree in forest habitats, over 30–60 m in height and up to 1.5 meters in diameter. The plant bark is blackish-brown, and the leaves are simple, oval-shaped, alternate, shiny light green on the underside and deep green on the top side. The flowers are greenish to white in color. The fruits are pinkish-brown, bi-lobed, and spherical, measuring approximately 7 mm in length and 1.3 cm in width, and when they are ripe, the thin fruit pulp turns dark red to reddish-brown.8 It grows in humid and semi-humid conditions at an altitude of about 900–3400 m above sea level, with a mean annual rainfall of 890–2600 mm and a mean annual temperature of 18–26 °C.9
The tree is found in the rainforests of the equatorial region in Africa which includes, Angola, Congo, Cameroon, Ghana, Kenya, Ethiopia, Madagascar, Mozambique Malawi, South Africa, Uganda, Tanzania, Zimbabwe, and Zambia.10 Traditional healers across Africa use P. africana as a medicine to treat many ailments including diarrhea, dysmenorrhea, epilepsy, impotency, infertility, mental illness, eye disorders, pneumonia, arthritis, hemorrhage, hemorrhoids, hypertension, anthelmintic, anti-inflammatory, antimalarial, anti-rheumatic diseases, etc. In Ethiopia, P africana is found in the northwest and southeast highlands including Harerge, Illubabor, Kefa, Arsi, and Wallaga.11 Different ethnobotanical studies in Ethiopia have reported that the stem bark of the plant was used for the treatment of wounds, benign prostate hyperplasia, sunken fontanel, and skin rash.6,7,12–14
Materials and Methods
Collection of Plant Material
The stem bark of P. africana was collected from the natural habitat around Gimbi, West Wallaga, Oromia region, 441 km away from Addis Ababa, West Ethiopia. The plant was identified and authenticated by a taxonomist at the National Herbarium, College of Natural and Computational Sciences, AAU, where a voucher number (Code: SH001) was deposited for future reference.
Experimental Animals
Healthy, adult Swiss albino mice of both sex (6–8 weeks of age) weighing 25–35g were obtained from the animal house of the School of Pharmacy, AAU. The animals were housed in cages at standard environmental conditions (12h light/dark cycle) with free access to standard laboratory pellets and clean drinking water ad libitum. They were all acclimatized to the working environment one week prior to the commencement of the experiments. The study was carried out following the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.15
Crude Extraction
The stem bark was cleaned, washed under running tap water, and allowed to dry in the open air for 1 month under shade at room temperature. Then, the dried bark was crushed into small pieces and grounded to a coarse powder using a wooden mortar and pestle. The powdered material (800 g) was macerated with 80% methanol (3200 mL) at a solid-to-solvent ratio of 1:4 (w/v) in a conical flask with occasional shaking for three consecutive days. The extract was first filtered through a funnel plunged with nylon cloth, and the filtrate was passed through Whatman filter paper (No. 1) under a vacuum. This procedure was performed two more times, each time with a different fresh solvent added to the leftover residue or marc. The resulting combined extract after successive filtration was concentrated using a rotor evaporator with a temperature set at 40 °C. The concentrated extract was then frozen in a deep freezer and dried in an oven. Finally, solid crystals were obtained and percent yields were determined and then powdered, transferred into a tight container, labeled, and stored in a refrigerator at −4 °C until used for the formulation of ointments and solvent fractionation.16
Preparation of Solvent Fractions
The 80% methanol bark extract was fractionated with varying polarities in a series of solvent–solvent fractionations (chloroform, n-butanol, and water). With the use of a tiny orbital shaker, 60 grams of crude extract were dissolved in 180 milliliters of distilled water. The mixture was transferred into a separator funnel, and an equal volume of chloroform was added to it. The new mixture was gently shaken to mix it, then allowed to rest for a few minutes to form a distinct layer, after which the chloroform fraction (CF) was separated by eluting the bottom layer. The process was repeated three times. After that, n-butanol (in an equal proportion with distilled water) was added to the aqueous residue, and the two fractions were mixed and separated in the same way as before, with the exception that the aqueous fraction was eluted first. The chloroform and n-butanol fractions’ filtrates were then concentrated using a rotary evaporator, and the three fractions were dried in a dry oven at 40 °C. The proportion yield was then calculated and stored in airtight containers at 4 °C until the experiment began.17
Ointment Formulation
Simple ointment and medicated ointments of 80% methanol crude extract, chloroform, n- butanol, and aqueous fractions were prepared as per British Pharmacopeia. A simple ointment base of 100gm was prepared by placing 5gm of hard paraffin in a beaker and melting it over a water bath. Then, 5gm of Cetostearyl alcohol, 85gm of white petrolatum, and 5gm of wool fat were added in descending order of their melting point, respectively (Table 1). All of the components were melted in a water bath while being constantly stirred until they were completely homogenous. The mixture was then taken out of the water bath and stirred until it became cool. By mixing 10gm and 5gm of powdered extracts into 90gm and 95gm of simple ointment base, respectively, 10% (w/w) and 5% (w/w) crude extract ointments were prepared. Both medicated ointments were prepared by levigation on the ointment slab’s surface to produce an ointment with a homogeneous consistency and smooth texture. The same procedures were also used for each fraction of ointment preparation. For negative control, 100g of simple ointment base was prepared without the active ingredient. Furthermore, 80% methanol crude extract and solvent fraction ointment and simple ointment base were transferred to a clean closed container for topical application during the experiment. Finally, a routine baseline color and odor change for the formulated ointment was observed, followed by a new ointment formulation every 5 days to aid formulated ointment stability.
