Back to Journals » Nanotechnology, Science and Applications » Volume 17
Evaluation of the Antimicrobial, Cytotoxic, and Physical Properties of Selected Nano-Complexes in Bovine Udder Inflammatory Pathogen Control
Authors Wierzbicki M , Kot M, Lange A, Kalińska A, Gołębiewski M, Jaworski S
Received 2 November 2023
Accepted for publication 6 February 2024
Published 20 March 2024 Volume 2024:17 Pages 77—94
DOI https://doi.org/10.2147/NSA.S447810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Mateusz Wierzbicki,1 Magdalena Kot,2 Agata Lange,1 Aleksandra Kalińska,2 Marcin Gołębiewski,2 Sławomir Jaworski1
1Department of Nanobiotechnology, Warsaw University of Life Sciences, Warsaw, 02-786, Poland; 2Animal Breeding Department, Warsaw University of Life Sciences, Warsaw, 02-786, Poland
Correspondence: Sławomir Jaworski, Department of Nanobiotechnology, Warsaw University of Life Sciences, Ciszewskiego 8 street, Warsaw, 02-786, Poland, Email [email protected]
Purpose: Mastitis in dairy cows is a worldwide problem faced by dairy producers. Treatment mainly involves antibiotic therapy, however, due to widespread antibiotic resistance among bacteria, such treatments are no longer effective. For this reason, scientists are searching for new solutions to combat mastitis, which is caused by bacteria, fungi, and algae. One of the most promising solutions, nanotechnology, is attracting research due to its biocidal properties. The purpose of this research was to determine the biocidal properties of nanocomposites as a potential alternative to antibiotics in the control of mastitis, as well as to determine whether the use of nanoparticles and what concentration is safe for the breeder and the animal.
Patients and Methods: In this study, the effects of Ag, Au, Cu, Fe, and Pt nanoparticles and their complexes were evaluated in relation to the survival of bacteria and fungi isolated from cattle diagnosed with mastitis, their physicochemical properties, and their toxicity to bovine and human mammary epithelial cells BME-UV1 and HMEC (human microvascular endothelial cells). Moreover, E. coli, S. aureus, C. albicans, and Prototheca sp. invasion was assessed using the alginate bead (bioprinted) model. The NPs were tested at concentrations of 25, 12.5, 6.25, 3.125, 1.56 mg/l for Au, Ag, Cu and Fe NPs, and 10, 5, 2.5, 1.25, 0.625 mg/l for Pt.
Results: With the exception of Fe and Pt, all exhibited biocidal properties against isolates, while the AgCu complex had the best effect. In addition, nanoparticles showed synergistic effects, while the low concentrations had no toxic effect on BME-UV1 and HMEC cells.
Conclusion: Synergistic effects of nanoparticles and no toxicity to bovine and human cells might, in the future, be an effective alternative in the fight against microorganisms responsible for mastitis, and the implementation of research results in practice would reduce the percentage of dairy cows suffering from mastitis. The problem of increasing antibiotic resistance is posing a global threat to human’s and animal’s health, and requires comprehensive research to evaluate the potential use of nanoparticles – especially their complexes – as well as to determine whether nanoparticles are safe for the breeders and the animals. The conducted series of studies allows further consideration of the use of the obtained results in practice, creating a potentially new alternative to antibiotics in the treatment and prevention of mastitis in dairy cattle.
Keywords: cattle, mastitis, bacteria, fungi, nanocomposites
Introduction
The most common and expensive issue occurring in cattle herds is mastitis, ie, inflammation of the udder tissue. Despite advancing science and some innovative solutions, this problem is faced by breeders worldwide. Mastitis reduces the animal’s health condition, negatively affects their welfare, and results in financial losses.1
Mastitis is a multifactorial disease that is primarily caused by microorganisms such as bacteria: gram-positive Streptococcus agalactiae, Staphylococcus spp. and Streptococcus uberis, and gram-negative Escherichia coli, Enterococcus spp. and Mycoplasma spp.2 as well as fungi, especially the yeast Candida albicans. In recent years, there have also been cases involving algae, such as Prototheca sp., which is highly resistant to all available commercial therapeutics, among other antibiotics.3
Despite the implementation of mastitis control strategies and an increasing knowledge of this disease, it is an ongoing issue with an increasing frequency of occurrence. Particularly alarming is the fact that there is increasing antibiotic resistance among bacteria, due to their overuse and incorrect usage. Currently available agents—among others, antibiotics, antifungal, and antibacterial agents—are not very effective,2 therefore, it is crucial to search for new, effective alternatives.
In recent years, an increasing number of studies have targeted the use of metal nanoparticles (NPs). They are especially appreciated because of their numerous properties. These include drug delivery,4 antiviral properties,5 antifungal,6 anti-parasitic,7 therefore, they are very often used in medicine,8 pharmacy,9 and cosmetology.10 In vitro studies also confirm anticancer activities of nanocomposites.11
The scope of recent research using nanoparticles has been developing specifically in the context of bacterial control, due to their unique properties and affinity for bacterial cells, which is dependent on the morphological and physicochemical properties of the nanoparticles.12,13 There are different methods of nanoparticle synthesis. These can be biological method, which is the most environmentally friendly of all possible synthesis methods. This method uses various living organisms including: bacteria, viruses,14 but also yeasts,15 fungi,16 algae,17 as well as land plants, their extracts,18,19 and even submerged plants,20,21 which exhibit biocidal properties. Nanoparticles can also be synthesized by chemical methods using various chemicals, and by physical methods using, for instance, UV radiation, ultrasound, and laser rays as well as various reducing agents.22,23 Unfortunately, both chemical and physical methods of synthesis use toxic chemicals, which have a significant negative impact on the environment. Nanoparticle synthesis works based on two methods, first is top-down which involves breaking down a large mass into smaller ones, and the second method is bottom-up, which works the opposite way – materials of nano-size are combined into larger structures.24 However, the most important feature of nanoparticles in the context of their use in combating pathogens, is the bacteria’s inability to develop resistance to them.25
Previous studies have also shown that metal nanoparticles interact with the external structures of bacteria (the membrane and cell wall), which can lead to disruption of the membrane potential, damage to the cell membrane, and activation of oxidative stress.26,27
The previously described literature reports suggest that the application of several types of nanoparticles (Au, Ag, Cu, Fe, and Pt) and the exploitation of their synergistic effects as complexes, might, in the future, be an effective alternative in the fight against the microorganisms responsible for mastitis. The implementation of research results in practice would help breeders around the world and reduce the percentage of dairy cows suffering from mastitis, thereby increasing their welfare. The nanoparticles’ lack of toxicity to human mammary epithelial cells (HMEC) and the bovine mammary epithelial cell-line BME-UV1 has recently been confirmed,28 however, there is a shortage of information in the literature about the cytotoxic effects of nanoparticle complexes. In addition, there have been no studies indicating the effect of nanocomposites (Au, Ag, Cu, Pt, and Fe NPs) on pathogens isolated from the milk from cows with subclinical mastitis.
