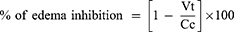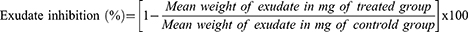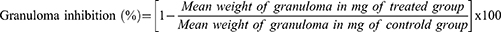Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of Anti-Inflammatory Activity of the Methanol Extracts of Premna schimperi Engl (Lamiaceae) Leaves in Rats
Authors Arega M , Nardos A , Debella A , Dereje B , Terefe L , Abebe A
Received 9 September 2023
Accepted for publication 9 November 2023
Published 14 November 2023 Volume 2023:15 Pages 437—447
DOI https://doi.org/10.2147/JEP.S432615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Melese Arega,1 Aschalew Nardos,2 Asfaw Debella,3 Beyene Dereje,4 Lidet Terefe,2 Abiy Abebe3
1Department of Pharmacy, Pawi Health Sciences College, Pawi, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 3Department of Traditional and Modern Medicine Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 4Department of Pharmacology, School of Medicine, College of Medicine and Health Sciences, Dire Dawa University, Dire Dawa, Ethiopia
Correspondence: Melese Arega, Email [email protected]
Background: Even though it is a protective reaction, inflammation continues to be one of the most challenging medical disorders. The current conventional anti-inflammatory drugs have many undesirable health effects and are in need of newer drugs. The purpose of this study was to evaluate the anti-inflammatory effects of an aqueous methanol crude extract of Premna schimperi leaves.
Methods: Premna schimperi leaf was extracted with 80% methanol and concentrated; the concentrated extract was used to evaluate the acute toxicity and anti-inflammatory effects. For the acute toxicity study, a single dose of Premna schimperi extract at a dose of 2000 mg/kg was administered and observed for 14 days. Acute, sub-acute, and chronic anti-inflammatory models were employed to evaluate the anti-inflammatory effect of the extract compared to the standard drug. Data were analyzed with SPSS V. 27, and the significance was established with a one-way ANOVA followed by a post hoc Tukey’s test.
Results: Acute oral toxicity testing at a dose of 2000 mg/kg did not show any sign of toxicity. According to the phytochemical study, the plants contained flavonoids, terpenoids, tannins, cardiac glycosides, steroids, phenolics, and anthraquinones. The extract doses of 200 mg/kg, 400 mg/kg, and 800 mg/kg of extracts effectively (p< 0.001) reduced paw edema in the acute and sub-acute models of inflammation. When compared to the negative control group, all tested doses in the chronic model significantly (p< 0.05) decreased the production of exudates and the amount of granuloma tissue.
Conclusion: Premna schimperi displayed significant anti-inflammatory activity. The tested doses inhibit the formation of edema, granulomas, and exudates.
Keywords: anti-inflammation, carrageenan, indomethacin, paw edema, Premna schimperi
Background
Inflammation is a body’s preventive response that produces redness, heat, edema, pain, and loss of function as a result of infectious, chemical, and physical agents.1 Hemostasis, inflammation, proliferation, and remodeling or maturation are four distinct and highly planned processes by which tissues in the body can heal, either through regeneration or repair mechanisms.2
Serious inflammatory conditions that can worsen if inflammation is left untreated include reperfusion injury, hypersensitivities, rhinitis, atherosclerosis, rheumatoid arthritis, asthma, autoimmune disease, chronic inflammation, glomerulonephritis, inflammatory bowel disease, pelvic inflammatory disease, and glomerulonephritis. They are considered the major clinical, social, and economic problems in most communities around the world.3
More than half of all deaths are now thought to be caused by chronic inflammatory disorders, which are now regarded as the leading cause of death worldwide.4
Around the world, NSAID prescriptions for various inflammatory diseases total up to 100 million per year. Today’s NSAIDs and corticosteroids have side effects that limit their therapeutic use, including gastro esophageal reflux disease, peptic ulcer disease, osteoporosis, iatrogenic Cushing’s syndrome, and cardiovascular adverse effects.5,6