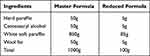 |
Table 1 Reduced and Master Formula Employed for Simple Ointment Preparation |
Selection of Dosage Form and Dose
Based on prior preliminary test on powder (Crude extract alone) and ointment base form, it was observed that the ointment base form was more effective that it was selected for this study. On the other hand, for the study of wound healing activity; 2%, 5%, and 10% ointment dose levels of extract were conducted, and the two effective doses ie, 5% (w/w) and 10% (w/w) were selected for this study.
Acute Dermal Toxicity Study
The test was performed according to the OECD draft guideline number 402.18 A total of 6 female healthy adult mice showing normal skin texture were randomly selected and marked to provide individual identification. They were given five days to acclimate to the laboratory environment before the test. IP injections of ketamine (50 mg/kg) and diazepam (5 mg/kg) were used to anesthetize the mice the day before the test chemical was administered, and by clipping fur from the dorsal/flank area of the mice, about 10% of their body surface area was shaved off. The 10% (w/w) extract ointments were applied uniformly to the entire shaved site of two animals at a limit-test dose of 200mg/kg. Animals were monitored immediately after medication and at regular intervals for the first 24 hours, with a focus on the first 2 to 6 hours. The residual test substances were removed after 24 hours, and the animals were inspected once again. After 48 hours, the rest of the animals were given the same treatment as the preceding animals, with a limit dose of 1000mg/kg and then 2000mg/kg. For the next 14 days, all animals were monitored for signs and symptoms such as tremors, convulsions, salivation, diarrhea, lethargy, and coma.
Grouping and Dosing of Animals
Both crude extract and solvent fractions were evaluated with excision and incision wound models. Excision wound model animals were separated into four groups, each with six animals, as follows: Simple ointment was given to the first group as a negative control, whereas Nitrofurazone ointment (0.2%) was given to the second group as a positive control. The third and the fourth groups were given 5% (w/w) and 10% (w/w) ointments of 80% methanol stem bark extract of P. africana, respectively. In the incision model, five groups of mice (6 per group) were employed. Animals in groups I to IV were treated similarly with an excision wound model; however, those in group V were not (served as untreated negative control).
Eight groups of mice (6 per group) were employed to test the solvent fractions (chloroform, n-butanol, and aqueous) in an excision wound model. Group I received simple ointment (negative control), group II received 5% (w/w) chloroform fraction (CF), group III received 10% (w/w) CF, group IV received 5% (w/w) butanol fraction (BF), group V received 10% (w/w) BF, group VI received 5% (w/w) aqueous fraction (AF), group VII received 10% (w/w) AF, and group VIII received Nitrofurazone (0.2%) ointment (positive control). The animals were divided into nine groups of six mice each for the incision wound model, with the same grouping and dosage as the excision wound model except for the inclusion of an untreated group.
Excision Wound Model
Before excising the wound, the mice were given 50 mg/kg ketamine and 5 mg/kg diazepam intraperitoneally. The animals’ dorsal fur was shaved with a shaving machine, and the expected area of the wound was marked on the backs of the animals. As shown in Figure 1, approximately about 314 mm2 of circular wound area was carefully created using forceps and sterilized scissors after shaving.19 The wound was blotted with a cotton swab dipped in normal saline to achieve hemostasis and leave it open to the environment. Then, the wounding day was considered as day-0. All ointments were applied once daily to the wounded area after 24 hours of wound creation until complete healing was attained according to the applicable grouping specified in the section on grouping and dosing. Every two days, wound contraction was measured as a percent contraction until the wound was completely closed.
 |
Figure 1 Photograph of circular excision wound on day 0. |
Measurement of Wound Contraction
The wound contraction rate was assessed by measuring the wound margin area using a transparent plastic sheet and a permanent marker. The tracing paper was placed on a 1 mm2 graph sheet and traced out. The traced-out wound area was used to calculate the percentage of wound contraction. Animals were monitored for wound closure, and the area was measured at 2, 4, 6, 8, 10, 12, 14, 16, and 18th post-wound days. Using the following formula, the percentage of wound contraction was calculated using the evaluated wound area, with the initial size of the wound set to 100%.20
Where; n = 2nd, 4th, 6th……18th number of measurement days.