The aim of this study was to determine the biocidal effects of commercially available nanoparticles (Ag, Cu, Au, Pt, and Fe) obtained by physical methods, on bacteria, fungi, and algae isolated from cows diagnosed with mastitis. The conducted study also determined the effect of their physicochemical properties (morphology, size of selected nanoparticles their zeta potential, and hydrodynamic diameters). The purpose of this study was also to test nanocomposites of the above-mentioned NPs due to their potential synergistic effects against mastitic pathogens. The aim was also to determine the applicability of this technology not only in terms of the effectiveness of the biocidal properties of nanoparticles against mastitic pathogens but also in terms of safety against other cells. Therefore, studies were conducted to determine the toxicity of single nanoparticles and their complexes against bovine and human mammary epithelial cells BME-UV1 and HMEC. Thus, according to the stated purpose it was possible to determine whether the use of nanoparticles and what concentration is safe for the breeder and the animal. Among the main goals was also determining the level of reactive oxygen species, which are responsible for inducing oxidative stress, making the nanoparticles exhibit antibacterial activity.
In this study, we assessed the toxicity of Au, Ag, Cu, Pt, and Fe NPs and their complexes on human and bovine mammary epithelial cells as well as bacterial and fungal isolates such as Streptococcus uberis, Staphylococcus sciuri, Escherichia coli, Serratia marcescens, Staphylococcus aureus, Candida albicans, Prototheca sp., Citrobacter koseri, Candida glabrata, and Candida parapsilosis. The biological material used was mastitic milk collected from Holstein-Friesian cows with diagnosed mastitis (SCC > 400,000/mL). Subsequently, the cultured colonies were submitted for identification using a MALDI-TOF MS apparatus (Bruker, Poznań, Poland), resulting in precise and reliable identification. For selected strains of microorganisms, the study of the invasion of microorganisms and cell viability in biofilm was carried out in a method utilizing bioprinted alginate beads.
Materials and Methods
Morphology of Nanoparticles
Hydrocolloidal Ag, Au, Cu and Pt nanoparticles were purchased from the Nano-Tech Poland (Warsaw, Poland), while the Fe nanoparticles were purchased from 3d-nano (Cracow, Poland), with a purity >97.0%. It was produced by a nonexplosive, high-voltage method, using a high-purity metal (99.9999%) and high-purity demineralized water. To determine the morphology of the nanoparticles, transmission electron microscopy (TEM) images were acquired using a JEM-1220 microscope (Jeol, Tokyo, Japan) at 80 kV with a Morada 11-megapixel camera (Olympus Soft Imaging Solutions, Münster, Germany). Samples were prepared by placing droplets of hydrocolloids at a concentration of 10 mg/l onto formvar-coated copper grids (Agar Scientific, Stansted, UK) that were air-dried before observation.
Physicochemical Properties of Selected Nanoparticles (Size, Zeta Potential, and Average Hydrodynamic Size)
Zeta potential measurements were performed by microelectrophoresis with Smoluchowski approximation and size distribution measurements by dynamic light scattering (DLS) using a Zetasizer Nano-ZS90 analyzer (Malvern Instruments, Worcestershire, UK) after stabilization of the colloids for 120 s at 25 °C in triplicate. FT-IR analysis of Ag and Cu nanoparticles has been carried out in our recent publications.29,30
The Cell Culture of BME-UV1 and HMEC Cells
The BME-UV1 cells were purchased from the Cell Bank of The Lombardy and kindly provided by Prof. M. Gajewska (Institute of Veterinary Medicine, Warsaw University of Life Sciences, Warsaw, Poland) and were maintained in DMEM/F12, RPMI-1640, and NCTC 135 medium (Thermo Fisher Scientific) at a ratio of 5:3:3, which was enriched with alpha lactose (0.1%, Merck), glutathione (1.2 mM, Merck), bovine insulin (1 μg/mL, Merck), bovine holo-transferrin (5 μg/mL, Thermo Fisher Scientific), hydrocortisone (1 μg/mL, Merck), L-ascorbic acid (10 μg/mL, Merck), 10% FBS (Thermo Fisher Scientific), and an antibiotic and an antimycotic Thermo Fisher Scientific).
HMEC cells were obtained from Thermo Fisher Scientific and were maintained in HuMEC Ready Medium (Thermo Fisher Scientific) containing bovine pituitary extract (BPE) at a concentration of 50 μg/mL (Thermo Fisher Scientific), HuMEC Supplement Kit (Thermo Fisher Scientific), and an antibiotic and an antimycotic (Thermo Fisher Scientific). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Cell Viability Assay
To determine cell viability, a PrestoBlue HS assay (Thermo Fisher Scientific) metabolic activity analysis was performed. The cells were plated in a 96-well plate at 1×104 cells per well and incubated for 24 hours. The culture medium was then removed, and dilutions of nanoparticles in a new medium were added at final concentrations of 0.1, 0.5, 1, 2, and 2.5 mg/l at 100 μL per well. The control group consisted of cells in a medium without the addition of nanoparticles. After 24 hours of incubation, the culture medium was removed and 100 μL of new culture fluid containing 10% PrestoBlue HS reagent was added. After two hours of incubation in a cell culture incubator, the fluorescence (excitation wavelength 560 nm, emission wavelength 590 nm) was measured using a microplate reader (Infinite M200, Tecan, USA). The study was conducted as three independent experiments with three replications.
Prior to the actual analysis of cell metabolic activity, the potential for interference of nanoparticles with the PrestoBlue assay (Thermo Fisher Scientific) was assessed. The interference test was performed in exactly the same way as the actual analysis but without the presence of cells. The results of the interference tests are presented in the Supplementary Figure S1A.