In studies on the adverse effects of NSAIDs, upper gastrointestinal bleeding was found to occur 5.5 times more frequently in NSAID users than in non-users. Increased NSAID doses, especially ibuprofen, diclofenac, and piroxicam, increase the risk of bleeding. Along with the gastroduodenal mucosal injury, NSAIDs have the potential to harm the distal portions of the small and large intestines. Additionally, NSAID medications may raise the chance of lower gastrointestinal hemorrhage and perforation.7,8 Despite the availability of adequate medications, inflammation and pain continues to be one of the most difficult and devastating health problems, impacting 80% of the adult population worldwide.3
Various plants are traditionally used in Ethiopia to cure conditions that involve pain, such as headaches, stomachaches, inflammation, and wounds.9,10 One plant from the genus Premna that has been utilized for a variety of therapeutic purposes in Ethiopia is Premna schimperi. Studies on its leaf extracts’ anti-leishmanial, anti-plasmodial, and anti-microbial properties have been conducted.11,12 In folkloric medicines, it has been used to treat inflammatory issues, but to date, no further research has been recorded to validate this folkloric usage against inflammation, and no scientific investigations have been done to support this traditional claim for treatment of different inflammatory disorder.13–16 The current study aims to evaluate Premna schimperi leaf crude 80% methanol extract’s anti-inflammatory activity.
Methods and Materials
Equipments
Electronic weighing balance (Kern-Alj 220–4, Germany), mini orbital shaker (SSM1-STUART), rotary evaporator (Heidolph, Germany), oven (Medite Medizintechnik, Germany), plethysmometer (Ugo Basile-Cat no. 7140, Italy), syringe with needles, oral gavage, silk round-bodied (0/4 round −1/2 Circle), Whatman filter paper no. 1, gloves, mortar and pestle, round flask, erlenmeyer conical flask, measuring cylinder, water bath, beaker, surgical blade, and cotton.
Drugs and Chemicals
Methanol (Carlo Erba, Italy), indomethacin (Cadila, Ethiopia), normal saline (Addis Pharmaceutical Factory, Ethiopia), Tween 80 (Sigma Aldrich, Germany), Dragendorff reagents (Fisher Scientific, UK), glacial acetic acid (Basell, India), chloroform (Bulex Laboratory, India), 10% ammonium (BDH Laboratory Supplies Poole, England), formalin (Research-Lab Fine Chem Industries, India), carrageenan (Sigma Aldrich, Germany), thiopental sodium (NEON Labs, India), distilled water, 2% sodium hydroxide, 10% lead acetate, 10% sodium hydroxide, concentrated sulfuric acid, and ferric chloride.
Study Design and Sample Size Determination
In the experiment involving lab animals, randomization was employed to determine treatment groups, exposure to test changes, placement within an environment, and the sequencing of measurements. G-power analysis was used to determine the sample size (n = 6).
Collection and Approval of Plant Materials
Premna schimperi Engl (Lamiaceae) leaves were collected in January 2023 from Yabelo Woreda in the Oromia region of Ethiopia. Mr Melaku Wondafrash (Botanist) authenticated the plant material at Addis Ababa University’s College of Natural Sciences and Addis Ababa National Herbarium with voucher number MA-001.
Preparation of Crude Extracts
The plant’s crude extract was extracted using an 80% methanol solvent (1:5 w/v) by macerating it at room temperature for 72 hours while occasionally shaking it to ensure that all of the soluble material had dissolved. The mixture was filtered using Whatman No. 1 filter paper. The process described above was used to extract the marc twice more consecutively, for a total of nine days.17,18
After thorough filtration, the methanol solvent was evaporated in a rotary evaporator at 40°C under decreased pressure to produce an 80% methanol extract. After evaporation, the raw extract was dried in a 40°C oven. Until they were needed, the dried extract products were stored in tight containers in a deep freezer.18
Study Animals
A total of 110 Wistar albino rats weighing 200–300 g and 8–12 weeks old of either sex were used during the experiment.19,20 The animals were raised and supplied by the Ethiopian Public Health Institute. Rats were housed in plastic cages. Rodent pellets made up the rats’ diet, and they had unrestricted access to water. 22 ± 2°C temperature and 50–60% relative humidity were maintained. There were 12 hours of artificial light and 12 hours of complete darkness. The OECD’s 2008 and Arrive recommendations were followed when handling and caring for the animals. The week before the trial, the animals were acclimated.21,22
Experimental Units
Rats were randomly divided into five groups of six rats, consisting of three test groups, one positive control group, and one negative control group, which were drawn from the sample size determined using G-power analysis.