Epithelialization Period Measurement
The period of epithelialization was calculated using the number of days required for the dead tissue to fall without any residual raw wound.21
Hydroxyproline Content Estimation
In hydroxyproline content measurement, the same approach was previously utilized to inflict an excisional wound. Following treatment with the formulations for 10 days of the circular wound created in the excision model, each animal from the respective group was euthanized on the 11th day using a high dose of anesthesia. The tissues were removed from the mice using a surgical blade and dried in an oven at 60 °C for 12 hours at a constant weight. In sealed glass tubes, the dry tissue was hydrolyzed with 6N hydrochloric acid for 24 hours at 110 °C. After that, the hydrolysates were neutralized to pH 7.0.20 One mL of the supernatant solution was taken from each and treated in the same way as the standard hydroxyproline, and absorbances were determined at a wavelength of 572 nm using a UV-spectrophotometer. The hydroxyproline content in each sample solution was calculated using the equation obtained from the calibration curve.
For the determination of standard hydroxyproline, the Neuman and Logan technique was used.22 Accordingly, 0.05 g of hydroxyproline was dissolved in 480 mL of water and 20 mL of concentrated hydrochloric acid to form 100 µg/mL. One mL of the solution was diluted to give 5, 10, and 15 g/mL of hydroxyproline, with triplicate solutions of each concentration and a blank solution prepared. Each tube was filled with one mL of 0.05 M copper sulfate, followed by one mL of 2.5 N sodium hydroxide, and the contents were gently swirled together. After immersing the tubes in a water bath at 40 °C for 3–5 minutes, 1 mL of 6% hydrogen peroxide was added and quickly mixed by swirling the contents of one tube before going on to the next. The tubes were kept in the bath for 10 minutes, with the contents being collected and swirled on occasion. Four milliliters of 3N sulfuric acid and 2 mL of 5% p-dimethylamino-benzaldehyde solution were added to the tubes after they were cooled with tap water, rotating the contents of the tubes after each addition. The solutions were cooled and mixed, and their absorbance was measured at 572 nm after being sealed and immersed in a water bath at 70 °C for 16 minutes.23
Incision Wound Model
In this model, a straight line was marked at a distance of 1 cm from the paravertebral region after the mice were anesthetized, and their dorsal fur was shaved similar to that of the excision model. Then, via the skin and subcutaneous tissue, a four-centimeter-long longitudinal paravertebral incision was created. On day 0, the parted skin was sutured one centimeter apart with a surgical thread and curved needle (Figure 2). After 24 hours of wound creation (on day 1), animals were treated as described under the grouping and dosing section, with a topical formulation of simple ointment, medicated ointment, and standard drug daily for nine days, leaving out the last group without applying any of the interventions.24 The sutures were removed on the eighth post-incision day, and tensile strength was assessed on the tenth post-wounding day using a continuous water flow technique (Figure 3).
 |
Figure 2 Photograph of the incision wound on day 0. |
 |
Figure 3 Photograph of the measurement of tensile strength. |
where; TS = Tensile strength, SO = Simple ointment.
Quantification of the Total Phytochemical Contents of the Extract and the Fractions
The total phytochemical contents of the plant extract, n-butanol, and aqueous fractions were determined using different methods. The total phenol contents of the plant extract, the n-butanol, and aqueous fraction fractions were determined by the Folin–Ciocalteu method using gallic acid as standard.25 Similarly, the total flavonoid content of the plant crude extract, n- butanol fraction, and aqueous fraction was determined using an aluminum chloride complex-forming assay and quercetin as a reference standard,26 while the total alkaloid contents were determined according to a method described by Ajanal et al, using atropine as a reference standard.27 All the procedures were performed in triplicate; the same procedure was repeated with the plant extract and fractions at (1 mg/mL) and also for the blank solutions for the respective tests.
Statistical Analysis
The experimental results of the study were expressed as the mean (±SEM). The data were entered, coded, and analyzed using SPSS version 20 software. A one-way ANOVA followed by the Tukey post hoc test was used to compare the different experimental groups. And p < 0.05 was considered statistically significant.
Results
Yields of Crude Extract and Solvent Fractions
The percentage yield value of the crude extract was determined and found to be 16.96%. Aqueous, n-butanol, and chloroform fraction yields were 47.66%, 40.83%, and 6.33%, respectively.
Acute Dermal Toxicity
In this acute dermal toxicity study, the maximum concentration of the ointment (10% w/w) administered at a limit dose of 2000 mg/kg was found to be safe. After 24 hours of application in the shaved area, there was no sign of tremors, convulsions, salivation, diarrhea, lethargy, or coma, with special attention given for the first 2 to 6 hours. There were also no overt signs or symptoms observed when the animals were monitored for 48 hours. Moreover, no signs of toxicity, as well as no mortality, were observed during the 14-day observation.
