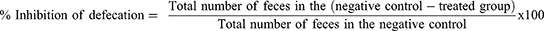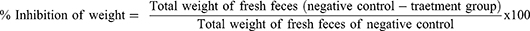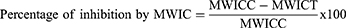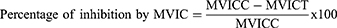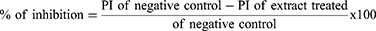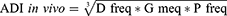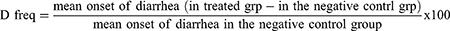Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of Anti-Diarrheal Activities of the 80% Methanol Extract and Solvent Fractions of Maesa lanceolata Forssk (Myrsinaceae) Leaves in Mice
Authors Megersa A , Dereje B , Adugna M, Ayalew Getahun K , Birru EM
Received 5 August 2023
Accepted for publication 19 October 2023
Published 25 October 2023 Volume 2023:15 Pages 391—405
DOI https://doi.org/10.2147/JEP.S429403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Alemayehu Megersa,1 Beyene Dereje,1 Meaza Adugna,2 Kefyalew Ayalew Getahun,2 Eshetie Melese Birru2
1Department of Pharmacology, School of Medicine, College of Medicine and Health Science, Dire Dawa University, Dire Dawa, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Alemayehu Megersa, Email [email protected]
Background: Due to the limits of present antidiarrheal medications, it is critical to seek novel, safe, and inexpensive antidiarrheal agents. Thus, the goal of this study was to assess the antidiarrheal activity of 80% methanol crude extract and solvent fractions of Maesa lanceolata leaves in mice.
Methods: Leaf powder was extracted by 80% methanol and then fractionated with n-hexane, ethyl acetate, and distilled water. At 100, 200, and 400 mg/kg, the effects of the crude extract on castor oil-induced diarrhea, enteropooling, and gastrointestinal motility tests were investigated. Tween 2% and atropine used as negative and positive controls, respectively. A gastrointestinal motility test was used to explore the anti-motility effects. Data were analyzed with SPSS V. 26, and the significance was established with a one-way ANOVA followed by a post hoc Tukey’s test.
Results: The crude extract delayed the onset of diarrhea and significantly reduced the number of fecal drops at 100 (p< 0.05), 200 and 400 mg/kg (p< 0.001). Similarly, the number and weight of wet feces, as well as total fresh feces, were reduced at 200 (p< 0.05) and 400 mg/kg (p< 0.001) compared to Tween 2%. The enteropooling test demonstrated that the extracts significantly reduced the volume and weight of intestine content at 200 (p< 0.05) and 400 mg/kg (p< 0.001). The anti-motility activity test revealed that the all extracts decreased gastrointestinal motility significantly (p< 0.001). The ethyl acetate fraction significantly reduced gastrointestinal transit time at all doses (p< 0.001). At 400 mg/kg, the activities of the n-hexane fraction were significant (p< 0.01). The efficacy of the residual aqueous fraction on gastrointestinal motility was significant at 200 (p< 0.05) and 400 mg/kg (p< 0.001).
Conclusion: The 80% methanol extract of Maesa lanceolata Forssk leaf and solvent fractions were shown to exhibit potent antidiarrheal activity in the current study.
Keywords: castor oil, crude extract, diarrhea, Maesa lanceolata Forssk, solvent fraction
Introduction
Diarrhea is described as passing three or more loose or liquid stools per day or passing stools more frequently than is normal for the individual.1 Regularly passing formed feces is not diarrhea, nor is the passage of loose, “pasty” stools by breastfeeding babies.2 Along with frequency and consistency, the weight of the feces should be evaluated. As a result, diarrhea is defined as more than 200 g of feces per day.3,4 Diarrhea is a major preventable cause of death and morbidity, particularly in communities with limited resources. Childhood diarrhea affects approximately 1.7 billion children worldwide each year, resulting in half a million fatalities among children under the age of five, making diarrhea the second largest cause of death among children under the age of five. The majority of deaths from diarrheal sickness occurred in Sub-Saharan Africa and South Asia, where sanitation and hygiene are weak.5,6
A variety of viruses, bacteria, and parasites can cause diarrhea. Pathogens vary according to a variety of characteristics, including but not limited to host factors, socioeconomic situations, geographic and climatic settings, and others.7,8 Noninfectious factors include pancreatic exocrine insufficiency, endocrine disorders, tumors, chronic malassimilation states, bile acid malabsorption, and intoxications; medication-related factors such as laxatives, antibiotics, chemotherapy, and radiotherapy; and diet-related factors such as tube feeding and oral diet.9
The digestive system regulates electrolyte and water intake and secretion to meet physiological needs and maintain hydration.10 During diarrhea, the usual net absorption of electrolytes and water reverses into output.11 All diarrheas share the same underlying pathophysiology, which is either inadequate fluid absorption from the intestine or osmotic water retention inside the lumen.12 The four different mechanisms that produce diarrhea are osmotic load escalation, secretory diarrhea, inflammation, inflammatory diarrhea, and poor absorption time as a result of disturbed intestinal motility.13
The most important goal of diarrhea treatment is to keep the patient hydrated, to effectively counter electrolyte and fluid losses and to prevent morbidity and mortality. Fluid therapy is the most life-saving treatment measure, and it is administered to patients based on their medical history, physical examination, laboratory findings, and an appropriate understanding of electrolyte and fluid dynamics, with the goal of restoring fluid balance.14
Other treatment approaches to achieve these goals include probiotics, which are nonpathogenic organisms, and prebiotics, which are fermented or indigestible nutrients.