Grouping and Dosing of Animals
Five groups of six rats each were allocated at random from the total number of rats. The fifth group of randomly chosen rats serves as the negative control and is given Tween 80 (10 mL/kg). The first, second, and third groups received 200mg/kg, 400mg/kg, and 800mg/kg of 80% methanol crude extracts, respectively. The fourth group received indomethacin 10mg/kg as the positive control group. All of the therapies were administered orally by oral gavage. The animal was given thiopental sodium (25mg/kg) to alleviate its pain. Rats were sacrificed via cervical dislocation at the end of the experiment.12,23
Acute Oral Toxicity Test
Based on the limit test requirements of OECD/OCDE Guideline 425, an acute oral toxicity test was conducted.21 Prior to giving a dose of the extract containing 2000 mg/kg to single, nulliparous, and non-gestational female rats, they were fasted for four hours. Following the administration of the extracts, food was limited for an additional 4 hours. Following that, the rat was kept under close observation for the next 24 hours, with a particular focus on the first four hours, for a total of 14 days. Before giving the extract in a single dose of 2000mg/kg to the remaining four female rats, they were starved for four hours. They underwent the same inspection as the previous rats. The following 14 days were spent observing the rats for any signs of toxicity. Changes in somatomotor activity, behavioral patterns, and the respiratory, circulatory, autonomic, and central nervous systems were among the factors that were observed.21
Carrageenan-Induced Paw Edema
The reduction of carrageenan-induced rat hind paw edema served as the foundation for measuring the anti-inflammatory effect in vivo. Before being employed in the tests, rats were fasted for 12 hours while having unlimited access to water. Five groups of both sexes of rats, each with six rats, were employed in this experiment. The right hind paw was painted with ink at the level of the lateral malleolus. To ensure it could always be immersed to the same depth in the measuring chamber of the plethysmometer.23
The extracts were administered to each study group one hour before formalin injection on the 1st and 3rd days and administered for 7 consecutive days once daily. Carrageenan in 0.1 mL of 1% sodium chloride solution was injected into the right hind paw’s plantar side. The paw volume was measured using a plethysmometer at 0, 1, 2, 3, 4, and 5 hours after inducing inflammation by carrageenan, and the basal volume was taken before inducing inflammation. A different dose of the extract and tween 80, as well as Indomethacin 10mg/kg, were administered 1 hour prior to the injection of carrageenan.23
The percentage inhibition of inflammation was calculated as follows:
- Vt and Vc are the mean edema volumes in the treated and control groups, respectively.
Formalin-Induced Paw Edema
With freshly prepared 0.1 mL of 2% v/v formalin in distilled water, hind paw edema was induced. With the exception of the dosing, basal paw volume measurement, and therapy, these are similar to carrageenan-induced rats’ paw edema. Formalin was injected into the right hind paw of overnight fasted rats by sub-plantar injection on the first and third days of observation.3 The extract, the reference medication, and the vehicle were each given according to their groupings one hour prior to the measurement of paw edema for seven consecutive days.3,23
On the first, third, second, third, fourth, fifth, sixth, and seventh days of therapy, the paw volume was measured. Daily measurements of the rat paw volume were taken using a plethysmometer after one hour of drug administration; however, on day one, the measurement was conducted three hours after formalin injection.23,24
- The percent inhibition was calculated as:

- Vt and Vc are the mean edema volumes in the treated and control groups, respectively.