Wound Healing Activity of Crude Extracts
Excision Wounds
Wound Contraction
On the mice, topical applications of ointments containing 80% methanol extract of P. africana bark demonstrated wound healing efficacy. The progressive wound contraction induced by treatment with 5% and 10% (w/w) P. africana bark of 80% methanolic extract, simple ointment base, and nitrofurazone 0.2% (w/w) ointment is shown in Table 2.
 |
Table 2 The Effect of a Topical Ointment Made from 80% Methanol Stem Bark Extract of P. africana on Wound Contraction in a Mouse Excision Wound Model |
The 10% (w/w) extract and standard drug exhibited significant wound contraction (p < 0.05 to 0.001) starting from the 4th day of administration compared to the control. Similarly, 10% (w/w) extract facilitated significant (p < 0.05) wound contraction from the 8th to 12th days of treatment as compared with 5% (w/w) extract, while it showed comparable efficacy with the standard drug. On the other hand, a significant wound contraction effect was observed with 5% (w/w) extract from the 6th day onward as compared to the control group (p < 0.001). However, the effect of the 5% (w/w) extract was not significantly different from that of the standard.
The maximum percentages of wound contraction were observed with 10% (w/w) extract on the 12th and 14th day, which were 94.38% and 98.38%, respectively. Comparable percentages of wound contraction (93.12% and 98.01%) were also observed with the standard drug on the 12th and the 14th day, respectively. The 10% (w/w) extract and standard drug-treated groups showed a high percentage of wound closure. Furthermore, complete wound closure in the 10% (w/w) extract and the standard drug was observed on the 16th day, while it took about 18 days in the 5% (w/w) extract (Figure 4).
Epithelialization Period
In extract ointments and nitrofurazone-treated groups, the time for complete epithelialization was short (p < 0.01 to 0.001) compared with the control (Table 3). The 10% (w/w) extract and standard drug-treated groups showed a significant reduction (p < 0.001) in the epithelialization period when compared with the control. Likewise, the 5% (w/w) treated group exhibited a significant decrease (p < 0.01) in the epithelialization period as compared to the control group. However, the epithelialization periods of the extracts between each other and with the standard were not statistically significant.
 |
Table 3 The Effect of a Topical Ointment Made from P. africana 80% Methanol Stem Bark Extract on Wound Epithelialization Time |
Hydroxyproline Content Estimation
In the present study, both the extract ointments and the standard drug resulted in significantly higher hydroxyproline content than the control (p < 0.001). Similarly, the hydroxyproline level was found to be significantly elevated (p < 0.01) in 10% (w/w) of extract ointment and the standard drug-treated groups in comparison to 5% (w/w) extract ointment. Looking at the percentage increase in hydroxyproline, nitrofurazone achieved the maximum increase (96.09%) followed by 10% (86.76%) and 5% (52.71%) of the extract ointment (Table 4).
 |
Table 4 Hydroxyproline Contents of Excision Wound Following Topical Application of an Ointment Containing 80% Methanol Stem Bark Extract of P. africana |
Incision Wounds
In the group treated with the standard drug, 5% (w/w), and 10% (w/w) extract ointments recorded a significant increase in tensile strength (p < 0.001) when compared to the negative control (simple ointment). Likewise, the 10% w/w extract was found to be significantly (p < 0.05) enhanced in tensile strength as compared to the 5% w/w extract. In this finding (Table 5), the observable increase in tensile strength was found to be higher in 10% extract ointment as compared to nitrofurazone-treated groups. However, this finding failed to reach statistical significance. It also appears that no significant difference in tensile strength was noted when nitrofurazone was compared with 5% (w/w) extract-treated groups.
 |
Table 5 The Tensile Strength of Incision Wounds After Topical Administration of an Ointment Prepared from 80% Methanol Stem Bark Extract of P. africana |
Wound Healing Activity of Solvent Fractions
Excision Wounds
Wound Contraction
The aqueous and n-butanol fractions showed significant wound healing activity in a dose-dependent manner comparable with that of nitrofurazone (Table 6). From the 4th post-wounding day onward, aqueous fractions (5% and 10% w/w), n-butanol fractions (5% and 10% w/w), and nitrofurazone 0.2% ointment-treated group recorded significant (p < 0.05 to 0.001) wound contraction as compared with the control. In addition, both the strong chloroform fractions showed significant (p < 0.05 to 0.01) wound contraction from the 8th to the 14th post-wounding days as compared with the control. On the other hand, both the strong aqueous fraction and nitrofurazone 0.2% ointment exhibited significant (p < 0.05 to 0.001) wound contraction from the 4th day onward as compared with 5% (w/w) chloroform fraction treated groups. Likewise, aqueous fractions (5% and 10% w/w), n-butanol fractions (5% and 10% w/w), and nitrofurazone 0.2% ointment-treated groups showed significant (p < 0.05 to 0.001) wound contraction from the 6th day of administration onward as compared with 10% (w/w) chloroform fraction ointment. There was a slightly higher effect with 10% (w/w) ointment preparations than 5% (w/w) ointment preparations between each solvent fraction, but it failed to reach statistical significance. Furthermore, 10% (w/w) aqueous fraction ointment demonstrated comparable efficacy with the standard drug.