Probiotics, which are nonpathogenic organisms, prebiotics, which are fermented or indigestible nutrients; antisecretory agents, such as loperamide, diphenoxylate, and bismuth subsalicylate, which act by stimulating absorption and decreasing water secretion in the gastrointestinal tract; and anti-motility agents, which inhibit intestinal motility by acting as opiate receptor agonists, such as opiates, are other treatment approaches to achieve these goals.15,16 Anticholinergics such as atropine, propantheline, methscopolamine, and glycopyrrolate are usually successful treatment options for irritable bowel syndrome diarrhea (IBS-D) due to their antispasmodic and acid secretion-reducing properties. Additionally, dietary support and antimicrobial intervention (if needed) are therapeutic possibilities.17 When administered properly, specific or empiric antibiotic therapy for diarrhea caused by shigellosis, campylobacteriosis, C. difficile colitis, and protozoal infections is effective.4,14
Maesa lanceolata, from the genus Maesa and the family Myrsinaceae, was the experimental plant tested for antidiarrheal activity. The Myrsinaceae family contains approximately 30 genera and 1000 species. Maesa lanceolata (Myrsinaceae) is a 10 meter tall shrub or tree with greenish-brown stems. The leaves are oval with coarsely serrated margins, and the inflorescence is a single or complex raceme. This plant is utilized in medicinal formulations by traditional healers to treat a number of ailments.18 In many parts of our world, Maesa lanceolata froskk leaves are used for the treatment of malaria,19 ascariasis,20 dermatosis, scabies,21 and the management of difficult childbirth to augment labor.22 It has also been used to manage wounds,23 and its roots are used to treat jaundice.24 Additionally, fruits and seeds can also treat intestinal worms, stomach aches, and sore throats.25 The plant can be found all over Eastern Africa, up into Arabia, and all over India.20
Triterpenoid saponins26 and benzoquinones27 have been identified as active components in this plant in phytochemical research thus far. Saponins contain biological properties such as virucidal activity against enveloped viruses, hemolysis, molluscicidal activity, moderate fungistatic activity, antimutagenicity and vasoconstriction.26 One study also determined the antimicrobial activities of the seed oil extract of the study plant because of the availability of different phytochemicals that have antimicrobial activities, such as alkaloids, flavonoids, tannins, terpenoids, saponins, and phenols.28
This plant has also shown antifungal,29 antioxidant,27 antiviral,18 and anticancer30 activities. Experimental studies have confirmed that the methanol extract of Maesa lanceolata showed anthelmintic activity.25 Another in vitro experimental study additionally showed that chloroform extract from the leaves of this plant had the highest antiplasmodial activity.31 In Ethiopia, this medicinal plant is known by different local names, including Kalawa and Sowereya in Amharic, Abayii in Afaan Oromoo, and kawacho in Sidama.32 To treat diarrhea, the leaves of this medicinal plant are crushed, soaked, and taken three times a day orally. A decoction of this plant is also used by others to treat this ailment. It has been documented and reported that Maesa lanceolata is used traditionally for the treatment of diseases such as diarrhea in Ethiopia around Ankober,32 without clear scientific proof for its safety and efficacy. The current study aimed to assess the antidiarrheal activity of Maesa lanceolata Forssk (Myrsinaceae) leaves 80% methanol extract and solvent fractions in mice.
Materials and Methods
Experimental Animals
The experiment utilized a total of 143 apparently healthy Swiss albino mice of either sex, weighing between 25–30 g and aged 8–12 weeks. The study animals were obtained from the laboratory animal center of the Pharmacology Department at the University of Gondar, School of Pharmacy. The study animals were housed in standard cages at room temperature with free access to food and water on a 12-hour light and dark cycle. The animals were introduced to the testing area one week before the trial.33 The study was performed at the Pharmacology Laboratory of the University of Gondar, Ethiopia.
Plant Material
On May 15, 2021, fresh Maesa lanceolata Forssk leaves were taken from Ankober Woreda, North Shewa Zone, Amhara region, which is located 40 kilometers east of Debre Birhan and approximately 140 kilometers northeast of Addis Ababa, Ethiopia’s capital city. A botanist recognized and authenticated the plant, and a voucher specimen with voucher number 01/AM/2022 was deposited in the Herbarium, College of Computational and Natural Sciences, University of Gondar.
Equipment, Drugs and Chemicals
Rotary vapor (Yamato Scientific Co. Ltd, Japan), dry oven, deep freezer, mortar and pestle, measuring cylinders, funnel, transparent plastic ruler, beaker, surgical scalpel blade - No. 24, oral gavage with needle, forceps and Erlenmeyer flask were used for the current experiment. Drugs such as castor oil (Amman Pharm., Jordan), loperamide (Daehwa Pharm., Republic of Korea), charcoal meal (Acuro Organics, New Delhi), atropine (Humanwell Pharmaceutical Ethiopia), ketamine (Trit Taij, Germany), Tween 80 (Care Laboratory’s and Medical Supply, India), methanol (Follium Pharmaceuticals, Ethiopia), n-hexane (Lopa Chemie, India), ethyl acetate (Sisco Research Lab., India), and distilled water were used in the experiment.
Preparation of 80% Methanol Extracts
After collecting the leaves, they were carefully rinsed with running tap water to eliminate dust. To avoid solar damage to the plant’s contents, the plant was then dried in the house at room temperature. Finally, the dried plant leaves were mashed with a mortar and pestle. Cold maceration was used to remove the plant material. Methanol 80% was chosen over other solvents due to its universality, lower boiling point, higher volatility, and higher extraction efficiency, depending on the intended secondary metabolite composition.34 Powdered leaves (900 g) were steeped for 72 hours at room temperature in an Erlenmeyer flask with 80% methanol in a 1:5 (w/v) ratio. Following muslin cloth filtration, the crude extract was filtered with Whatman No. 1 filter paper, and the marc of extract was then remacerated twice more with an equal volume of different solvents. The filtrate yields from extractions were mixed, and methanol was extracted from the extract using a Rota Vapor set to 40 °C and evaporation under vacuum. The yields were stored in the refrigerator after being lyophilized with a freeze dryer and deposited in a deep freezer overnight. The yield was then calculated as a percentage.
Solvent Fractionation
Stages of fractionation were performed on the 80% methanol crude extract. According to their increasing polarity, the solvents utilized in the fractionation procedure were n-hexane, ethyl acetate, and distilled water. After measuring out 70 g of the crude extract, 250 mL of distilled water and an equal amount of n-hexane were added to a beaker. A 500-mL separating funnel was then used to contain the suspension, which was shaken occasionally while it stood there. Because n-hexane has a lower density than water, it was taken on the upper phase and collected. The residue was macerated twice more using the same ratio of n-hexane and procedure as the first maceration. The n-hexane fraction (NHF) was created by combining the filtrate fractions produced in the preceding process. The residue was then treated with 250 mL of ethyl acetate, and the method was repeated as with hexane. The residue was then removed from the ethyl acetate layer and utilized to create the fraction (EAF). The residue aqueous fraction (RAQF) was formed from the final residue. The RAQF was lyophilized after the NHF and EAF were dried in an oven at 40 °C.35
Grouping and Dosing of Animals
The 80% methanol extract was tested using three models, and each fraction was tested using a gastrointestinal motility test, castor oil-induced enteropooling test and antidiarrheal index. Animals were randomly assigned to one of five groups: negative control (G-I), positive control (G-II), and the remaining three test groups (Groups III–V), with six animals in each group. The first group received 2% Tween 80 (10 mL/kg) for crude extract, NHF, and EAF, while G-I for the aqueous fraction received distilled water (DW) at 10 mg/kg; the second group received loperamide (3 mg/kg) for castor oil-induced diarrhea and enteropooling assays. Atropine at 5 mg/kg was administered for tests of gastrointestinal motility; groups III to V were treated with different doses (100, 200, and 400 mg/kg, respectively) of Maesa lanceolata Forssk extracts. The doses were determined based on the results of an acute oral toxicity test.36
Qualitative Phytochemical Screening
The existence of secondary phytochemicals such as alkaloids, flavonoids, terpenoids, saponins, tannins, steroids, phenols and cardiac glycosides was assessed using established techniques and reagents.37 The tests used for this experiment were for alkaloids (Wagner’s test), flavonoids (alkaline reagent test), cardiac glycosides (Keller-Kiliani test), phenols (ferric chloride test), saponins (foam test), steroids (Salkowski’s test), terpenoids (Salkowski’s test), tannins (Braymer’s test), and terpenoids (Salkowski’s test).