Cotton Pellet-Induced Granuloma Method
The cotton pellet-induced granuloma in rats was evaluated as specified by Afsar et al and Belay and Makonnen. The rats were given free access to water but fasted for the previous night prior to receiving the vehicles, medicines, and test substances. The rats were put into five groups of six animals each at random. Animals in group IV receive 10mg/kg of indomethacin, whereas those in group V receive Tween 80. Groups I, II, and III received doses of 80% methanol crude extract of 400mg/kg, 800mg/kg, and 200mg/kg, respectively.23,25
The rats were given thiopental sodium (25mg/kg, i.p.) 20 minutes after administering the standard drug, the vehicle, and the extracts. A subcutaneous tunnel was then created aseptically in each rat’s previously shaved groin using a surgical blade and blunted forceps. The subcutaneous tunnel was subsequently implanted bilaterally with two sterilized cotton pellets weighing 10 mg each, and the tunnel was closed with non-absorbable silk rounded bodes (0/4 rounded, 1/2 Circle).23
A standard drug, a vehicle, and the 80% methanol crude extract were given for seven days. On the eighth day, the rats were sacrificed by cervical dislocation, and the cotton pellets were carefully taken out and thoroughly cleaned of extraneous tissues. In order to attain a constant dry weight, the pellets were dried in an incubator for 24 hours at 60°C before being weighed again.23,25,26
To determine the weight of the wet exudate, the constant dry weight of the pellet was subtracted from the immediate wet weight of the pellet. Granuloma tissue formation was measured by subtracting the weight of a cotton pellet (10 mg) from the constant dry weight of the pellet.23 According to Afsar et al, Belay, and Makonnen, the percentage inhibitions of exudate and granuloma tissue formation were calculated.23,25
Preliminary Phytochemical Screening
The phytochemical screening revealed that the 80% methanol crude extract included a variety of secondary metabolites, including flavonoids, terpenoids, steroids, phenols, cardiac glycosides, tannins, alkaloids, and anthraquinones.23,27,28
Statistical Analysis
Data was entered and analyzed with the IBM statistical package for social sciences (SPSS). The data were tabulated and expressed as mean ± standard errors of the mean (SEM) (n = 6). The statistical analysis was carried out using a one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test. The result was considered significant when p <0.05.29
Results
Yields of Extraction
A total of 73.04g of crude extract (16.6% of leaf powder) was produced from 440g of plant powder that had been macerated with 80% methanol.
Acute Oral Toxicity Test
The acute oral toxicity investigation found that rat given up to 2000 mg/kg of an 80% methanol extract of Premna Schimperi Engl leaves did not die within the first 24 hours or after 14 days. As a result, the oral LD50 exceeds 2 g/kg. The experimental rat’s physical and behavioral evaluations revealed no apparent acute poisoning symptoms, such as loss of appetite, tremors, hair erection, salivation, or diarrhea.
Phytochemical Screening
The preliminary phytochemical screening showed that different secondary metabolites such as flavonoids, terpenoids, steroids, phenolics, cardiac glycosides, tannins, alkaloids, and anthraquinones were available in the 80% methanol crude extract.
Anti-Inflammatory Activity on Carrageenan-Induced Paw Edema
The 80% methanol crude extracts significantly (p< 0.05) reduced paw edema at all tested doses, beginning with the first hour of edema measurements, in contrast to the negative controls. When evaluated by linear regression, it was discovered that the effect at the fifth hour increased in a dose-dependent way (R2 = 0.955). At the fifth hour after edema induction, the dose of 800mg/kg extract significantly differed from the dose of 400mg/kg (p <0.05), but it did not differ significantly from the doses of 200mg/kg and 400mg/kg at the first and second hours of edema measurement (Table 1 and Figure 1).
 |
Table 1 Anti-Inflammatory Activity of 80% Methanol Crude Extracts of Premna Schimperi on Carrageenan-Induced Rat Paw Edema |
 |
Figure 1 Percentage protection of 80% methanol extracts of Premna Schimperi Engl on carrageenan-induced paw edema model in rats. Note: Points 1–5, indicates the experimental duration in hours. |
Anti-Inflammatory Activity on Formalin-Induced Paw Edema
In comparison to the negative control group, all tested extract doses significantly inhibited paw edema, commencing on day 1 of treatment (p <0.001) and continuing through day 7 (p <0.001). The anti-inflammatory effect on the sixth day of treatment grew in a dose-dependent way, according to linear regression analysis (R2 = 0.949). 800 mg/kg of 80% methanol crude extracts and 10 mg/kg of indomethacin effectively (p <0.001) decreased paw edema from day 1 to day 7 of therapy when compared to the negative control group (Table 2 and Figure 2).