Membrane Perforation Analysis
To evaluate the integrity of the BME-UV1 and HMEC cell membranes, a CyQUANT Cell Proliferation Assay (Thermo Fisher Scientific) was used to determine the amount of lactate dehydrogenase (LDH) released from the cells. Cells were plated in a 96-well plate at a density of 1×104 cells per well, and then incubated for 24 hours. After incubation, the culture medium was removed and 100 μL per well of fresh medium containing 10% nanoparticles was added at final concentrations of 0.1, 0.5, 1, 2, and 2.5 mg/l. In the case of the BME-UV1 cells, in order to reduce interference by the LDH present in the FBS culture medium, the FBS content was reduced to 2%. The control group consisted of cells cultured in a medium without the addition of nanoparticles. After 24 hours of incubation, the culture plates were centrifuged; 50 μL of culture medium was transferred to a new 96-well plate and a reaction mixture was added. The plate was incubated for 20 minutes at room temperature in the absence of light. The reaction was then stopped by adding 50 μL of reaction-stopping solution per well. Absorbance was measured at 490 nm and 680 nm using a plate reader (Infinite M200, Tecan, USA). The absorbance value measured at 680 nm was subtracted from the value measured at 490 nm. The study was conducted as three independent experiments with three replications.
Prior to the actual analysis of membrane perforation, the potential for NDs to interfere with the assay was assessed. The interference test was performed in exactly the same way as the actual analysis but without the presence of cells. The results of the interference tests are presented in Supplementary Figure S1B.
Evaluation of the Morphology of BME-UV1 and HMEC Cells After Treatment with Nanoparticles
To obtain morphological images of the BME-UV1 and HMEC cell lines, 5×104 cells were transferred to 24-well plates. After 24 hours of incubation, nanoparticles were added to a culture media to produce a final concentration of 2.5 μg/mL. After 24 hours, cell morphology was analyzed using a CKX41 inverted light microscope (OLYMPUS, Japan) with a 20x phase-contrast objective.
Bacteria Cultures
Samples of mastitic milk were collected from farms from July 2021 to March 2022. The collected samples were submitted to standard microbiological procedures for initial multiplication and preliminary species identification: strains were isolated and then cultured on solid and liquid media. Specialized microbiological media (Biomaxima, Poland) were used to culture the strains: nutrient broth for general microbial culture, Mannitol Salt Lab-Agar, Edwards Lab-Agar with the addition of bovine blood, Chromogenic Uri-Color Lab-Agar, MacConkey Lab-Agar, Salmonella-Shigella Lab-Agar, Chromogenic Candida Lab Agar, Rose Bengal Lab-Agar, TBX Lab-Agar, Chromogenic Coliform Lab-Agar, LB Broth, Rogosa LS Lab-Agar, Reinforced Clostridial Medium, Legionella CYE Lab-Agar Base, Listeria acc., Oxford Lab-Agar Base, Enterococcus Confirmatory Lab-Agar, Eijkman Lactose Medium, Prototheca Isolation Medium according to Pore (1973),31 and Sabouraud dextrose agar (all from Argenta, Poland). Subsequently, the selected colony samples were subjected to identification on a MALDI-TOF MS (Bruker, Poznań, Poland).
Determination of the Survival of Microorganisms Isolated from Mastitic Milk Treated with Au, Ag, Pt, Cu, and Fe Nanoparticles and Their Complexes
The viability of microorganisms treated with Ag, Au, Cu, Fe, and Pt nanoparticles at selected concentrations was determined. For this purpose, cell suspensions with an optical density (OD) of 0.5 on the McFarland scale were prepared. The microorganism suspension, in a volume of 100 μL, was transferred to 96-well plates. Concentrated nanoparticle hydrocolloids were added to the wells to obtain the following nanoparticle concentrations (after mixing): 25, 12.5, 6.25, 3.125, 1.56 mg/l for Au, Ag, Cu and Fe, and 10, 5, 2.5, 1.25, 0.625 mg/l for Pt. The incubation of the microorganisms with nanoparticles was carried out for 24 hours at 37 °C. Then, 20 μL of tetrazolium salt solution was added to each well. The amount of formazan produced (spectrophotometric measurement at 450 nm) was compared to the control group, in which the microorganisms had not been treated with nanoparticles, and the percentage of cell viability in the test group was calculated on this basis. After analyzing the results, dual-nanoparticle complexes were prepared, which showed inhibitory effects against the tested microorganisms. An analogous analysis to that described above was carried out for the complexes.
Determination of Reactive Oxygen Species (ROS)
The detection of intracellular ROS was determined using DCFDA (2′,7′-Dichlorofluorescin Diacetate) (Cat. No. 287,810, Sigma, St Louis, MO, USA). The microbial strains were cultured in a Mueller–Hinton broth medium (BioMaxima, Lublin, Poland) in a shaking incubator at 37 °C overnight. The nanocomposites were prepared by dilution in deionized water (final concentration 12.5 μg/mL) and sonicated for 30 min before use. The inoculum of microorganisms (1.5 × 108 cells per well) with the tested nanoparticles (final volume 100 μL) was placed in 96-well plates and incubated at 37 °C for 2 hours. A DCFDA reagent was prepared according to protocol, and 10 μL of the mixture was added to each well. Shortly after, the fluorescence measurements were conducted with an excitation wavelength at 495 nm and an emission wavelength at 527 nm using an Infinite M200 microplate reader (Infinite M200, Tecan, Durham, NC, USA). The results were replicated a minimum of three times for each group.
Assessment of Microbial Invasion
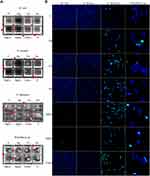
Beads were printed in a µ-Slide 8-well-chambered coverslip (Ibidi, Gräfelfing, Germany) with 6 µL of alginate with E. coli, S. aureus, C. albicans, or Prototheca sp. Alginate beads were crosslinked with 50 mM calcium chloride (Cellink, Göteborg, Sweden) for 1 minute. Subsequently, appropriate microbiologic media containing nanoparticles with a final concentration of 2.5 mg/l were added to the chambers and incubated for 48 hours. Media without nanoparticles were considered to be the control. After incubation, images of the chambered coverslip were made using a Scan 4000 colony counter (Interscience, Saint Nom la Brétèche France). For the confocal microscope analysis of biofilm formation after cell migration from alginate beads, each chamber was washed twice with phosphate-buffered saline (PBS) (Thermo Fisher Scientific) so that the alginate beads and non-adherent microorganisms were washed away. The dead cells were then stained with Image-IT DEAD Green Viability Stain (Thermo Fisher Scientific; final concentration 0.1 μg/mL) for 30 minutes. After washing with PBS, the dead and live cells were stained with Hoechst 33,342 for 15 minutes (Thermo Fisher Scientific; final concentration 5 μg/mL). Cells were imaged using a FV-1000 confocal microscope (Olympus Corporation, Tokyo, Japan) equipped with an incubation chamber that maintained a temperature of 37 °C (Solent Scientific Ltd, Portsmouth, United Kingdom). Determination of invasion of microorganism from the alginate gel bead after treatment with AuNPs, AgNPs, CuNPs, and their complexes, was assessed using turbidity analysis and confocal microscopy assessment of the produced biofilm.