Acute Oral Toxicity Test
To assess acute oral toxicity, the OECD 425 guidelines were used to test 5 female mice.38 Prior to the administration of 80% MEML (80% methanol extract of Maesa lanceolata leaves), the animals were fasted but had access to water for 4 hours. Then, 2 g/kg of the crude extract was administered to the mouse orally. The mice were fasted for two hours after administration of the crude extract. For four hours, the mouse was observed every half-hour for any indications of toxicity or early mortality. Based on the findings of the first mouse, since there was no observed acute toxicity or mortality during the first 24 hours, the extract was sequentially administered (2000 mg/kg) to the next four mice. After that, mice were housed separately and watched for 4 hours, followed by a 30-minute break, before being checked daily for two weeks for indications of toxicity in the same way as the first animal.
Antidiarrheal Activity Determination
Castor Oil-Induced Diarrhea
For this model, the methods described by Fokam Tagne et al were used.27 The mice of either sex were randomly allocated and given the treatments outlined in the grouping and dose section after fasting for 18 hours. One hour after administration, all mice were given 0.5 mL of castor oil orally through oral gavage and placed in a separate clear cage with a white, nonwetting, transparent paper-lined bottom. For a total of four hours, the white, nonwetting, clean paper was changed every hour. During the observation period, the onset of diarrhea, the quantity and weight of wet stools, and the overall number and weight of fecal output were all documented. Finally, the percentage inhibition of weight, percentage inhibition of diarrhea, and percentage inhibition of feces were calculated using the procedures mentioned below.
Castor Oil-Induced Enteropooling
For this model, the methods described by Patil et al39 were followed. After fasting for 18 hours, animals of either sex were divided into groups and given the appropriate treatments as outlined in the section on grouping and dosing. Castor oil (0.5 cc) was ingested after an hour. One hour later, the mice were slaughtered and died via cervical dislocation. The intestine from the pylorus to the cecum was methodically dissected, ligated, and removed from each mouse’s abdomen. The weights of the small intestines before and after milking were recorded, and the contents of the small intestine were milked into a graduated tube. Finally, the weight of the intestinal contents and the % reduction in intestinal output were determined using the following formulas:
where: MWIC – mean weight of intestinal content; MWICC - mean weight of intestinal content of control group; MWICT - mean weight of intestinal content of test group
where: MVIC – mean volume of intestinal content; MVICC – mean volume of intestinal content of the control group; MVICT – mean volume of intestinal content of the test group
Gastrointestinal Motility Test by Charcoal Meal
This study was performed in accordance with the method stated by Patil et al.39 All mice were grouped and administered treatments in accordance with the grouping and dosing section after being fasted for 18 hours with free access to water. Each mouse received castor oil, 0.5 cc an hour after treatment. All mice received 0.5 cc of a charcoal meal suspension an hour after receiving castor oil. The entire length of the intestine, from the pylorus to the cecum, was then removed and laid lengthwise on a flat platform after the animals were sacrificed after waiting 30 minutes. The length of the intestine as a whole and the distance covered by the charcoal meal were then measured using a transparent plastic measuring ruler. The formula below was used to determine the peristaltic index and percentage of inhibition:
Anti-Diarrheal Index (ADI)
The positive control and extract-treated groups’ in vivo antidiarrheal index (ADI) was calculated using various data from the aforementioned experiments using the following formula:40
where:
- D freq is delay in defecation time or diarrhea onset from castor oil diarrhea test,
- G meq is the gut meal travel reduction obtained from the charcoal meal test,
- P freq = the purging frequency or reduction in the number of wet stools obtained from the castor oil–induced diarrheal model.
Statistical Analysis
SPSS version 26.0 was used to enter and analyze all data. To identify statistical variances between all study groups, a one-way analysis of variance (ANOVA) was conducted. Tukey’s post hoc multiple comparison tests were used for pairwise comparisons. The results are presented as the mean ± SEM. p values < 0.05 were considered statistically significant.
Results
Crude Extracts and Fraction Yields
Out of 900 g of leaf powder, 128 g of dried methanol extract was obtained, and the total percentage yield was 14.2%. The aqueous, ethyl acetate and n-hexane fraction percentage yields were 53.47% (37.43 g), 22.57% (15.80 g), and 9% (6.30 g), respectively.
Acute Oral Toxicity
The results showed that mice exposed to crude methanol extract up to 2 g/kg did not die within the first 24 hours or for the next 14 days, indicating that the oral LD50 is more than 2 g/kg. Gross physical and behavioral examinations of the experimental mice revealed no noticeable acute poisoning symptoms, such as vomiting, diarrhea, or loss of appetite.
Qualitative Phytochemical Screening
The qualitative phytochemical screening test results showed the existence of alkaloids, tannins, and phenols in both crude extracts of Maesa lanceolata and solvent fractions (Table 1). Steroids are absent in both crude and solvent fractions. In addition to the abovementioned results, saponins are absent in NHF and EAF. Flavonoids are absent in NHF and RAQF. Cardiac glycosides are only absent in RAQF.
 |
Table 1 Preliminary Phytochemical Screening Test Results of Maesa lanceolata Leaf Extracts |
Effects of Crude Extracts on Castor Oil-Induced Diarrhea
When compared to the negative control, the crude leaf extract of Maesa lanceolata considerably delayed the onset of diarrhea and the total amount of fecal drops. The extract showed a significant delay in the onset of diarrhea at 400 mg/kg (125.67±4.79 minutes). When compared to the negative control, the effects of the extract on the overall number of fecal drops were statistically significant at doses of 100 mg/kg (p<0.05), 200 mg/kg and 400 mg/kg (p<0.001). The extract had a profound effect at 400 mg/kg on fecal drop totals equivalent to that of the conventional medication loperamide (3 mg/kg). With an extract of 80% methanol at a dose of 200 mg/kg (p<0.05) and 400 mg/kg (p<0.001), it was discovered that there were fewer moist feces compared to the negative control. However, at 100 mg/kg in this parameter, the extract was statistically insignificant compared to the negative control.