 |
Table 2 Anti-Inflammatory Activity of 80% Methanol Crude Extracts of Premna Schimperi on Formalin-Induced Rat Paw Edema |
 |
Figure 2 Percentage protection of 80% methanol extracts of Premna Schimperi Engl on formalin-induced paw edema model in rats. Note: Points 1–7, indicates the experimental duration in days. |
Anti-Inflammatory Activity on Cotton Pellet-Induced Granuloma
The findings of this investigation demonstrated that the 80% methanol crude extracts at all tested concentrations significantly (p < 0.001) inhibit the exudate and granuloma formation brought on by cotton pellets. Additionally, when evaluated using linear regression, it was found that the extract’s anti-inflammatory effectiveness increased in a dose-dependent way in the reduction of exudate development (R2 = 1) and granuloma formation (R2 = 0.937) (Table 3 and Figure 3).
 |
Table 3 Anti-Inflammatory Activity of 80% Methanol Extracts of Premna Schimperi on Exudate and Granuloma Tissue Formation in Cotton Pellet-Induced Granuloma in Rats |
 |
Figure 3 Percentage inhibition of 80% methanol extracts of Premna schimperi Engl and indomethacin on cotton pellet-induced exudate formation and granuloma mass in rats. |
Discussion
Premna schimperi leaves have long been used in Ethiopia as an anti-inflammatory drug.14–16 In experimental models of inflammation, there is no proof of this plant’s anti-inflammatory properties. The current study looked at the anti-inflammatory properties of Premna Schimperi leaf extract in order to support its traditional claims. Rats were given 80% methanol crude extracts at doses of 2000mg/kg. Following the 2008 Organization for Economic Cooperation and Development, no signs of acute toxicity were observed in the rats. An injection of carrageenan causes localized inflammation that develops in two stages: the first stage, which begins 0 to 2.5 hours after the injection and is characterized by a high expression of histamine, serotonin, and bradykinin, and the second phase, which begins at a later time.20 The second phase, which starts 2.5 to 5 hours after the injection of carrageenan, is brought on by the overproduction of COX-2 and its pro-inflammatory PGs products as well as the invasion of polymorphonuclear leucocytes (neutrophils).30
In line with the studies mentioned, the second phase of carrageenan-induced paw edema, which is primarily dictated by COX2 and its pro-inflammatory PGs, is inhibited to a greater extent by the 80% methanol crude extracts of this plant.23,31,32
The extract’s inhibitory effect on mediators like histamine and serotonin may account for the anti-inflammatory effects of the extracts shown within the first two hours of inflammation caused by carrageenan. After the third hour of inflammation induction, the paw edema significantly decreased, suggesting that the extracts may have an effect on the cyclooxygenase enzyme and its pro-inflammatory mediators (PGs). The largest anti-inflammatory impact was observed on the fifth hour relative to their respective inhibitory values in the first phase (0–2.5 hours), and all tested extract doses significantly (p < 0.05) suppressed edema on all measurement hours of the inflammation.
Injection of formalin causes biphasic localized pain and inflammation in the hind paw of rats. Pain is assumed to start during the initial neurogenic phase (0–5 minutes) by the direct chemical stimulation of nociceptors, and substance-P and bradykinin are proposed as its mediators.33 Histamine, serotonin, prostaglandin, and bradykinin appear to be the main mediators of the second phase (15 minutes after induction), which is an inflammatory phase that can be reduced by anti-inflammatory medications with peripheral activity. The most conspicuous activity in this inflammatory response is leukocyte migration into the inflamed area, which starts late in this period (2.5–6 hours).34
In comparison to the negative control group, the 80% methanol crude extracts at dosages of 200, 400, and 800mg/kg showed a statistically significant (p < 0.001) suppression of paw edema from day 1 to day 7 of therapy, as shown in Table 2. Both 10mg/kg of indomethacin and 800mg/kg of 80% methanol crude extracts had comparable anti-inflammatory effects. This may be caused by specific changes in the inflammatory response, which may have anti-arthritic effects on par with those of the commonly used medication indomethacin.