Statistical Analysis
The results of the tests on the toxicity of Ag, Cu, Au, Pt, and Fe nanoparticles and their complexes on bovine and human mammary epithelial cells—BME-UV1 and HMEC (respectively)—were statistically processed using multivariate analysis of variance ANOVA using GraphPad Prism 8 software. Differences between groups were analyzed using Dunnett’s test. The results were expressed as mean values with standard deviations. Differences with a p-value of ≤0.05 were considered statistically significant.
The statistical analysis used in the study of the survival rate of microorganisms isolated from mastitic milk treated with Au, Ag, Pt, Cu, and Fe nanoparticles and their complexes was developed using one-way analysis of variance (ANOVA) using STATGRAPHICS® Centurion XVII software, version 17.2.05 (StatPoint Technologies, Inc., Warrenton, VA, USA).
Results
Morphology of the Nanoparticles Analyzed from TEM Images
Transmission electron microscope (TEM) images was analyzed to evaluate morphology of the nanomaterials and their complexes (Figure 1). All the nanoparticles had a spherical morphology and a size range of approximately 20–60 nm (AgNP), 10–50 nm (AuNPs), 10–20 nm (CuNPs), 10–70 nm (FeNPs), 5–100 nm (PtNPs).
Physicochemical Properties of Selected Nanoparticles and Their Complexes
Table 1 shows the zeta potential of the nanoparticles and complexes used, which allows the stability of the dispersion and its durability over time to be assessed. Of the tested nanoparticles, all had a negative zeta-potential, while the most negative potential was found for FeNPs and AgNPs (about −38 mV). The AgCuNPs and CuAuNPs complexes had a potential closest to 0 (−13.2 and −18.9 respectively), which may account for their poorer dispersion stability performance. All the values established for hydrodynamic diameter reached positive values: from 143.8 to 538 nm. The largest values were found for CuNPs (538 nm), while a slightly smaller value was obtained for the CuAuNP complex (400.6 nm). The smallest hydrodynamic diameter was obtained for AgNPs. The sizes of the nanoparticles used in the experiment are shown in Table 1. Small measurements were obtained for AgAuNP and AuNP complexes (up to 50–60 nm), as well as for CuNP (10–50 nm); however, the size of the nano-copper NP was difficult to determine due to impurities and poor quality. The AgCuNP and CuAuNP complexes reached the largest sizes (up to 100–120nm).
 |
Table 1 Zeta Potential (mV), Hydrodynamic Diameter (Nm), and Size (Nm) of the Used Nanoparticles and Complexes |
Nanoparticle Interference Study Using the PrestoBlue HS Test, and CyQUANT LDH Cytotoxicity Assay (Thermo Fisher Scientific)
The results of the interference test, with nanoparticles at concentrations of 0.1, 0.5, 1, 2, and 2.5 mg/l, are shown in Supplementary Figure S1. There was no significant observable interference between the nanoparticles and their complexes, and the PrestoBlue HS test for any of the tested concentrations. This indicated that this assay does not interfere with the results and could be used for the rest of the experiment with BME-UV1 and HMEC cells.
Testing the Viability and Cell Membrane Integrity of the BME-UV1 and HMEC Cells After Nanoparticle Treatment
The viability of BME-UV1 and HMEC cells was determined using a PrestoBlue HS assay that measured cell oxidoreductive activity. The results of cell viability after treatment with nanoparticles at concentrations of 0.1, 0.5, 1, 2, and 2.5 mg/L are shown in Figure 2, where A is the viability of the BME-UV1 cells and B is the viability of HMEC cells. Significant decreases in cell viability were observed for BME-UV1 and HMEC cells after treatment with copper nanoparticles at concentrations higher than 0.1 mg/L and for copper nanoparticle complexes (AgCuNPs, CuPtNPs, CuAuNPs, CuFeNPs). For other nanoparticles, toxicity to BME-UV1 cells did not occur or occurred only at the highest concentration tested, for example, for AgPtNP and AgFeNP complexes. For HMEC cell viability after treatment with Pt and Fe nanoparticles and their complexes, a toxicity of 10–15% was observed for the higher tested concentrations. For other nanoparticles, toxicity for either cell lines was not observed.
The integrity of cell membranes after the treatment of the epithelial BME-UV1 and HMEC cells with nanoparticles at concentrations of 0.1, 0.5, 1, 2, and 2.5 mg/l was assessed using an assay that measured the lactate dehydrogenase (LDH) enzyme secreted from the cells, an elevated level of which indicates cell damage. The results are shown in Figure 2, where C is the integrity of the BME-UV1 cell membrane and D is the integrity of HMEC cell membrane. In relation to the influence of nanoparticles on BME-UV1 cells, CuNPs and its complexes reduced LDH secretions at all concentration. A similar effect was observed for FeNPs and PtFeNPs. However, increased perforation of cell membranes occurred at a concentration of 0.1 mg/L with AgPtNP, AgAuNP, AgFeNP, and AuFeNP complexes. In contrast, in the case of the HMEC cell line, CuNPs and its complexes as well as FeNPs decreased LDH secretions. The higher concentrations tested (2 and 2.5 mg/l) for single AgNPs as well as for AgCuNP, AgAuNP, and AgFeNP complexes also resulted in a decrease in spontaneous LDH secretions. Increased perforation of cell membranes for this line was observed for concentrations of 0.1–0.5 mg/l for AgNPs, and 0.1mg/l for AuFeNPs complex.
Morphology Analysis of the BME-UV1 and HMEC Cells After Nanoparticle Treatment
The morphology of the BME-UV1 cells was evaluated after treating the cells with nanoparticles at a concentration of 2.5 mg/l. Images were taken at 200x magnification. The images are shown in Supplementary Figure S2. The morphology of the cells after treatment with nanoparticles did not change; however, an increase in the number of rounded cells was observed for the cells that were treated with AgNPs or its complexes, indicating potential toxicity. HMEC cells were also treated with nanoparticles at a concentration of 2.5 mg/L, and their morphology was evaluated by taking images at 200x magnification. The morphology of the cells did not change after treatment with nanoparticles, as shown in Supplementary Figures S2 and S3.