Defecation was inhibited by 20.51%, 35.9% and 55.13% by the extract at 100, 200 and 400 mg/kg, respectively. Data from the experiment also showed that at dosages of 100, 200 and 400 mg/kg, the percentages of diarrheal inhibition were 17.39%, 32.61%, and 54.35%, respectively. This model was also utilized to assess how the test extract affected the weight of wet and overall fresh feces. The activity was significant at doses of 200 mg/kg and 400 mg/kg (p<0.001) in reducing the number of wet feces as well as the total weight of all fresh feces. The extract had no significant difference from the negative control at 100 mg/kg. At test doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg, the percent reduction in weight of fecal output was similarly reduced, with 20.88%, 36.26%, and 54.95%, respectively (Table 2).
 |
Table 2 Effects of 80% Methanol Extract of Maesa lanceolata on Castor Oil-Induced Enteropooling |
Effects of Crude Extracts on Castor Oil-Induced Enteropooling
Studies on the current model showed that the test crude of Maesa lanceolata extract at 200 mg/kg (p<0.05) and 400 mg/kg (p<0.001) significantly decreased the intraluminal volume of fluid buildup when compared to the negative control. On the other hand, the activity of the test extract was not statistically significant at 100 mg/kg. The maximal percentage inhibition of the volume of intestinal content was measured at a dose of 400 mg/kg of the test extract (42.82%). In line with this, at 200 mg/kg (p<0.05) and 400 mg/kg (p<0.001), the test extract significantly decreased the weight of intestinal contents in comparison to the negative control. The percentage inhibition in the weight of intestinal content at these corresponding doses was calculated to be 25.56% and 48.89%, respectively (Table 3).
 |
Table 3 Effects of 80% Methanol Extract of Maesa lanceolata on Gastrointestinal Motility |
Effects of Crude Extracts on Gastrointestinal Motility
The results showed that the marker’s distance was significantly decreased by all three doses of the extract at 100, 200, and 400 mg/kg (p<0.01). In mice administered extracts of 100 mg/kg, 200 mg/kg, and 400 mg/kg, the peristalsis index of the marker was 42.72%, 33.92%, and 28.96%, respectively. When groups of animals were given 100, 200, and 400 mg/kg crude extract, the mean percent inhibition of charcoal meal propulsion was 38.45%, 51.12%, and 58.27%, respectively. When compared to the positive control, the test extracts at 200 mg/kg and 400 mg/kg had comparable effects. However, for 100 mg/kg, no statistically significant difference was observed (Table 4).
 |
Table 4 Effects of the Solvent Fractions of Maesa lanceolata Leaves on Gastro-Intestinal Motility |
Effects of the Solvent Fractions on Gastrointestinal Motility
Solvent fractions were effective at slowing intestinal motility in the small intestinal transit test. When compared to the negative control, the ethyl acetate fraction significantly slowed the gastrointestinal transit time of charcoal meal at 100 mg/kg (37.4%, p<0.001), 200 mg/kg (47.67%, p<0.001), and 400 mg/kg (53.68%, p<0.001).
On the other hand, at 100 mg/kg and 400 mg/kg, the activity of the n-hexane fraction did not differ substantially from that of the negative control. However, at 400 mg/kg, it drastically decreased gastrointestinal motility (23.37%, p<0.01). When compared to the negative control, the residual aqueous fraction activity on gastrointestinal motility was significant at 200 mg/kg (21.12%, p<0.05) and 400 mg/kg (32.74%, p<0.001) (Table 5).
 |
Table 5 The Effects of the 80% Methanol Extract of Maesa lanceolata Leaves on the Castor Oil-Induced Diarrhea Model |
Effects on in vivo Antidiarrheal Index
Combining the purging frequency or decrease in the quantity of wet stools, the delay in defecation time, and the decrease in gastrointestinal meal travel, the test extract’s in vivo antidiarrheal index was calculated. The ADI values were 26.8, 47.37, and 56.07 at test dosages of 100 mg/kg, 200 mg/kg, and 400 mg/kg, respectively. These results showed that the extracts of Maesa lanceolata had significant antidiarrheal properties, with 400 mg/kg showing the strongest antidiarrheal properties (Figure 1).
 |
Figure 1 Effect of Maesa lanceolata leaf extracts on the in vivo antidiarrheal index. Note: MEML = 80% methanol extract of Maesa lanceolata leaves. |
Discussion
This study examined the antidiarrheal efficacy of an 80% methanol leaf extract of Maesa lanceolata in tests for gastrointestinal motility, enteropooling, and motility after castor oil-induced diarrhea. According to the study’s findings, the plant extract possesses antidiarrheal properties in animal models.
Although the current medicinal plant is extracted using water as a solvent in the treatment of diarrhea in the traditional setup, the current investigation used 80% methanol as the solvent. Studies have demonstrated that hydroalcoholic solvent mixtures have a high extraction yield.25 Additionally, because methanol is naturally highly water soluble, a hydromethanol solvent mixture can be utilized to extract a variety of chemicals with varying polarity.41 Along with the previously mentioned factors, methanol also has the potential to inhibit the growth of pathogenic microorganisms (self-preservation), which enables the protection of the extract from contamination to some extent (70). Therefore, 80% methanol was employed in this study as the solvent for the first extraction of Maesa lanceolata leaves.
An acute oral toxicity test was performed to identify any potential side effects from a single dose of MEML to assess the plant’s safety profile. At a dose of 2 g/kg of the extract, there were no fatalities or acute toxicities, indicating that MEML has a larger safety margin and an LD50 value higher than 2 g/kg in mice. This outcome is likewise consistent with a test of acute oral toxicity performed previously on three male Swiss albino mice.42
Since many plants produce secondary metabolites with antidiarrheal activity in their tissues, it has become crucial to perform phytochemical screening. Alkaloids, tannins, flavonoids, phenols, saponins, terpenoids, and flavonoids are the primary secondary metabolites responsible for the antidiarrheal activity of medicinal plants.43 Their antisecretory, antispasmodic, or antimotility effects may be responsible for this. MEML tested positive for alkaloids, flavonoids, cardiac glycosides, phenols, saponins, tannins, and terpenoids in a preliminary qualitative phytochemical analysis. With the exception of saponins, all phytochemicals identified in the crude extract were likewise discovered in the ethyl acetate fraction. Alkaloids, phenols, saponins, tannins, and terpenoids were found in significant amounts in the residue’s aqueous fraction.
Alkaloids, cardiac glycosides, phenols, and tannins were all detected in the n-hexane fractions. The semipolar ethyl acetate fraction extracted more secondary metabolites than the other fractions. Castor oil is the substance that causes experimental diarrhea the most frequently.44 Na+/K+-ATPase, a vital intestinal enzyme, is needed for the intestinal absorption of water and electrolytes. Diarrhea may develop from any interference with or inhibition of this enzyme that increases intestinal fluid flow. By modifying the intestinal mucosa’s permeability to electrolytes, ricinoleic acid, a component of castor oil, produces diarrhea by resulting in abnormal intestinal absorptive and secretory mechanisms.45,46 Because castor oil replicates pathophysiologic processes and enables the observation of quantifiable changes in feces quantity, consistency, intestinal motility, and fluid buildup, its use as a diarrhea inducer in all models is therefore feasible.