This work used this model to further validate the anti-inflammatory effects of 80% methanol crude extracts on proliferative and transudative aspects of chronic inflammation.23,35,36 According to these findings, every dose examined in the cotton pellet-generated granuloma model showed a statistically significant (p< 0.001) decrease of both inflammatory exudates and granuloma formation when compared to the negative control groups.
Secondary metabolites such as alkaloids, flavonoids, and terpenoids possess anti-inflammatory activities due to their ability to reduce COX expression and PGE2 generation. Those metabolites limit the production of pro-inflammatory cytokines (Interleukin-1, Interleukin-6, and Tumor necrosis factor), as well as intracellular adhesion molecule and vascular cell adhesion molecule-1 expression.37–39 According to this study, Premna schimperi’s anti-inflammatory properties may be indicated by the presence of alkaloids, flavonoids, and terpenoids in its 80% methanol crude extracts.
Methanol, which can extract a variety of polar and non-polar phytochemical components from medicinal plants, was specially chosen for this study’s extraction solvent because of its wide polarity range and ability to provide high extraction yields.40–42 It is also thought that methanol can successfully permeate cell membranes, allowing for the extraction of significant amounts of endocellular components. Additionally, due to its relative safety, methanol is frequently employed in the herbal medicine industry to make crude extracts of phytochemicals for therapeutic purposes.19,43
In addition to the current study, more investigation on the anti-inflammatory properties of Premna schimperi is required, including the identification and purification of the active ingredients responsible for the anti-inflammatory effect, clarification of the mechanism of action of the active ingredients, identification of inflammatory biomarkers through histopathological analysis, and assessment of the plant’s long-term safety profile. To offer a comprehensive profile of the substance that was screened from this plant for medicinal use, more pharmacological and toxicological research should be conducted.
Conclusion
According to the results of the current investigation, higher doses of Premna schimperi leaf extract have a higher anti-inflammatory impact on carrageenan-induced paw edema in rats that is comparable to that of standard drugs. The extracts decrease the development of granulomas caused by cotton pellets and paw edema brought on by formalin. The claimed anti-inflammatory activity may have been aided by the presence of different secondary metabolites. Therefore, it is suggested that the active ingredient in Premna schimperi be extracted for more study.
Abbreviations
IRB, Institutional Review Board; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; PGs, Prostaglandins; OECD, Organization for Economic Cooperation and Development.
Data Sharing Statement
The corresponding author will provide all the data sets used and analyzed during the current work upon reasonable request.
Ethical Approval
The ethical approval and clearance were obtained from the institutional review board (IRB) with reference number IRB/151/14 at Hawassa University College of Medicine and Health Sciences. Experimental animals were handled ethically according to the animal handling standard requirements and guidelines for animal use (OECD, 2008).
Acknowledgments
For their assistance during the study, we are grateful in particular to the Ethiopian Public Health Institute (EPHI), Pawi Health Science College, and Hawassa University College of Medicine and Health Science. The full thesis of this manuscript is available on the Hawassa University repository at this link: http://etd.hu.edu.et/handle/123456789/3650.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Pawi Health Science College, Pawi, Ethiopia.
Disclosure
The authors declare that they have no competing interests.
References
1. Karrat L, Yaser M, Nayal R. Heliyon Investigating the anti-inflammatory and analgesic properties of leaves ethanolic extracts of Cedrus libani and Pinus brutia. Heliyon. 2022;8:e09254. doi:10.1016/j.heliyon.2022.e09254
2. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
3. Yimer T, Birru EM, Adugna M, Geta M, Emiru YK. Evaluation of analgesic and anti-inflammatory activities of 80 % methanol root extract of echinops kebericho M.(Asteraceae). J Inflamm Res. 2020;Volume 13:647–658. doi:10.2147/JIR.S267154
4. Crops A, Crops V, Sad N. Anti-inflammatory properties of plants from Serbian traditional medicine. Life. 2023;2023:1–17.