Identification of Selected Pathogens
The identification of cultured pathogens was carried out using a MALDI-TOF instrument (Bruker, Poznan, Poland), which compares the molecular weight of biomolecules in the sample to a database, allowing the species or type of microorganism present in the sample to be determined. The results of the identification process are presented in Supplementary Table S1.
Viability Analysis of the Microorganisms Isolated from Mastitic Milk Treated with Au, Ag, Pt, Cu, and Fe Nanoparticles
Among the tested nanoparticles, AgNPs exhibited the strongest antibacterial properties as shown in Table 2. S. uberis were the most susceptible bacteria to its effect: for concentrations above 6.25 mg/l the survival rate was 0%. The same results were obtained using a concentration of 25 mg/l against S. marcescens and C. koseri. Though S. sciuri were the most resistant bacteria to the influence of nano-silver, even for the lowest concentration (1.56 mg/l) of applied nanoparticles the survival rate decreased to 16%. Slightly weaker results were observed for CuNPs, with S. sciuri being most susceptible to their effects. Gold nanoparticles were less effective than AgNPs and CuNPs. The greatest resistance to AuNPs’ action was shown by S. sciuri, where, for the highest concentration, the survival rate was still 66%. E. coli was also resistant to AuNP concentrations of 1.56–12.5 mg/l, having no effect or slightly reducing survival by a maximum of 3%; while at the highest concentration of 25 mg/l, survival dropped by as much as 83%. The most sensitive bacteria to nano-gold were S. aureus, where even the lowest concentration (1.56 mg/l) reduced their survival rate by 74%. S. uberis, on the other hand, exhibited resistance to AuNP concentrations of 1.56–3.125 mg/l, but for higher concentrations than 6.25 mg/l, the survival rate was 0%. PtNPs and FeNPs showed no or minor antibacterial properties (the maximum reduction in survival rate was 9%) even for the highest concentrations; therefore, they were excluded from the next part of the experiment, in which nanoparticle complexes were used.
 |
Table 2 Viability Analysis of the Bacteria, Fungi, and Algae Isolated from Mastitic Milk Treated with Au, Ag, Pt, Cu, and Fe Nanoparticles |
In the case of mastitic fungi, PtNPs mainly showed no or minor biocidal properties; however, for a concentration of 10 mg/l the survival rate of C. glabrata decreased by 61%. C. albicans were most susceptible to FeNPs at a concentration of 25 mg/l, reducing their survival rate by 75%. For the remaining concentrations’ effectiveness on the isolated fungi and algae, no reduction in survival greater than 8% was observed after the application of FeNPs and PtNPs. Poor bioactive properties were also shown by AuNPs. The most resistant fungi to its effects were C. albicans, where a reduction in survival was observed for concentrations of 1.56 and 25 mg/l. C. parapsilosis were the most sensitive fungi to nano-gold, while the 25 mg/l concentration reduced the survival of C. glabrata by up to 62%, which was the greatest decrease in viability observed after the application of this nanoparticle. Slightly higher antifungal properties were exhibited by CuNPs, especially against C. glabrata, C. parapsilosis, and Prototheca sp., in which their viability decreased by 23–34% after applying the lowest concentration (1.56 mg/l), while for the highest concentration (25 mg/l), a decrease in survival to 2.5–12% was observed. The strongest antifungal properties were exhibited by AgNPs, and the most sensitive fungus to these nanoparticles were Prototheca sp., where a concentration of 1.55 mg/l decreased survival rate to 21%, while, when the highest tested concentration of 25 mg/l was applied, survival fell to 0%. The most resistant fungus to AgNPs were C. albicans; however, the biocidal properties of the nanoparticles were only observed for concentrations higher than 6.25 mg/l, decreasing viability by 95%.
Viability Analysis of Microorganisms Isolated from Mastitic Milk Treated with Au, Ag, and Cu Nanoparticle Complexes
Among the tested nanoparticle complexes presented in Table 3, CuAuNPs showed the lowest biocidal properties. E. coli, S. marcescens, and C. koseri were the most resistant bacteria to the effect of this complex, where the tested concentrations showed biocidal activity above 6.25 mg/l. Concentrations of 0.78–3.125 mg/l for these pathogens showed no or minor antibacterial properties, and the maximum decrease in survival rate reached 12% for S. marcescens. The most sensitive bacteria to CuAuNPs were S. sciuri and S. aureus, in which survival rates at the highest concentration were reduced to 1.5–1.8%. Slightly superior antibacterial properties were characterized by the AgAuNP complex, which fully destroyed the bacterial cells of S. uberis at concentrations 6.25–12.5 mg/l. Also susceptible to this complex were E. coli, which, for the highest concentration of nanoparticles, had a survival rate of 1%, and also S. aureus, with a survival rate of 1.7%. However, the most resistant bacteria were C. koseri, for which the survival rate decreased by only 6% for the lowest concentration (0.78 mg/l), while for 12.5 mg/l it reached 7.6%, which is comparable to the other results. The strongest biocidal properties were demonstrated by the AgCuNP complex, which at the lowest concentration of 0.78 mg/l reduced the survival rate of pathogens such as S. uberis, S. sciuri, E. coli, and S. aureus by 95–97%. S. uberis, for concentrations of 6.25–12.5 mg/l for this complex showed 0% survival, as did S. marcescens for concentrations of 12.5mg/l. C. koseri proved to be the most resistant bacteria for the lowest concentration (0.78 mg/l), however, the survival rate nevertheless decreased by about 60% after using this concentration.
 |
Table 3 Viability Analysis of the Bacteria, Fungi, and Algae Isolated from Mastitic Milk Treated with Au, Ag, and Cu Nanoparticle Complexes |
The results of the viability analysis of fungi and algae treated with nanoparticle complexes were similar to the results obtained from the nanoparticle complexes used against mastitic bacteria. The weakest antifungal activity was exhibited by the CuAuNP complex. The most resistant fungi were C. parapsilosis, where the application of the highest concentration (12.5 mg/l) reduced survival by 13%. Although C. albicans were resistant to this complex, applying the highest concentration led to a decrease in survival rate to 2.58%. C. glabrata were the most sensitive of the fungi to CuAuNPs, where the lowest concentration reduced their survival rate by 50%, while the highest concentration caused an 80% reduction. The fungi that was most sensitive to the AgAuNP and AgCuNP complexes were Prototheca sp., where the highest concentration of 12.5 mg/l resulted in overall cell death. For the other pathogens, as the concentration of the AgAuNPs increased, it exhibited stronger antifungal properties. Of these pathogens, C. parapsilosis showed the greatest resistance to this most active complex, but nevertheless, showed a 95% decrease in viability at the highest concentration.