The crude extract was statistically significant in the evaluated parameters for the onset of diarrhea, the number of fecal drops, the number and weight of wet feces, and the overall weight of all fresh feces, as shown in Table 2. This model, the castor oil-induced diarrheal model, was used to evaluate the extract’s overall antidiarrheal activity. This considerable activity in castor oil-induced diarrhea was likely brought on by intestinal lipase enzyme activity, which prevented ricinoleic acid from being released and freed. As a result, the Na+/K+-ATPase’s normal absorptive capacity was indirectly restored, and there was a corresponding increase in the intestinal absorption of Na+ as well as other electrolytes and water. Numerous herbal extracts have been shown to improve the activities of Na+/K+-ATPase, which is followed by an apparent increase in intestinal absorption and a decrease in secretory capacity.47–49 Therefore, MEML may contain phytochemicals that have the ability to stimulate the Na+/K+-ATPase on the intestinal mucosa both directly and/or indirectly. Furthermore, ricinoleic acid causes localized intestinal mucosa irritation and inflammation, which causes the release of PG and an increase in the net secretion of water and electrolytes into the small intestine.50,51 It is indicated that prostaglandin biosynthesis inhibitors prolong the time of onset of diarrhea caused by castor oil.51,52
Another study revealed that the leaf extract of Maesa lanceolata has in vitro Cyclooxygenase 1 and 2 inhibitory activity.53 Previous research suggested that the antipyretic properties of the hydroethanolic extract of Maesa lanceolata were caused by interference with the biosynthesis of prostaglandins.27 These details support the hypothesis that MEML may reduce PG-induced electrolyte and water release by reducing prostaglandin synthesis.
The stimulation of the nitric oxide (NO) pathway is another potential mechanism by which ricinoleic acid causes diarrhea.48 Ricinoleic acid causes enhanced mucosal permeability, which is connected to an increase in intraluminal fluid collection and may be harmful to intestinal epithelial cells. As a defense mechanism against intestinal damage brought on by cytotoxins such as ricinoleic acid, nitric oxide is produced in reaction to these toxins.54 The finding that castor oil-induced intestinal fluid buildup and Na+ secretion in mice pretreated with NO synthase inhibitors are reduced55,56 supports the theory that interfering with the NO pathway may be the mechanism behind the antidiarrheal effects of MEML. Previous research showed that Maesa lanceolata leaf extracts in various solvents significantly inhibited the formation of nitric acid.57,58 Therefore, it makes sense to make assumptions that the phytochemical components of MEML may have disrupted the NO pathway, reducing NO production and, consequently, intestinal fluid secretion.
It was important to suggest which mechanism (a reduction in intestinal motility and/or suppression of intestinal secretion) was responsible for this activity once MEML’s antidiarrheal activity was established. As a result, the MEML contributions to enteropooling caused by castor oil were assessed. A model was created to assess whether castor oil treatment for diarrhea induction would impede secretory elements in the gastrointestinal system. As shown in Table 3, the weight and volume of intestinal content were significantly reduced in this model compared to the negative control at 200 mg/kg and 400 mg/kg. The results suggest that the benefits of MEML may be due to an increase in the absorption of electrolytes and/or a reduction in the intestine’s hypermotility, which may improve its capacity to retain fluids, a process comparable to that of loperamide. As a result, the presence of phytochemicals that promote the absorption of electrolytes and water by lowering castor oil-mediated effects may be responsible for the apparent decrease in enteropooling caused by castor oil in the crude extract.
To evaluate the effects of extracts on castor oil-induced gastrointestinal transit in mice, the gastrointestinal propulsion test by charcoal meal, or charcoal meal test, is used. In comparison to the negative control, all tested doses of MEML considerably reduced the propulsive movement or gastrointestinal transit of charcoal meal (p<0.001) (Table 4).
Peristalsis in the GI system is increased in cases of diarrhea, according to numerous studies.13,59,60 One of the mechanisms through which antidiarrheal drugs can work is by reducing GI peristalsis. For instance, the usual medication (atropine) employed in this trial inhibited the propulsive activity in the charcoal meal due to its anticholinergic action, lengthening intestinal transit time.61,62 This prolongs the period that nutrients remain in the colon, delaying the absorption of gastrointestinal contents. This may also increase the absorption of water and electrolytes, reducing the watery texture of diarrheal stools.63 Therefore, a reduction in the intestinal transit caused by castor oil may be caused by the extracts’ anti-motility and antispasmodic qualities, which also contribute to the plant’s antidiarrheal effects. The similar percentages of motility inhibition between the 200 mg/kg and 400 mg/kg doses of the extract and atropine imply that the plant extract may exert its anti-motility effect in a manner similar to that of atropine.
The first model in this study showed that MEML has strong antidiarrheal activity based on the results from the three models. The plant extracts have anti-enteropooling activity, according to the second model (castor oil-induced enteropooling), even if it was statistically insignificant at 100 mg/kg in all parameters. When compared to the negative control, the third-model charcoal meal test displayed statistically significant action at all doses. Atropine, the positive control, had effects that were comparable to those of the drug at 200 and 400 mg/kg (Table 4). Therefore, to evaluate the impact of different solvent fractions, the charcoal meal test was utilized.
EAF was shown to have stronger anti-motility activity among the fractions than the negative control (Table 5), and RAQF showed a statistically significant effect at 200 and 400 mg/kg. Only at 400 mg/kg did the anti-motility action of n-hexane become apparent. Alkaloids, flavonoids, phenols, and terpenoids are examples of apparently secondary metabolites found in the EAF that may contribute to the reported substantial activity of these plants, either independently or in concert with other plants.
The aqueous fraction also revealed anti-motility effects, which may be explained by its secondary metabolites, such as alkaloids, phenols, and terpenoids. The difference in the percentage of inhibition might be due to the concentration (amount) of the secondary metabolites available in both the aqueous solution and EAF. Additionally, the proportion of inhibition in all fractions rose as the test dose increased, supporting the ideas presented above. The variability in the distribution and quantity of the secondary metabolites is likely what causes the variation in rank order of potency.
Overall, the presence of secondary metabolites in this medicinal plant, including flavonoids, alkaloids, tannins, saponins, phenols, and terpenoids, may be the cause of the crude extract’s antidiarrheal actions. Alkaloids, flavonoids, tannins, saponins, phenols, and terpenoids all tested positive in this study’s MEML phytochemical screening test. Each of these phytochemicals works to alleviate diarrhea using a different mechanism.
Tannins inhibit fluid secretion by denaturing proteins and coating the surface of the intestinal mucus with a tannate complex, which increases mucus resistance to chemical changes and lowers secretion.64,65 They also reduce the intracellular Ca2+ inward current and facilitate the calcium pumping mechanism, which has a spasmolytic and relaxing impact on smooth muscle.62 Prostaglandin E2 synthesis and COX-2 expression, which are crucial components of inflammatory cascades, are inhibited by flavonoids.64 Additionally, through the activation of alpha 2-adrenergic receptors and inhibition of the inward calcium current, flavonoids generate anti-motility action (97). Terpenoids inhibit the release of prostaglandins and interfere with the peristaltic movements of the intestine.64,66 Intestinal secretion and motility are inhibited by phenols.64
Alkaloids may interfere with the peristaltic movement of the intestine, slowing down the absorption of electrolytes and water.67 Saponins also prevent the release of histamine.68
The combined effects of extracts for Dfreq (delay in diarrhea start), Gmeq (reduction in gut meal motility as% inhibition), and Pfreq (reduction in the amount of wet stools as% inhibition) are measured by the antidiarrheal index (ADI). The extract’s significant antidiarrheal action is indicated by its high ADI. The 400 mg/kg crude extract has the greatest antidiarrheal index (ADI), which suggests that it has a higher level of antidiarrheal activity than the other two test levels. The increase in the effect is likely due to the presence of phytochemical components (alkaloids, flavonoids, phenols, tannins, and terpenoids) in high concentrations, which may be responsible for the antidiarrheal activity.