5. Gulab G, Kumar A, Rizvi W, Tripathi CD, Khan RA. Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. J Tradit Chinese Med Sci. 2016;6(2):172–175.
6. Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–132. doi:10.1016/j.bpg.2009.11.005
7. Sharma JN, Jawad NM. Adverse effects of COX-2 inhibitors. Scientif World J. 2005;5:629–645. doi:10.1100/tsw.2005.82
8. Roshi D, Toçi E, Burazeri G, SchröderBck P, Brand H. Users’ knowledge about adverse effects of non-steroidal anti-inflammatory drugs in Tirana, Albania. Mater Socio Medica. 2017;29(2):138. doi:10.5455/msm.2017.29.138-142
9. Leaf M, Bak F, Tamiru W, Engdawork E. Experimental evaluation of analgesic and anti-inflammatory activity of 80% methanolic leaf extract of Moringa stenopetala Bak. F. in Mice. Ethiop Pharmac J. 2015;31(1):15–26. doi:10.4314/epj.v31i1.2
10. Alemu A. Evaluation of the in vivo analgesic and anti-inflammatory activities of 80 % methanol extract of Leonotis ocymifolia (Burm. F.) iwarsson leaves asnakech alemu a thesis submitted to the department of pharmacology and clinical pharmacy, school of pharma; 2017.
11. Dianita R, Jantan I. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Premna: a review. Pharm Biol. 2017;55(1):1715–1739. doi:10.1080/13880209.2017.1323225
12. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Evaluation of the antiplasmodial properties of selected plants in southern Ethiopia. BMC Complement Altern Med. 2015;15:1–12. doi:10.1186/s12906-015-0520-z
13. Tigray S. Study of plants traditionally used in public and animal health management in Seharti SamreDistrict, Southern Tigray, Ethiopia. J Ethnobiol Ethnomed. 2015;11:1–25.
14. Tafese Awulachew M. Hand book of common Ethiopian traditional medicinal plants: their parts and uses for human and animal treatments. J Dis Med Plants. 2021;7(3):48.
15. Banchiamlak NT, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;7:1–21.
16. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–525. doi:10.1016/j.jep.2006.10.011
17. Hossain MA, Al-Hdhrami SS, Weli AM, Al-Riyami Q, Al-Sabahi JN. Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in Sultanate of Oman. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S368–72. doi:10.12980/APJTB.4.2014C1051
18. Poojar B, Ommurugan B, Adiga S, et al. Methodology Used in the Study. Asian J Pharm Clin Res. 2017;7(10):1–5.
19. Bagri P, Ali M, Aeri V, Sultana S, Bhowmik M. Evaluation of anti-inflammatory and analgesic activity of Punica granatum linn. Int J Drug Dev Res. 2010;2(4):698–702.
20. Masresha B, Makonnen E, Debella A. In vivo anti-inflammatory activities of Ocimum suave in mice. J Ethnopharmacol. 2012;142(1):201–205. doi:10.1016/j.jep.2012.04.041
21. Guidelines O. The For, Of T. Oecd guidelines for the testing of chemicals; 2008.
22. Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci. 2020;4(1):1–7.
23. Belay R, Makonnen E. Anti-inflammatory activities of ethanol leaves extract and solvent fractions of Zehneria scabra (Cucurbitaceae) in rodents. Asian J Natural Prod Biochem. 2020;18(1):42–56. doi:10.13057/biofar/f180105
24. Alemu A, Tamiru W, Nedi T, Shibeshi W. Analgesic and anti-inflammatory effects of 80 % methanol extract of Leonotis ocymifolia (Burm. f.) iwarsson leaves in rodent models. Evidence Based Complem Alternat Med. 2018;2018:1.