Determination of Reactive Oxygen Species (ROS)
Figure 3 shows the level of reactive oxygen species (ROS) after the application of AgCuNP, AgAuNP, and CuAuNP complexes. The highest number of reactive oxygen species analyzed across all tested bacteria and the yeast C. albicans, was observed after adding the AgCuNP complex to the culture medium. It was this complex that showed the strongest biocidal properties in reducing the survival rate of the tested pathogens. In the case of other tested microorganisms, such as C. parapsilosis, C. glabrata, and Prototheca sp., the greatest increase in the amount of ROS was observed after the application of the AgAuNP complex.
Assessment of Microbial Invasion After Treatment with AgNPs, AuNPs, CuNPs, and Their Complexes
The invasion of E. coli, S. aureus, C. albicans, and Prototheca sp. was assessed with an alginate bead model in which the beads were bioprinted. Determination of invasion of microorganism from the alginate gel bead after treatment with AgNPs, AuNPs, CuNPs, and their complexes was assessed using turbidity analysis and confocal microscopy assessment of the biofilm produced. Turbidity analysis, which also shows alginate beads, demonstrated that, in the case of E. coli and S. aureus, invasion was inhibited by AgNPs and AgNP complexes (AgCuNPs, AgAuNPs) (Figure 4A). Biofilm analysis, which was carried out using a confocal microscope after the staining of live and dead cells, confirmed the effectiveness of the AgNPs and AgNP complexes in inhibiting invasion in the case of E. coli and S. aureus (Figure 4B). In addition, in the case of E. coli, an increase in the number of dead cells was observed after the use of CuNPs, AuNPs, and the CuAu complex. Concerning S. aureus, the same nanoparticles led to a reduction in the number of cells in the biofilm that had been formed. Treating C. albicans with nanoparticles significantly increased the resulting number of dead cells, which was particularly apparent in the cases of CuNPs and AuNPs, and their complexes. Furthermore, AgNPs and AgNP complexes, as well as the CuAu complex, increased the number of dead Prototheca sp. cells in the biofilm.
Discussion
In studies evaluating the biocidal effectiveness of nanoparticles against pathogens, in the context of their potential use in practical applications, it is essential to determine not only their effect on pathogen survival but also to assess the survival of cells—in this case, bovine BME-UV1 cells and human mammary epithelial cells (HMEC). This is a crucial issue, as highly toxic nanoparticles in high concentrations could lead to cell death, damaging animals’ and humans’ health. For this reason, this study was conducted to determine the effects of Ag, Au, Cu, Fe, and Pt NPs, and their complexes, against cells that would be exposed to nanoparticles in practical use. The study showed that, indeed, certain nanoparticles exhibit toxicity against BME-UV1 bovine cells, especially CuNPs in concentrations above 0.1 mg/l. All tested complexes containing copper nanoparticles acted similarly. AgPtNP and AgFeNP complexes were also toxic, however only at the highest concentration—2.5mg/l. Cu nanoparticles also showed toxicity to human HMEC cells, at the same concentration observed for BME-UV1 bovine cells. The available literature is limited with respect to such studies; while there are reports presented by Ahmed et al,32 stating that AuNPs show toxicity to a variety of tissues from mammalian cells, this result was not actually confirmed by the authors’ performed analyses. The toxicity of gold nanoparticles against human cells was also tested by Connor et al.33 The results obtained by these authors showed a lack of cytotoxicity against the cells, which is consistent with the results obtained in the current experiment.
The cell-membrane integrity assay determines the level of the enzyme lactate dehydrogenase (LDH), which increases with cell damage. In the case of the BME-UV1 cells, CuNPs and all of the Cu complexes decreased LDH secretions; however, elevated levels of this enzyme were observed for AgPtNPs, AgAuNPs, AgFeNPs, and AuFeNPs. In a study by Kalinska et al, elevated levels of LDH were observed after treatment with AgNPs and AgCuNPs,28 which is partially consistent with the results obtained here. In the current study, analysis conducted on the HMEC cell line showed that increased cell perforation occurred after the application of low concentrations of AgNPs and AuFeNPs. The results obtained by Kalinska et al only confirmed increased leakage for 0.5mg/l AgNPs.28 In recent years, due to the growing problem of antibiotic resistance, there has been a growing interest in new antibacterial agents based on metal nanoparticles and metal ions. The literature also shows studies on the antibacterial effect of some new metal chelates containing Cu(II), VO(II), Pd(II), Fe(III), Zn(II), and Mn(II) ions. Zones of growth inhibition were observed after administration of the complexes in various microorganisms, both gram-positive and gram-negative bacteria such as E. coli, B. subtilis, S.marcescens, M. luteus.34,35
The survival of mastitic pathogens was studied by Wernicki et al.36 Among the pathogens isolated by these authors were E. coli, S. aureus, S. uberis, C. albicans, and C. crusei. In their research, the authors tested the biocidal properties of Au, Ag, Pt and Cu NPs. Their study showed that the applied nanoparticles’ strongest biocidal properties were exhibited by AgNPs and CuNPs against all pathogens, which is consistent with the results obtained in the current experiment. In these authors’ study, AgNPs at a concentration of 12.5 mg/l inhibited the growth of all pathogens, while concentrations above 6.25 mg/l inhibited the growth of S. aureus and S. uberis. In contrast, in the current study, a concentration of 12.5 mg/l resulted in the survival rate of S. aureus being reduced by 86%. In the case of S. uberis, its survival rate was reduced to 0 even at the lower concentration of 6.25 mg/l, in comparison to S. aureus, in the studies by Wernicki et al.36 E. coli viability decreased to 5% for a concentration of 12.5 mg/l AgNPs. The study by Wernicki et al showed that 25 mg/l CuNPs fully inhibited the growth of all pathogens. Results obtained by the authors of the current study showed that these nanoparticles, at the same concentration of 25 mg/l, reduced the survival of pathogens by 86–98%. According to these authors, AuNPs showed weaker antimicrobial activity in comparison to AgNPs and CuNPs, which is consistent with the current study’s obtained results. PtNPs showed the weakest biocidal properties in the study by Wernicki et al,36 while the current study’s results showed that PtNPs not only showed poor biocidal properties, only causing a decrease in pathogen viability at the highest tested concentration (10 mg/l), but also, in some cases, showed no biocidal properties at all. A study by Dehkordi et al37 showed that 10 µg/mL AgNPs inhibited the growth of S. aureus by 90%. A study carried out by Abdel-Rahman et al38 also indicated the antibacterial properties of AgNPs against S. aureus, B. subtilis, S. marcescens, E. coli; however, used NPs were synthesized with biogenic method using extract of Moringa Oleifera.