 |
Table 6 The Effects of a Topical Ointment Made from Solvent Fractions of P. africana Stem Bark on Wound Contraction in a Mouse Excision Wound Model |
A higher percentage of wound contraction was observed from the 12th post-wounding day onwards in the group treated with aqueous fractions (5% and 10% w/w), n-butanol fractions (5% and 10% w/w), and nitrofurazone 0.2% ointment. However, both chloroform fractions (5% w/w and 10% w/w) and simple ointment showed a higher percentage of wound contraction on the 16th post-wounding day. The 10% (w/w) aqueous fraction and the standard drug showed complete wound closure on the 16th day, while the 5% (w/w) aqueous fraction and n-butanol fractions (5% and 10% w/w) showed on day 18. However, the remaining fractions and simple ointment were completed with their wound closure beyond the 18th post-wounding day.
Epithelialization Period
The time for complete epithelialization was short in both the aqueous fraction, n-butanol fraction, and nitrofurazone 0.2% ointment-treated groups compared to the control. Groups treated with 10% (w/w) aqueous fraction, 10% (w/w) n-butanol fraction, and the standard drug could shorten the epithelialization period more significantly (p < 0.001) as compared with simple ointment. Likewise, both the 5% (w/w) n-butanol and aqueous fraction treated groups showed a significant decrease (p < 0.01) in the epithelialization period as compared with the control. However, there was no significant difference between the chloroform fractions (5% and 10% w/w) and the negative control. On the other hand, both the strong aqueous fraction, 10% (w/w) n-butanol fraction, and standard drug-treated groups showed a significant decrease (p < 0.05 to 0.001) in the epithelialization period as compared to the 5% (w/w) chloroform fraction. It also appears that the effects of 10% (w/w) aqueous fraction and the standard drug-treated group exhibited a significant decrease (p < 0.01) in the epithelialization period as compared to 10% (w/w) chloroform fraction. There was a modest difference between the epithelialization periods of the 5% and 10% (w/w) preparations of each fraction, but they lacked significance when compared with each other (Table 7).
 |
Table 7 The Tensile Strength of Incision Wounds and Epithelialization Period After Topical Administration of an Ointment Made from Solvent Fractions of P. africana Stem Bark |
Incision Wounds
In the incision wound model, aqueous fractions (5% and 10% w/w), n-butanol fractions (5% and 10% w/w), and standard drug-treated groups showed significant (p < 0.001) increase in breaking strength when compared to the negative control group (simple ointment). Similarly, both the 5% (w/w) and 10% (w/w) chloroform fraction-treated groups recorded a significant (p < 0.01) increase in tensile strength as compared with a simple ointment base. On the other hand, both the strength of the aqueous and n-butanol fractions and the standard drug-treated group significantly (p < 0.001) increased the tensile strength as compared with the chloroform fraction (5% w/w and 10% w/w) treated groups. The percent tensile strength of 10% (w/w) aqueous fraction, 5% (w/w) aqueous fraction, 10% (w/w) n-butanol fraction, 5% (w/w) n-butanol fraction, and nitrofurazone 0.2% ointment treated groups were 71.83, 59.88, 63.68, 56.76, and 71.02, respectively (Table 7).
Quantification of Phytochemical Constituents
The total phenolic content of the plant extract and fractions were determined using the Folin-Ciocalteu reagent, depending on the standard gallic acid. From the results obtained, the aqueous fraction was found to have the highest total phenolic content (49.71 mg/g), followed by crude extract (45.3 mg/g) and the n-butanol fraction (42.37 mg/g) (Table 8). The total flavonoid content of the 80% methanol crude extract and both fractions of P. africana stem bark extract were determined by the aluminum chloride method based on the quercetin standard. The total flavonoid content in crude extract, aqueous fraction, and n-butanol fraction were found to be 37.64 mg/g, 39.58 mg/g, and 37.36 mg/g, respectively (Table 8). The total alkaloid content was calculated using atropine equivalent based on the equation resulting from the calibration curve. The highest amount of alkaloid was measured in the n-butanol fraction (9.05 mg/g), followed by crude extract (7.76 mg/g), while the aqueous fraction contained the lowest amount of alkaloid (3.89 mg/g), as indicated in Table 8.
 |
Table 8 Results of Total Phenolic, Flavonoid and Alkaloid Content in P. africana Stem Bark Crude Extract, n-Butanol Fraction, and Aqueous Fraction |
Discussion
Wound contraction is the rate at which the wound area shrinks over time throughout the healing process, reducing the wound size and the amount of ECM needed to repair the problem. Contraction also aids re-epithelialization by reducing the distance traveled by migrating keratinocytes.28 As a result, if the treatment is more effective, the wound will close at a faster rate. In this regard, the 10% (w/w) ointment of P. africana stem bark crude extracts and aqueous fractions exhibited an equivalent wound-healing effect to that of the standard drug, increasing the percentage of wound contraction, hydroxyproline content, and wound-breaking strength, as well as shortening the period of epithelialization compared to the control. This wound healing ability of Prunus africana bark observed in the present study might be due to the antimicrobial,29,30 anti-inflammatory,6 and antioxidant activity30 of the plant, as previous studies reported for the stated activities.