Conclusions
According to the findings of the current investigation, the 80% methanol extract of Maesa lanceolata Forssk leaves has anti-diarrheal properties. Additionally, in the current study, the anti-motility activity of the three solvent fractions varied in strength, with the ethyl acetate fraction being the most active, followed by the aqueous fraction and the n-hexane fraction. The combined inhibitory effects on castor oil-induced gastrointestinal motility and fluid secretion were connected to the overall antidiarrheal activity of MEML. The anti-diarrheal effects could be caused by the existence of bioactive secondary metabolites, which can act singly or in combination to produce the overall anti-diarrheal effect. With the alternative use of hydromethanolic solvents for the extraction of diarrheal medicines from the plant, this research supports the traditional use of the medicinal plant as an antidiarrheal agent.
Abbreviations
ANOVA, Analysis of Variance; EAF, Ethyl Acetate Fraction; MEML, 80% methanol extract of Maesa Lanceolata leaves; NHF, n-Hexane Fraction; OECD, Organization for Economic Cooperation and Development; PI, Peristalsis Index; RAQF, Residue Aqueous Fraction; SEM, Standard Error of the Mean; WHO, World Health Organization.
Data Sharing Statement
The datasets generated and analyzed during the current work are accessible from the corresponding author upon reasonable request.
Ethical Approval
The research and ethics committee of the Department of Pharmacology, University of Gondar, granted ethical clearance with Ref. No: SOP4/55/2014. The animals were housed in cages in an animal home that had a 12-hour light/dark cycle and were fed normal pellets and water on an ad libitum basis. All investigations were conducted in a quiet laboratory setting. The study were carried out in line with the Laboratory Animal Care and Use Handbook.69
Acknowledgments
The authors are happy to acknowledge the University of Gondar, School of Pharmacy, Department of Pharmacology, for letting us use the pharmacology laboratory settings, chemicals, and equipment for the current study.
Author Contributions
AM, BD, EM, MA and KA conceptualized the study and contributed to the original idea. AM, MA and KA had carried out the experiments. AM, EM and KA drafted the manuscript. AM, BD and MA participated in drafting the manuscript. AM, MA and KA recorded the data-related experiments. AM, BD, EM and KA performed the data analysis and drafted the results. The manuscript was reviewed and edited by AM, EM, KA, BD and MA. AM, EM and KA managed the entire project, from the creation of activity tools to data analysis. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was funded by the Department of Pharmacology, School of Pharmacy, College of Medicine and Health Science, University of Gondar.
Disclosure
The authors declare that they do not have any conflicts of interest.
References
1. World Health Organization. Diarrheal disease fact sheet [Internet]. World Health Organization Media Center; 2022:1–4. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
2. Nemeth V, Pfleghaar N. Diarrhea - StatPearls - NCBI Bookshelf. StatPearls Publishing; 2021.
3. Poitras P. The digestive system: from basic sciences to clinical practice; 2022.
4. Dereje B, Yibabie S, Keno Z, Megersa A. Antibiotic utilization pattern in treatment of acute diarrheal diseases: the case of Hiwot Fana Specialized University Hospital, Harar, Ethiopia. J Pharm Policy Pract. 2023;16(62):1–10. doi:10.1186/s40545-023-00568-7
5. Feleke DG, Chanie ES, Admasu FT, Bahir S, Amare AT, Abate HK. Two-week prevalence of acute diarrhea and associated factors among under five years’ children in Simada Woreda, South Gondar Zone, Northwest Ethiopia, 2021: a multi-central community based cross-sectional study. Pan Afr Med J. 2022;42:12. doi:10.11604/pamj.2022.42.12.32599
6. Mahyar A, Ayazi P, Shaftaroni MR, Oveisi S, Dalirani R, Esmaeili S. The effect of adding honey to zinc in the treatment of diarrhea in children. Korean J Fam Med. 2022;43(3):188–192. doi:10.4082/kjfm.21.0080
7. Colston JM, Faruque ASG, Hossain MJ, et al. Associations between household-level exposures and all-cause diarrhea and pathogen-specific enteric infections in children enrolled in five sentinel surveillance studies. Int J Environ Res Public Health. 2020;17(21):8078. doi:10.3390/ijerph17218078
8. Öner SZ, Kaleli İ, Demi RM, Mete E, Çalişkan A. Rotavirus and adenovirus prevalence in patients with acute viral gastroenteritis in Denizli, Turkey, 2017–2021. J Med Virol. 2022;94(8):3857–3862. doi:10.1002/jmv.27834
9. Reintam Blaser A, Deane AM, Fruhwald S. Diarrhoea in the critically ill. Curr Opin Crit Care. 2015;21(2):142–153. doi:10.1097/MCC.0000000000000188
10. Keely SJ, Barrett KE. Intestinal secretory mechanisms and diarrhea. Am J Physiol Gastrointest Liver Physiol. 2022;322(4):G405–20. doi:10.1152/ajpgi.00316.2021
11. Estella OU, Nneoma UT, Patrick O, Ikenna C. Quantitative phytochemical analysis and antidiarrhoeal activity of methanol leaf extract of Combretum Bauchiense Hutch. World J Pharm Res. 2021;11(1):1607–1622.
12. Nigam Y, Knight J, Williams N. Gastrointestinal tract 5: the anatomy and functions of the large intestine. Nurs Times. 2019;115(10):50–53.
13. Anbazhagan AN, Priyamvada S, Alrefai WA, Dudeja PK. Pathophysiology of IBD associated diarrhea. Tissue Barriers. 2018;6(2):e1463897. doi:10.1080/21688370.2018.1463897
14. Bruzzese E, Giannattasio A, Guarino A. Antibiotic treatment of acute gastroenteritis in children. F1000Research. 2018;7:193. doi:10.12688/f1000research.12328.1
15. Feng X, Zhuang L, Chen L, Zhao H, Huang R, Guo Z. Comparison of different probiotics in the treatment of acute diarrhea in children: a protocol for systematic review and network meta-analysis. Medicine (Baltimore). 2022;101(11):e28899.