In the current study, a concentration of 12 mg/l of AgNPs was tested on S. aureus: the survival rate was reduced by 86%; thus the values are similar to those obtained by Dehkordi et al.37 Tests on AgNPs suspended in gel conducted by Jain et al39 also showed biocidal properties against E. coli, S. aureus, and also C. albicans at 12.5 μg/mL. The authors reported that gram-negative bacteria were more susceptible to the NPs’ effects than gram-positive bacteria. Nanoparticles exhibit antifungal activity against C. glabrata and C. parapsilosis as proven by Mekky et al.40 Mastitis caused by Prototheca sp. is a particular problem in dairy herds due to its high resistance to all disinfectants. A study conducted by Ely et al41 suggested that AgNPs may provide a solution in the control of this algae, and this was in accordance with the results obtained in the current study, since the survival rate of Prototheca sp. for these nanoparticles at a concentration of 25 mg/l, decreased to 0, which demonstrates their strong biocidal properties. Similar results were observed for NP complexes of AgCu and AgAu. Nanoparticles have the ability to interact synergistically, allowing their complexes to exhibit stronger biocidal properties at lower concentrations than the higher concentrations of single nanoparticles.28 This theory was confirmed in the studies by Kot et al,42 as well as those performed by Lange et al,27 and is also consistent with the results obtained in the current study. One of the main purposes of the conducted research, was to determine the biocidal properties of nanoparticles against mastitic pathogens. However, the literature also indicates that there are other nanostructures such as metal ions that exhibit antimicrobial, antifungal and anticancer properties, which may also provide a new solution for combating pathogens.43,44
Conclusion
There is a growing number of research reports exploring alternative substances for the prevention and treatment of bovine udder inflammation caused by bacteria, fungi and even algae, or a combination of these etiological factors, which may find application in dairy cattle. One of the most promising approaches is to target research toward nanotechnology due to their biocidal properties. In vitro experiments have shown that silver, copper, and gold nanoparticles, and their complexes, exhibit no toxicity to bovine or human cells while showing the greatest biocidal potential against pathogens causing mastitis in cattle. AgCuNP concentrations of 6.25 and 12.5 mg/l have been shown to inhibit the proliferation of mastitis pathogens such as S. uberis, E. coli, C. albicans, and Prototheca sp. by up to 100%. Candida sp. and Prototheca sp. are pathogens that are difficult or impossible to control with antibiotics in herds. Mastitis induced by Prototheca sp. currently has no developed treatment; therefore, the obtained results have the potential for global innovation; however, it is necessary to test the biocidal properties of nanoparticles under field conditions. A bacterial cell inhibition of 98% was observed for S. aureus, an infectious indicator pathogen of mastitis. Additionally, this indicates the high potential of these solutions under field conditions, as this bacterium is one of the most difficult to treat, especially in dairy cows, due to its resistance to the most common antibiotics. The biocidal properties of selected nanomaterials have proven to be active agents for mastitis prevention but are harmless to bovine and human cells. The results suggest that they could be used in practical applications by breeders worldwide; however, it is necessary to test the biocidal properties of nanoparticles in vivo.
Data Sharing Statement
Data are available upon request from the authors.
Funding
Financial support for this study was obtained from project nr. POIR.01.01.01-00-2127/20-00, entitled “NanoCow—Development and Implementation of a Line of Products for the Prevention of Mastitis and Lameness Against Bacterial and Fungal Infections with the Use of Nanomaterials” provided by the National Centre for Research and Development. The publication was cofinanced by Science development fund of the Warsaw University of Life Sciences - SGGW.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fogsgaard KK, Røntved CM, Sørensen P, Herskin MS. Sickness behavior in dairy cows during Escherichia coli mastitis. J Dairy Sci. 2012;95:630–638. doi:10.3168/jds.2011-4350
2. Cheng WN, Han SG. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas J Anim Sci. 2020;33:1699–1713. doi:10.5713/ajas.20.0156
3. Ranjan R, Swarup D, Patra RC, Nandi D. Bovine protothecal mastitis: a review. CAB Rev. 2015;1. doi:10.1079/PAVSNNR20061017
4. Adeyemi OS, Sulaiman FA. Evaluation of metal nanoparticles for drug delivery systems. J Biomed Res. 2015;29:145–149. doi:10.7555/JBR.28.20130096
5. Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S. Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol. 2016;42:46–56. doi:10.1128/am.26.4.648-649.1973
6. Cruz-Luna AR, Cruz-Martínez H, Vásquez-López A, Medina DI. Metal nanoparticles as novel antifungal agents for sustainable agriculture: current advances and future directions. J Fungi. 2021;7:1033. doi:10.1016/j.molstruc.2021.131017
7. Benelli G. Gold nanoparticles–against parasites and insect vectors. Acta Trop. 2018;178:73–80. doi:10.1016/j.actatropica.2017.10.021
8. Rai M, Ingle AP, BirlaS Y, Santos CAD. A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev Microbiol. 2016;42:696–719. doi:10.3109/1040841X.2015.1018131
9. Al-Farraj ES, Alahmadi M, Mohamed WS, Alsaedi WH, Abu-Dief AM. Development of VSe2@ Cu2Se nano-composites via facile one-pot hydrothermal method for pharmaceutical applications. Phys Scr. 2023;98:095004. doi:10.1088/1402-4896/aceada
10. Dhanjal DS, Mehra P, Bhardwaj S, et al. Mycology-nanotechnology interface: applications in medicine and cosmetology. Int J Nanomed. 2022;17:2505–2533. doi:10.2147/IJN.S363282
11. Abu-Dief AM, Alrashedee FM, Emran KM, Al-Abdulkarim HA. Development of some magnetic metal–organic framework nano composites for pharmaceutical applications. Inorg Chem Commun. 2022;138:109251. doi:10.1016/j.inoche.2022.109251
12. Khezerlou A, Alizadeh-Sani M, Azizi-Lalabadi M, Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb Pathog. 2018;123:505–526. doi:10.1016/j.micpath.2018.08.008
13. Bashal AH, Khalil KD, Abu-Dief AM, El-Atawy MA. Cobalt oxide-chitosan based nanocomposites: synthesis, characterization and their potential pharmaceutical applications. Int J Biol Macromol. 2023;253:126856. doi:10.1016/j.ijbiomac.2023.126856
14. Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262. doi:10.1016/j.nano.2009.07.002
15. Kowshik M, Ashtaputre S, Kharrazi S, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95. doi:10.1088/0957-4484/14/1/321
16. Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170.