25. Afsar SK, Rajesh Kumar K, Venu Gopal J, Raveesha P. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J Pharm Res. 2013;7(6):463–467.
26. Gupta M, Mazumder UK, Gomathi P, Selvan VT. BMC Complementary and Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Complement Altern Med. 2006;6:1–6. doi:10.1186/1472-6882-6-36
27. Sharma A, Patel S, Pradesh M, Pradesh M. Preliminary phytochemical screening and quantitative analysis of secondary metabolites of Mentha arvensis and Azadirachta indica. Int J Adv Research and Devel. 2018;2015:114–118.
28. Shaikh JR, Patil MK. Qualitative tests for preliminary phytochemical screening: an overview. Intern J Chem Stud. 2020;8(2):603–608. doi:10.22271/chemi.2020.v8.i2i.8834
29. Paramita S, Kosala K, Dzulkifli D, Saputri DI, Wijayanti E. Anti-inflammatory activities of ethnomedicinal plants from Dayak Abai in North Kalimantan, Indonesia. Biodivers J Bio Diver. 2017;18(4):1556–1561. doi:10.13057/biodiv/d180433
30. Coura CO, Souza RB, Rodrigues JAG, et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS One. 2015;10(3):1–18. doi:10.1371/journal.pone.0119319
31. Archer AC, Muthukumar SP, Halami PM. Anti-inflammatory potential of probiotic Lactobacillus spp. on carrageenan induced paw edema in Wistar rats. Int J Biol Macromol. 2015;81:530–537. doi:10.1016/j.ijbiomac.2015.08.044
32. Sachin P, Amit G, Navin SH. Solanum xanthocarpum (Yellow Berried Night Shade): A review. Der Pharmacia Lettre. 2011;2(4):373–383. https://bitly.ws/324mR.
33. Sanusi RAM, Ab Shukor NA, Sulaiman MR. Anti-inflammatory effects of Labisia pumila (Blume) F. Vill-Naves. aqueous extract. Sains Malaysiana. 2013;42(10):1511–1516.
34. Cui E, Zhi X, Chen Y, et al. Coptis chinensis and Myrobalan (Terminalia chebula) can synergistically inhibit inflammatory response in vitro and in vivo. Evidence-Based Complement Altern Med. 2014;2014:1–8. doi:10.1155/2014/510157
35. Andrade SF, Cardoso LGV, Carvalho JCT, Bastos JK. Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of Austroplenckia populnea. J Ethnopharmacol. 2007;109:464–471. doi:10.1016/j.jep.2006.08.023
36. Bagad AS, Joseph JA, Bhaskaran N, Agarwal A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of curcuma longa. Advan Pharmacol Pharmac Sci. 2013;2013:1.
37. Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66:227–233. doi:10.1016/S0378-8741(98)00162-7
38. Cui H, Hayasaka S, Zheng L, Hayasaka Y, Zhang X-Y, Chi Z-L. Effect of berberine on monocyte chemotactic protein-1 and cytokine-induced neutrophil chemoattractant-1 expression in rat lipopolysaccharide-induced uveitis. Ophthalmic Res. 2007;0194:32–39. doi:10.1159/000097904
39. Bellik Y, Boukraâ L, Alzahrani HA, et al. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2013;2013:322–353.
40. Kimura N, Kainuma M, Inoue T, et al. Botany, uses, chemistry and bioactivities of mangrove plants V: acrostichum aureum and A. speciosum. ISME/GLOMIS Elect J. 2017;15(1):1–6.
41. Tatke P, Rajan M. Comparison of conventional and novel extraction techniques for the extraction of scopoletin from convolvulus pluricaulis. Indian J Pharmac Educat Res. 2014;48(1):27–31. doi:10.5530/ijper.48.1.5
42. Gupta A, Kothari V. Modern extraction methods for preparation of bioactive plant extracts modern extraction methods for preparation of bioactive. Intern J Appl Natural Sci. 2012;1(1):8.
43. Patel M, Murugananthan G. In vivo animal models in preclinical evaluation of anti- inflammatory activity: a review. Int J Pharm Res Allied Sci. 2012;1:1–5.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.