16. Stefano G, Anil D. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition.
17. Shankar S, Rosenbaum J. Chronic diarrhoea in children: a practical algorithm-based approach. J Paediatr Child Health. 2020;56(7):1029–1038. doi:10.1111/jpc.14986
18. Apers S, Baronikova S, Sindambiwe JB, et al. Antiviral, haemolytic and molluscicidal activities of triterpenoid saponins from Maesa lanceolata: establishment of structure-activity relationships. Planta Med. 2001;67(6):528–532. doi:10.1055/s-2001-16489
19. Katuura E, Waako P, Ogwal-Okeng J, Bukenya-Ziraba R. Traditional treatment of malaria in Mbarara District, western Uganda. Afr J Ecol. 2007;45(SUPPL. 1):48–51. doi:10.1111/j.1365-2028.2007.00737.x
20. Julius G. Some pharmacological effects of the leaf extracts of Vernonia lasiopus and maesa lanceolata: plants traditionally used to treat common ailments in humans in East Africa. Am J Res Commun. 2009;5:12–42. doi:10.1016/j.jhydrol.2017.11.003
21. Abdela G, Girma Z, Awas T. Ethnobotanical study of medicinal plant species in Nensebo District, south-eastern Ethiopia. Ethnobot Res Appl. 2022;24:1–25.
22. Mercy GT, Amon A, Patrick EO, Casim UT, Clement OA, Esther K. Medicinal plants used in gynecological procedures in Uganda. J Med Plants Res. 2020;14(4):185–194. doi:10.5897/JMPR2019.6847
23. Haile A. Ethnobotanical study of medicinal plants used by local people of Mojana Wadera Woreda, North Shewa Zone, Amhara Region, Ethiopia. Asian J Ethnobiol. 2022;5(1):35–43. doi:10.13057/asianjethnobiol/y050104
24. Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of traditional medicinal plants and associated indigenous knowledge in Dawuro Zone of Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18(1):48. doi:10.1186/s13002-022-00546-4
25. Tadesse D, Eguale T, Giday M, Mussa A. Ovicidal and larvicidal activity of crude extracts of Maesa lanceolata and Plectranthus punctatus against Haemonchus contortus. J Ethnopharmacol. 2009;122(2):240–244. doi:10.1016/j.jep.2009.01.014
26. Sindambiwe JB, Calomme M, Geerts S, Pieters L, Vlietinck AJ, Vanden Berghe DA. Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J Nat Prod. 1998;61(5):585–590. doi:10.1021/np9705165
27. Fokam Tagne MA, Tchoffo A, Noubissi PA, et al. Effects of hydro-ethanolic extract of leaves of Maesa lanceolata (Mursinaceae) on acetic acid-induced ulcerative colitis in rats. Inflammopharmacology. 2021;29(4):1211–1223. doi:10.1007/s10787-021-00825-8
28. Mekonnen Bayisa Y, Seid Bultum M. Extraction of oil from Maesa lanceolata seeds and evaluation of its antimicrobial activities. S Afr J Chem Eng. 2022;40:126–133. doi:10.1016/j.sajce.2022.02.007
29. Okemo PO, Bais HP, Vivanco JM. In vitro activities of Maesa lanceolata extracts against fungal plant pathogens. Fitoterapia. 2003;74(3):312–316. doi:10.1016/S0367-326X(03)00039-X
30. Shin SY, Dekebo A, Dinku W, et al. Identification of an anticancer compound contained in seeds of Maesa lanceolata, a medicinal plant in Ethiopia. J Korean Soc Appl Biol Chem. 2014;57(4):519–522. doi:10.1007/s13765-014-4177-y
31. Katuura E, Waako P, Tabuti JRS, Bukenya-Ziraba R, Ogwal-Okeng J. Antiplasmodial activity of extracts of selected medicinal plants used by local communities in western Uganda for treatment of malaria. Afr J Ecol. 2007;45:94–98. doi:10.1111/j.1365-2028.2007.00864.x
32. Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara Region, Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):1–13. doi:10.1186/1746-4269-9-63
33. Kumar S, Rajput MK, Tickoo SB. Laws, regulations, policies and guidelines governing the care and use of laboratory animals. In: Nagarajan P, Gudde R, Srinivasan R, editors. Essentials of Laboratory Animal Science: Principles and Practices [Internet]. Singapore: Springer Singapore; 2021:23–38. doi:10.1007/978-981-16-0987-9_2
34. Walum E. Acute oral toxicity. Environ Health Perspect. 1998;106 Suppl(Suppl 2):497–503.
35. Rabiu Abubakar A, Sani IH, Malami S, Yaro AH. Quantitative determination of secondary metabolites of fractions obtained from Solanum aethiopicum (L.) Fruit. Dutse J Pure Appl Sci. 2020;6(3):25–33.
36. Mosisa Gudeta B, Melesie Taye G, Abula T, Alemayehu Gadisa D. Evaluation of anti-diarrheal activity of 80% methanol extracts of Vernonia amygdalina Delile (Asteraceae) leaves in mice. J Exp Pharmacol. 2020;12:455–462. doi:10.2147/JEP.S282669
37. Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020;8(2):603–608. doi:10.22271/chemi.2020.v8.i2i.8834
38. OECD. Organization of economic corporation and development‟s guideline for the testing of chemical No; 425. Manual. 2008;3(October):27.
39. Patil VP, Hugar S, Sukanya Pramod P, Hanakunti Math N, Pampanagouda Patil V. Evaluation of antidiarrhoeal activity of extract of Moringa Oleifera Pods. Artic Int J Pharm Phytopharm Res. 2019;9(3):128–134.
40. Birru EM, Asrie AB, Adinew GM, Tsegaw A. Antidiarrheal activity of crude methanolic root extract of Idigofera spicata Forssk.(Fabaceae). BMC Complement Altern Med. 2016;16:272. doi:10.1186/s12906-016-1252-4
41. Sisay M, Engidawork E, Shibeshi W. Evaluation of the antidiarrheal activity of the leaf extracts of Myrtus communis Linn (Myrtaceae) in mice model. BMC Complement Altern Med. 2017;17(1):103. doi:10.1186/s12906-017-1625-3
42. Timothy C, Lizzy M, Richard K, Angela M, Christine B. Antimicrobial activity and safety of Maesa lanceolata for the treatment and management of selected bacterial pathogens. J Adv Microbiol. 2018;8(3):1–8. doi:10.9734/JAMB/2018/39450
43. Nortjie E, Basitere M, Moyo D, Nyamukamba P. Extraction methods, quantitative and qualitative phytochemical screening of medicinal plants for antimicrobial textiles: a review. Plants(Basel, Switzerland). 2022;11(15). doi:10.3390/plants11152011
44. Erhirhie E, Emudainohwo T, Igboeme S, et al. Preclinical screening techniques for antidiarrheal drugs: a comprehensive review. Am J Physiol Biochem Pharmacol. 2018;7(2):61. doi:10.5455/ajpbp.20180329014330
45. Ogunro OB, Ofeniforo EB. Ethanol extract of Spondias mombin L. Leaves exhibits antidiarrhoeal activity by stimulation of Na+–K+ ATPase, inhibition of prostaglandins or suppression of nitric oxide; 2021.