17. Armendariz V, Herrera I, Peralta-Videa JR, et al. Size controlled gold nanoparticle formation by avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res. 2004;6:377–382.
18. Ekrikaya S, Yilmaz E, Celik C, et al. Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards enterococcus faecalis and candida albicans. J Biotechnol. 2021;341:155–162. doi:10.1016/j.jbiotec.2021.09.020
19. Abu‐Dief AM, Abdel‐Rahman LH, Abd‐El Sayed MA, Zikry MM, Nafady A. Green synthesis of AgNPs() ultilizing Delonix regia extract as anticancer and antimicrobial agents. ChemistrySelect. 2020;5:13263–13268. doi:10.1002/slct.202003218
20. Koca FD, Demirezen Yilmaz D, Duman F, Ocsoy I. Comparison of phytotoxic effects of bio-synthesised copper oxide nanoparticle and ionic copper on elodea canadensis. Chem Eco. 2018;34:839–853. doi:10.1080/02757540.2018.1494162
21. Ceylan R, Demirbas A, Ocsoy I, Aktumsek A. Green synthesis of silver nanoparticles using aqueous extracts of three sideritis species from Turkey and evaluations bioactivity potentials. sustain. Chem Pharm. 2021;21:100426. doi:10.1016/j.scp.2021.100426
22. Kumar H, Venkatesh N, Bhowmik H, Kuila A. Metallic nanoparticle: a review. Biomed J Sci Tech Res. 2018;4:3765–3775. doi:10.26717/BJSTR.2018.04.001011
23. Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020;13:223–245. doi:10.1080/17518253.2020.1802517
24. Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi:10.1016/j.cis.2010.02.001
25. Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7:doi:10.1002/adhm.201701503
26. Radzikowski D, Kalińska A, Ostaszewska U, Gołębiewski M. Alternative solutions to antibiotics in mastitis treatment for dairy cows - a review. Anim Sci Pap Rep. 2020;38:117–133.
27. Lange A, Grzenia A, Wierzbicki M, et al. Silver and copper nanoparticles inhibit biofilm formation by mastitis pathogens. Animals. 2021;11:1884. doi:10.3390/ani11071884
28. Kalińska A, Jaworski S, Wierzbicki M, Gołębiewski M. Silver and copper nanoparticles—an alternative in future mastitis treatment and prevention? Int J Mol Sci. 2019;20:1672. doi:10.3390/ijms20071672
29. Lange A, Sawosz E, Wierzbicki M, et al. Nanocomposites of graphene oxide—silver nanoparticles for enhanced antibacterial activity: mechanism of action and medical textiles coating. Materials. 2022;15(9):3122. doi:10.3390/ma15093122
30. Lange A, Sawosz E, Daniluk K, et al. Bacterial surface disturbances affecting cell function during exposure to three-compound nanocomposites based on graphene materials. Nanomaterials. 2022;12(17):3058. doi:10.3390/nano12173058
31. Pore RS. Selective medium for the isolation of Prototheca. Appl Microbiol. 1973;26(4):648–649. doi:10.1128/am.26.4.648-649.1973
32. Ahamed M, AlSalhi MS, Siddiqui MKJ. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411(23–24):1841–1848. doi:10.1016/j.cca.2010.08.016
33. Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi:10.1002/smll.200400093
34. Aljohani ET, Shehata R, Abu‐Dief AM. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+, and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. App Organomet Chem. 2021;35:e6169. doi:10.1002/aoc.6169
35. Abu-Dief AM, El-khatib RM, El Sayed SM, et al. Tailoring, structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn (II), Cu (II) and Fe (III) complexes. J Mol Struct. 2021;1244:131017. doi:10.1016/j.molstruc.2021.131017
36. Wernicki A, Puchalski A, Urban-Chmiel R, et al. Antimicrobial properties of gold, silver, copper and platinum nanoparticles against selected microorganisms isolated from cases of mastitis in cattle. Med Weter. 2014;70:564–567.
37. Dehkordi SH, Hosseinpour F, Kahrizangi AE. An in vitro evaluation of antibacterial effect of silver nanoparticles on staphylococcus aureus isolated from bovine subclinical mastitis. Afr J Biotechnol. 2011;10:10795–10797. doi:10.5897/AJB11.1499
38. Abdel-Rahman LH, Al-Farhan BS, Abou El-ezz D, Abd–El Sayed MA, Zikry MM, Abu-Dief AM. Green biogenic synthesis of silver nanoparticles using aqueous extract of moringa oleifera: access to a powerful antimicrobial, anticancer, pesticidal and catalytic agents. J Inorg Organomet Polym. 2022;32:1422–1435. doi:10.1007/s10904-021-02186-9
39. Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol Pharm. 2009;6:1388–1401. doi:10.1021/mp900056g
40. Mekky AE, Farrag AA, Hmed AA, Sofy AR. Antibacterial and antifungal activity of green-synthesized silver nanoparticles using Spinacia oleracea leaves extract. Egypt J Chem. 2021;64:5781–5792. doi:10.21608/EJCHEM.2021.74432.3673
41. Ely VL, Pereira DIB, da Costa MM, et al. Activity of biogenic silver nanoparticles against isolates of prototheca species from bovine mastitis. Lett Appl Microbiol. 2022;75:24–28. doi:10.3168/jds.2011-4350
42. Kot M, Kalińska A, Jaworski S, Wierzbicki M, Smulski S, Gołębiewski M. In vitro studies of nanoparticles as a potentially new antimicrobial agent for the prevention and treatment of lameness and digital dermatitis in cattle. Int J Mol Sci. 2023;24:6146. doi:10.1016/j.micpath.2018.08.008
43. Abu-Dief AM, El-Khatib RM, Aljohani FS, et al. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J Mol Struct. 2021;1242:130693. doi:10.5713/ajas.20.0156
44. Mohamad ADM, Abualreish MJA, Abu-Dief AM. Antimicrobial and anticancer activities of cobalt (III)-hydrazone complexes: solubilities and chemical potentials of transfer in different organic co-solvent-water mixtures. J Mol Liq. 2019;290:111162. doi:10.1002/adhm.201701503
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.