Also, wound healing is characterized by contraction, epithelialization, production, and deposition of new collagen, and scar formation.31 Many biochemical markers, such as total protein and hydroxyproline, are used as indicators of wound healing. Quick wound healing is characterized by rapid wound contraction, a shorter epithelialization period, and enough tensile strength gain.28 It is challenging to grasp all of these healing cascades using a single model or depending on an in vitro study.24 Hence, in the present study, two separate animal wound models (excision and incision) were used to determine the in-vivo wound healing potential of P. africana stem bark extract and solvent fractions in mice.
Traditionally, the bark of P. africana has been used for wound healing activities by applying the bark powder directly to the wounded area.6 In our pilot study, applying the poudered plant material (without solvent extraction) directly to the wound area did not result in any wound-healing activity. Thus, the bark of P. africana was extracted in 80% methanol using the maceration process to separate the medicinally active portion from the insoluble cellular marc to evaluate the wound healing activity of the plant bark in the mouse model. The resulting extract was then incorporated into simple ointment bases to achieve the desired effect through sustained release of extract at the application site. Based on earlier research, methanol was employed for extraction in this study.10,16,32 Ointment bases, on the other hand, provide a medium for the dissolution of the principles in the crude extract and its fractions and may also form a barrier for the moisture over the wound area by hydrating the stratum corneum, which is important for cellular migration and growth factor diffusion to the wound.17
In the excision wound model, treatment with both a 5% (w/w) and a 10% (w/w) ointment made from a crude 80% methanol stem bark extract of P. africana demonstrated faster wound contraction compared to the control. This could be due to one of the following possible factors. First, a previous study of P. africana methanolic stem bark extract demonstrated antibacterial activity against pathogens such as MRSA, S. aureus, and P. aeruginosa, which commonly infect wounds.29,33 This antimicrobial activity may aid in the speedy healing of a wound by preventing infections and further complications. The presence of phenols, flavonoids, saponins, glycosides, terpenoids, alkaloids, tannins, and carbohydrates, which were detected in the preliminary phytochemical screening of the plant bark extract in a previous study, could be the second reason for the enhanced wound healing effect.8,10,16 The high phenolic and flavonoid content of the plant could have contributed to its anti-inflammatory property. In turn, such property may play a part in the rapid wound healing action of the extract.8,34 Another possible explanation could be due to the greater antioxidant activity of the plant bark from the previous study.30 Agents that demonstrate significant antioxidant activity may enhance cell proliferation, wound closure, and speedy wound healing by preventing enzymes and ROS, which would be produced as a result of inflammation and grossly lead to damage of DNA, lipids, proteins, free radical scavenger enzymes, the ECM, and cytokines.
When compared to the control group, the 10% (w/w) ointment prepared from the crude stem bark extract of P. africana considerably reduced the epithelialization period from 18 to 14 days. This could be attributed to faster wound contraction, which shortens the distance traveled by migratory keratinocytes.28 As wound re-epithelialization is a characteristic of successful wound care, thus the better healing activity of the extract is displayed by a short epithelialization period.35 Furthermore, collagen is the predominant extracellular protein in the granulation tissue of a healing wound and its rapid synthesis in the wound area provides strength and integrity to the tissue matrix.20 Thus, based on the results of the hydroxyproline estimation of our study (Table 6), the extract may have augmented collagen synthesis and deposition which facilitates proliferation, migration, and increased viability of epithelial cells. Thus, shorter epithelialization periods in animals treated with the extract support the idea that P. africana has a potential as a wound-healing agent.
The findings from the incision wound model also consistently showed a significant increase in the measure of tensile strength, which revealed that the healed tissue resisted breaking under tension, particularly with the 5% and the 10% extract ointments. The tensile strength of a wound reveals how well the healed tissue resists breaking under tension.36 The literature suggests that this effect of extracts could be due to the increased collagen synthesis, maturation, angiogenesis, and stabilization of fibers with crosslinking of the protein.37 Hence, all these cumulative effects improve circulation by providing oxygen and nutrients to the wound area which is essential for the healing process of the wound.
The 80% methanol crude extract of P. africana stem bark was further fractionated with solvents of different polarities including chloroform, n-butanol, and water to identify which fraction of the plant extract was accountable for wound healing activity. The aqueous fraction produced the highest yield indicating that the bark of P. africana consists of a higher concentration of polar compounds which are better dissolved in water medium. This is consistent with the findings of a previous study that illustrated the enhanced extract yield as the polarity of the solvent increases.11
In the excision wound model, the aqueous fraction showed a superior wound healing activity compared to the negative control, the BF, and CF fractions. Both the 5% and 10% (w/w) aqueous fractions demonstrated a shorter duration of epithelialization and a high percentage of wound contraction. These effects could be linked to the fraction’s induction or promotion of cellular proliferation, enhanced collagen production, and protein cross-linking, increased anti-inflammatory activity, and antioxidant properties. Our study may suggest that the polar compounds largely found in AF could be linked with enhanced wound-healing activity (Table 8). Phenols and flavonoids are usually polar, highly soluble in water, and known for their antioxidant and anti-inflammatory activity. Thus, these compounds might be responsible for the enhanced wound healing activity by the aqueous fraction and BF.