46. Araújo TSL, de Oliveira TM, de Sousa NA, et al. Biopolymer extracted from Anadenanthera colubrina (Red Angico Gum) exerts therapeutic potential in mice: antidiarrheal activity and safety assessment. Pharmaceuticals (Basel). 2020;13(1):17. doi:10.3390/ph13010017
47. Shahed-Al-Mahmud M, Shawon MJA, Islam T, Rahman MM, Rahman MR. In vivo anti-diarrheal activity of methanolic extract of Streblus asper leaves stimulating the Na(+)/K(+)-ATPase in Swiss Albino Rats. Indian J Clin Biochem. 2020;35(1):72–79. doi:10.1007/s12291-018-0781-7
48. Ahmed MU, Arise RO, Umaru IJ, Mohammed A. Antidiarheal activity of catechol and ethyl 5, 8,11,14,17 - icosapentanoate-rich fraction of Annona senegalensis stem bark. J Tradit Complement Med. 2022;12(2):190–194. doi:10.1016/j.jtcme.2021.07.007
49. Dong CL, Qin Y, Ma JX, et al. The active ingredients identification and antidiarrheal mechanism analysis of Plantago asiatica L. Superfine powder. Front Pharmacol. 2020;11:612478. doi:10.3389/fphar.2020.612478
50. Ukwuani-Kwaja AN, Yakubu IL, Mustapha AS, Makun B. Antidiarrhoeal effects of hydromethanolic leaves Extract of Ipomea asarifolia in Albino Rat Model. J Complement Altern Med Res. 2019;1–7. doi:10.9734/jocamr/2019/v7i430106
51. Njideka F, Chidinma F, Nnah S, Ebaimoh F. Antimicrobial activity and anti-diarrheal potentials ofAntimicrobial activity and anti-diarrheal potentials of Psidium guajava Linn leaf extract in experimental rat models. Anim Res International. 2022;19(2):4530–4542.
52. Sarkar KK, Mitra T, Rahman MA, et al. In vivo bioactivities of Hoya parasitica (Wall.) and in silico study against Cyclooxygenase Enzymes. Biomed Res Int. 2022;2022:1331758. doi:10.1155/2022/1331758
53. Elgorashi EE, McGaw LJ. African plants with in vitro anti-inflammatory activities: a review. South African J Bot. 2019;126:142–169. doi:10.1016/j.sajb.2019.06.034
54. Olanrewaju Oyindamola J, Christian Akuodor G, Ifeanyi Obi M, Ogochukwu Nwachukwu E, Chilaka KC, Irinmwinuwa OE. Comparative assessment of the anti-diarrhoeal effects of the ethanol leaf and stem extracts of lantana camara in Wistar rats. Magna Sci Adv Res Rev. 2021;3(1):035–45. doi:10.30574/msarr.2021.3.1.0059
55. Degu A, Engidawork E, Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016;16(1):379. doi:10.1186/s12906-016-1357-9
56. Ferede YA, Zewdu WS, Zeleke MM, Alemu MA. Evaluation of antidiarrheal activity of 80% methanolic extract of the leaves of Cordia africana (Lamiaceae) in mice. Evid Based Complement Alternat Med. 2021;2021:3627878. doi:10.1155/2021/3627878
57. Elisha IL, Dzoyem JP, McGaw LJ, Botha FS, Eloff JN. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement Altern Med. 2016;16(1):307. doi:10.1186/s12906-016-1301-z
58. Adebayo SA, Amoo SO. South African botanical resources: a gold mine of natural pro-inflammatory enzyme inhibitors? South African J Bot. 2019;123:214–227. doi:10.1016/j.sajb.2019.03.020
59. Zhao SR, Ni XM, Zhang XA, Tian H. Effect of cognitive behavior therapy combined with exercise intervention on the cognitive bias and coping styles of diarrhea-predominant irritable bowel syndrome patients. World J Clin Cases. 2019;7(21):3446–3462. doi:10.12998/wjcc.v7.i21.3446
60. Anggraeni R, Aljaberi MAA, Nisha Nambiar N, Sansuwito TB, Wati NL. The relationship of supplementary feeding, breast milk (MP-ASI) to infants with the event of diarrhea. Int J Nurs Inf. 2022;1(1):1–9.
61. Obioma Obiorah E, Obu DC, Ezeanosike OB, Akuodor GC, Anusiem CA, Okorie AU. Ethanol leaf extract of Dialium guineense produces anti-diarrhoeal and gastrointestinal motility slowing activities in Wistar rats. Magna Sci Adv Res Rev. 2022;5(1):018–24. doi:10.30574/msarr.2022.5.1.0088
62. Douho Djimeli RC, Nchouwet ML, Poualeu Kamani SL, Tchoumba Tchoumi LM, Kamanyi A, Wansi Ngnokam SL. Antisecretory and spasmolytic activities of aqueous and ethanolic stem bark extracts of Nauclea diderrichii in Wistar rats. Biomed Res Int. 2022;2022:7569848. doi:10.1155/2022/7569848
63. Ahmad MH, Usman AG, Abba SI. Comparative performance of extreme learning machine and Hammerstein-Weiner models for modelling the intestinal hyper-motility and secretory inhibitory effects of methanolic leaf extract of Combretumhypopilinum Diels (Combretaceae). In Silico Pharmacol. 2021;9(1):31. doi:10.1007/s40203-021-00090-1
64. Desta GT, Adela Alemu M, Tsegaw A, Belete TM, Adugna BY. Antidiarrheal effect of 80% methanol extract and fractions of Clerodendrum myricoides (Hochst.) Vatke (Lamiaceae) Leaf in Swiss Albino Mice. Evid Based Complement Alternat Med. 2021;2021:9369173. doi:10.1155/2021/9369173
65. Bulbul IJ, Shanta AP, Rashid MA. Evaluation of antidiarrheal activity of Leea aequata L. (Family: Vitaceae) in mice models. Bangladesh Pharm J. 2022;25(2):180–187. doi:10.3329/bpj.v25i2.60969
66. Akter S, Roy R, Basher DMA. Methanolic extract of Pycreus polystachyos possesses potent antidiarrheal activity that varies in male and female mice. J Pharmacogn Phytochem. 2022;11(2):151–154. doi:10.22271/phyto.2022.v11.i2b.14381
67. Andargie Y, Sisay W, Molla M, Adela M. Evaluation of In vivo antidiarrheal activity of hydro-methanolic extract of the root of Rumex nepalensis in Swiss Albino mice. Metab Open. 2022;15:100197. doi:10.1016/j.metop.2022.100197
68. Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal activity of 80% methanolic leaf extract of Justicia schimperiana. Evid Based Complement Alternat Med. 2018;2018:3037120. doi:10.1155/2018/3037120
69. National Research Council. Guide for the Care and Use of Laboratory Animals.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.