Back to Journals » Patient Preference and Adherence » Volume 18
Evaluating the Feasibility of a Community Pharmacy-Delivered Behaviour Change Intervention to Reduce Reliever Reliance in Asthma
Authors Foot H, Beyene K , Horne R, Fingleton J, Harrison J , Chan AHY
Received 20 October 2023
Accepted for publication 31 January 2024
Published 7 February 2024 Volume 2024:18 Pages 361—371
DOI https://doi.org/10.2147/PPA.S445763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Holly Foot,1,2 Kebede Beyene,3 Rob Horne,4 James Fingleton,5,6 Jeff Harrison,1 Amy Hai Yan Chan1,4
1School of Pharmacy, The University of Auckland, Auckland, New Zealand; 2School of Pharmacy, The University of Queensland, Woolloongabba, QLD, Australia; 3Department of Pharmaceutical and Administrative Sciences, University of Health Sciences and Pharmacy, St Louis, MO, Unites States; 4Centre of Behavioural Medicine, School of Pharmacy, University College London, London, UK; 5Capital and Coast District Health Board, Wellington, New Zealand; 6Medical Research Institute of New Zealand, Wellington, New Zealand
Correspondence: Amy Hai Yan Chan, School of Pharmacy, The University of Auckland, Private Bag 92019, Auckland, 1142, New Zealand, Tel +6493737599, Email [email protected]
Purpose: The aim of this study was to evaluate the feasibility of a community pharmacy-delivered intervention to shift patients’ beliefs about short-acting beta2 agonists (SABA) in asthma management. The study targeted individual beliefs about SABA and assessed actual SABA use, focusing on reducing SABA use as well as adherence to inhaled corticosteroids (ICS) as a preventive measure.
Patients and Methods: This non-randomized, before-and-after feasibility study enrolled participants with asthma from four community pharmacies in Auckland, New Zealand. Eligible participants were aged 18 years and above and were prescribed a SABA for their asthma. The intervention included the SABA reliance questionnaire to determine the degree of SABA reliance, verbal discussions with pharmacists personalised according to the degree of SABA reliance identified, and referral to general practitioners as appropriate.
Results: Of the 44 patients who consented into the study, 19 were in the control group and 16 in the intervention group. Recruitment and retention were modest, with 10 control and five intervention participants completing the 90-day follow-up. Although not statistically significant, preliminary results indicated reduced SABA reliance and increased ICS adherence in the intervention group, and reduced SABA refill. Feedback showed that 78% of intervention participants found the information easy to understand, and 56% expressed intent to consult their general practitioners. Pharmacy staff found the intervention feasible but noted time constraints as a barrier to intervention delivery.
Conclusion: The study demonstrates that a community pharmacy-delivered intervention is feasible and acceptable to both patients and pharmacists. While preliminary results show a positive effect on reducing SABA reliance and improvement of ICS adherence, the results were not statistically significant due to the small numbers recruited. This suggests a larger randomised trial is indicated. This intervention holds promise for addressing the over-reliance on SABA in asthma management and improving adherence to preventive therapies.
Keywords: adherence, SABA, SRQ, feasibility, pharmacist, asthma
Introduction
The most recent asthma guidelines have recommended a shift away from the use of short-acting beta2 agonists (SABAs) for asthma management, with frequent SABA use (≥3 times per week) being an indicator of poorly controlled asthma.1 Because SABAs mask, rather than treat underlying inflammation, overuse can increase the likelihood of exacerbations and mortality.2 The negative effects associated with SABA overuse can be rapid; the odds of asthma-related admissions are increased by 1.45 in the three-months following SABA overuse, and SABA overuse increases asthma-related costs.3 Despite the risks, SABA over-reliance and overuse remains common, and is worsened by poor ICS adherence.2,4,5 In New Zealand, up to 50% of individuals using a SABA regularly are not using a preventer regularly.6 ICS adherence rates are typically only 25–35%, leaving many exposed to SABA-only treatment, thus reinforcing the risks of SABA over-reliance.7
Convincing patients to make such a fundamental change away from SABA is challenging, particularly when clinician time is already limited and most patients are prescribed SABA since childhood. Many patients are “attached” to their SABA, believing this to be the best way to control their asthma4,8 and thus need to be convinced of their personal need to change treatments. They may be unaware that their current ways of using SABA, which have become routine practice to them (e.g. daily), are considered overuse.