On the other hand, the faster wound-healing effects of both aqueous and n-butanol fractions could be linked with the antimicrobial activities of the fractions. Infection from S. aureus and anaerobic bacteria during the wound healing process might prolong the inflammatory phase of the wound, resulting in poor epithelialization and failure in wound repair.38 Hence, the robust wound healing effect of both aqueous and 10% (w/w) n-butanol fractions could be linked with their antibacterial, antioxidant, and antifungal activities as stated in different previous studies.29,30 Accordingly, P. africana bark has bactericidal activity against methicillin-resistant Staphylococcus aureus (MRSA), S. aureus, and P. aeruginosa, as well as antifungal activity.33 Therefore, the antibacterial and antifungal activity of the plant could be important to spice up wound healing by preventing infection.
In the incision model, an increase in the tensile strength by the aqueous fraction indicated enhanced collagen synthesis, maturation, and stabilization.31 Higher tensile strength in AF may be attributable to the antioxidant and antibacterial characteristics of phytochemicals contained in this fraction, which may help speed up wound healing. Flavonoids, for example, are powerful antioxidants and free radical scavengers that protect cells from oxidative damage.35 The present study reported that total flavonoids found in the aqueous fraction were higher compared with BF and crude extracts, as indicated in Table 8. Hence, a higher percentage of tensile strength may be due to the higher concentration of flavonoids in the aqueous fraction. The substantial amount of flavonoids in the aqueous fraction of P. africana stem bark, which has antiviral and antibacterial properties, may help to prevent secondary wound infections and enhance wound healing. On the other hand, a high amount of phenolic compounds present in the plant favors better venerability of collagen synthesis, which finally increases the tensile strength of the AF.20
Conclusion
The study indicated that the 80% methanol crude extract and the aqueous and n-butanol fractions of Prunus africana stem bark possessed wound-healing activity. The crude extract and its fractions demonstrated enhanced wound contraction, hydroxyproline content, a shorter epithelization period, and higher tissue-breaking strength. Hence, the observed findings support the traditionally acclaimed use of P. africana bark for wound treatment.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author and will be submitted when requested.
Ethical Approval
All of the experiments followed international guidelines for the care, use, and handling of laboratory animals.15 The Research and Ethical Review Board at the Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, granted ethical approval with the reference number ERB/SOP/178/13/2020.
Acknowledgments
The authors would like to extend their gratitude to the Department of Pharmacology and Clinical Pharmacy, Addis Ababa University, for allowing laboratory facilities and material support.
Funding
This research is funded by the Office of Graduate Studies of Addis Ababa University.
Disclosure
The authors declare that they have no competing interests to disclose in this work.
References
1. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
2. Saqallah F, Hamed W, Talib W. In vivo evaluation of antirrhinum majus’ wound-healing activity. Sci Pharm. 2018;86(4):45. doi:10.3390/scipharm86040045
3. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735. doi:10.3390/pharmaceutics12080735
4. Menke NB, Ward KR, Witten TM. Impaired wound healing. Am J Clin Dermatol. 2018. doi:10.1016/j.12.005
5. Sabale P, Bhimani B, Prajapati C, Sabale V. An overview of medicinal plants as wound healers. Pharm Sci. 2012. doi:10.7324/JAPS.2012.21127
6. Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiop J Health Dev. 2019;2019:1.
7. Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka District, East Welega Zone of Oromia Regional State, West Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):68. doi:10.1186/1746-4269-9-68
8. Mutuma GG, Joseph N, Alex M, Silas K. Phytochemical and anti-inflammatory analysis of Prunus africana bark extract. Res J Pharm. 2020;7:31–38. doi:10.22127/rjp.2020.229941.1583
9. Komakech R, Kang Y, Omujal F, Omujal F. A review of the potential of phytochemicals from Prunus africana (Hook f.) Kalkman stem bark for chemoprevention and chemotherapy of prostate cancer. Evid Based Complement Alter Med. 2017;2017:1–10. doi:10.1155/2017/3014019
10. Mwangi KJ, Kariuki KJ, Reuben T, Kibe KG. The phytochemical components and acute toxicity of methanolic stem bark extract of Prunus africana. Int Organ Sci Res Pharm. 2018;8(12):39–45.
11. Begeno TA. Phytochemical investigation and characterization on the root bark extract of Prunus africana. CMR. 2020. doi:10.7176/CMR/12-6-02
12. Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40. doi:10.1186/1746-4269-10-40
13. Birhanu T. Ethnobotanical study of medicinal plants in selected Horro Gudurru Woredas, Western Ethiopia. J Biol Agri Healthc. 2015;5(1):83.