Community pharmacists are ideally placed to motivate and enable patients to reduce SABA use and to provide personalised support to individuals with asthma. Community pharmacists are well-skilled in patient counselling, providing medication information, have regular contact and established rapport with their patients. However, motivating and enabling patients to reduce SABA use can be challenging for any health professional to address. Simply providing information is unlikely to be sufficient to change behaviour.9 Patients may require discussions with health professionals in a way that addresses the individual’s beliefs.10,11 A discussion that addresses misplaced beliefs about their personal need for SABA, and persuades them of the risks of harm is required.10 As there is often limited time in consultations, there is a need for a brief intervention that can quickly and accurately identify and address any misplaced beliefs that put patients at risk of SABA over-reliance and overuse.
One method of addressing SABA reliance could be the use of a self-completion tool to encourage patients to self-reflect on and challenge existing pre-conceptions prior to interaction with a health professional. The Risk of Reliance Test (RRT) is a recently developed, brief, online intervention for patients with asthma, designed to identify and change patient beliefs driving inappropriate SABA use.12 The RRT comprises two parts: the SABA Reliance Questionnaire (SRQ) along with behaviour change messages that are personalised depending on the participant responses to the SRQ. The SRQ is a validated questionnaire that identifies patient beliefs influencing SABA over-reliance and overuse.13 The SRQ responses can be used to guide the delivery of brief, behaviour change messages designed to shift patient beliefs about SABA based on their responses to the SRQ, as part of the RRT intervention. Previous work on an online sample of participants with asthma has shown that significant changes in beliefs driving SABA use were seen after exposure to the brief messages immediately and at 2-weeks after intervention exposure (p < 0.0001).14 However, it is not clear whether this intervention can be delivered in a clinical setting, for example by pharmacists, and whether pharmacists and patients will find this acceptable in practice.
The aim of this study was to investigate the feasibility of community pharmacists delivering the RRT to stimulate discussion and feedback with patients regarding SABA use. Secondly, a preliminary evaluation of the effect of the intervention on individual’s beliefs about SABA and on actual SABA use was explored as well as its effect on asthma control and adherence to ICS.
Materials and Methods
The study follows the Consolidated Standards of Reporting Trials (CONSORT) extension for randomised pilot and feasibility trials,15 and was registered with the Australian New Zealand Clinical Trials Registry (study no.: ACTRN12620001345976). This study complies with the Declaration of Helsinki. Ethics approval was granted by the New Zealand Northern B Health and Disability Ethics Committee (ref: 20/NTB/153). All participants involved gave informed consent, including to publish anonymous responses. The full protocol has been previously reported.16
This was a non-randomised, before- and after-feasibility study of individuals with asthma attending community pharmacies in Auckland, New Zealand. Individuals presenting to one of the four enrolled community pharmacies were eligible to participate in the study if they were aged 18 years or over and prescribed a SABA as a “reliever” for their asthma symptoms. Individuals were not eligible to participate if they were using a SABA for a reason other than asthma (eg viral respiratory infection, exercise-induced asthma) or did not manage their own medicines.
Recruitment
Community pharmacy study sites were selected based on an expression of interest process via advertisement through New Zealand’s professional pharmacy body – the Pharmaceutical Society of NZ (PSNZ) email newsletter. Interested pharmacies were selected based on their reason for wishing to participate, socio-demographics of the population they serve, pharmacy location, and number of SABA prescriptions in the last year.
Participants were a sample of patients who self-selected to be involved by responding to study advertisements at the enrolled community pharmacies. Pharmacy staff also personally invited patients who presented with a prescription for an asthma medication. Interested patients scanned a QR code or used the URL on the study advertisement to complete the study survey online. Patients also had the option to fill out a paper questionnaire if they preferred. All participants who completed follow-up surveys received a NZD$10 voucher for a national retail chain.