14. Kewessa G, Abebe T, Demessie A. Indigenous knowledge on the use and management of medicinal trees and Shrubs in Dale District. Sidama Ethnobot Res Appl. 2015;182(June):171–182. doi:10.17348/era.14.0.171-182
15. National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the Care and Use of Laboratory Animals.
16. Ngule MC, Ndiku MH, Ramesh F. Chemical constituents screening and in vitro antibacterial assessment of Prunus africana bark hydromethanolic extract. J Nat Sci Res. 2014;4(16):85–91.
17. Mengie T, Mequanente S, Nigussie D, Legesse B, Makonnen E. Investigation of wound healing and anti-inflammatory activities of solvent fractions of 80% methanol leaf extract of achyranthes aspera L. (Amaranthaceae) in rats. J Inflamm Res. 2021;14:1775–1787. doi:10.2147/JIR.S298244
18. OECD. Test No. 402: acute dermal toxicity; 2017. Available from: https://www.oecd-ilibrary.org/content/publication/9789264070585-en.
19. Lodhi S, Vadnere GP. Relevance and perspectives of experimental wound models in wound healing. Asian J Pharm Clin Res. 2017;10(7). doi:10.22159/ajpcr.2017.v10i7.18276
20. Nagar HK, Srivastava AK, Srivastava R, Kurmi ML, Chandel HS, Ranawat MS. Pharmacological Investigation of the wound healing activity of cestrum nocturnum (L.) ointment in Wistar Albino rats. J Pharm. 2016;2016:1–8. doi:10.1155/2016/9249040
21. Shenoy RR, Sudheendra AT, Nayak PG, Paul P, Kutty NG, Rao CM. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J Ethnopharmacol. 2011;133(2):608–612. doi:10.1016/j.jep.2010.10.045
22. Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184(1):299–306. doi:10.1016/S0021-9258(19)51149-8
23. Leach AA. Appendix —Notes on a modification of the Neuman & Logan method for the determination of the hydroxyproline. Biochem J. 1960;74(1):70–71. doi:10.1042/bj0740070
24. Gebremeskel L, Bhoumik D, Sibhat GG, Tuem KB. In vivo wound healing and anti-inflammatory activities of leaf latex of Aloe megalacantha baker (Xanthorrhoeaceae). Evid Based Complement Alter Med. 2018;2018:1–7. doi:10.1155/2018/5037912
25. Pandey B, Rajbhandari M. Estimation of total phenolic and flavonoid contents in some medicinal plants and their antioxidant activities. Nepal J Sci Technol. 2015;15:53–60. doi:10.3126/njst.v15i1.12010
26. Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exp Pharmacol. 2021;Volume 13:15–22. doi:10.2147/JEP.S285079
27. Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV ‑ Spectrophotometer. Anc Sci Life. 2018;31(4):2–5. doi:10.4103/0257-7941.107361
28. Mulisa E, Asres K, Engidawork E, Chen C-Y, Ke C-J. Evaluation of wound healing and anti- inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15:1–10. doi:10.1186/s12906-015-0878-y
29. Mwitari PG, Ayeka PA, Ondicho J, Matu EN, Bii CC. Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS One. 2013;8(6):4–12. doi:10.1371/journal.pone.0065619
30. Maina EG, Kairigo PK, Murigi M, Ogilo J. In vitro antioxidant and antimicrobial activity of Prunus africana (Hook. f.) Kalkman (bark extracts) and Harrisonia abyssinica Oliv. extracts (bark extracts): a comparative study. J Med Plants Econ Dev. 2018. doi:10.4102/jomped.v2i1.39
31. Murthy S, Gautam MK, Goel S, Purohit V, Sharma H, Goel RK. Evaluation of In vivo wound healing activity of bacopa monniera on different wound model in rats. Biomed Res Int. 2013;2013:1–9. doi:10.1155/2013/972028
32. Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. J Medicinal Aromat Plants. 2015;4(3):3–8. doi:10.4172/2167-0412.1000196
33. Bii C, Korir KR, Rugutt J, Mutai C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. J Med Plants Res. 2010;4(11):995–998. doi:10.5897/JMPR09.227
34. Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9). doi:10.5812/ircmj.19960
35. Taddese SM, Gurji TB, Abdulwuhab M, Aragaw TJ. Wound healing activities of hydromethanolic crude extract and solvent fractions of bersama abyssinica leaves in mice. Evid Based Complement Alter Med. 2021;2021:1–20. doi:10.1155/2021/9991146
36. Masson‐Meyers DS, Andrade TAM, Caetano GF, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. doi:10.1111/iep.12346
37. Murti K, Kumar U. Enhancement of wound healing with roots of Ficus racemosa L. in albino rats. Asian Pac J Trop Biomed. 2012;2(4):276–280. doi:10.1016/S2221-1691(12)60022-7
38. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.