Study Procedure
Pharmacies began in the control phase in January 2021, where patients were recruited and continued to receive usual care. After three months of control phase, pharmacists received training on the intervention and participants were then recruited into the intervention phase. Overall, the study was conducted over six months starting with the control phase from 19th January to 15th April 2021, followed by the intervention phase from 16th April to 16th July 2021.
All patients who met the eligibility criteria and agreed to the online consent form were asked to complete a survey at the pharmacy on their personal device. The survey elicited basic sociodemographic and patient characteristics, reliever overuse (SRQ),12 asthma control (Asthma Control Test [ACT]17) and self-reported adherence to ICS (Medication Adherence Report Scale [MARS-5]13), if applicable. Participants were also asked to complete the SRQ, ACT (90 days only) and MARS-5 at 30 days and 90 days after enrolment.
The SRQ assesses patient beliefs about SABA to identify patients at risk of SABA over-reliance and overuse.12 The SRQ is a validated questionnaire with a series of statements about SABA; participants indicate their level of agreement with each statement using a 5-point Likert scale, where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree and 5 = strongly agree. Higher scores indicate higher necessity beliefs for SABA, reflecting higher reliance on SABA. The ACT is a five-item questionnaire to assess asthma symptom control over the previous four weeks, with scores from 5 to 25.17 High scores indicate better asthma control. For participants who self-reported using a ICS, MARS-5 was used to assess adherence.13 MARS-5 consists of five statements regarding adherence-taking behaviours that are answered on a 5-point Likert scale, where 1 = always, 2 = often, 3 = sometimes, 4 = rarely and 5 = never, ranging from 5 to 25. A high MARS-5 score indicates better adherence.
Control
Pharmacists were asked to provide usual care to participants during the control phase. This may have involved education on asthma inhaler technique and reliever overuse. Pharmacists were blinded to control participants’ answers to the survey. Once the control participants had completed the 90-day follow-up, they were provided the results of their SRQ score. This ensured all participants had the opportunity to receive the SRQ standardised intervention (ie, the RRT).
Intervention
Participants recruited during the intervention phase of the study received usual care from their community pharmacy in addition to the intervention. The RRT is a brief, pragmatic intervention aimed to shift any misplaced beliefs identified from participant’s responses from the SRQ, with the aim of reducing SABA over-reliance and overuse and improving adherence to preventer medication.
The RRT intervention comprised three components:
- Delivery of standardised written information about SABA and asthma, tailored to the participant’s responses to the SRQ;18
- Personalised verbal discussion between the pharmacist and participant based on the specific responses to the study questionnaires; and
- Referral to general practitioner (GP) for those at risk of SABA overuse (SRQ score ≥18) or those who self-reported not using a preventer.
Participants in the intervention phase were provided with their score from the SRQ directly on their personal device after they had completed the questionnaire. The score ranged between 5 and 25 and based on this, participants were told they were either low (≤10), medium (11–17) or high (≥18) risk of relying on their reliever inhalers. Based on their personalised score, participants were also provided with information on their personal device about what their score might mean for their asthma. Intervention participants were asked to complete the SRQ immediately after receiving the intervention.
Participants were then asked to show their SRQ score to the pharmacist. The pharmacist then had a discussion with the participant regarding their responses to the SRQ. For participants who had a score of 11 or more (medium or high risk), the pharmacist gave behaviour-change messages verbally along with written information to shift beliefs, based on the specific responses to each statement in the SRQ. For participants with scores of less than 11 on the SRQ (low risk), the pharmacists provided messages that reinforced their current behaviour and strengthened their current treatment and asthma beliefs. These messages were developed by the research team, based on the extended Common Sense Model and Necessity Concerns Framework.19–21
Finally, all participants who indicated to the pharmacist that they were not using a preventer were referred to their GP. Pharmacists also encouraged the participants to have a discussion with their GP about their answers to the questionnaire and their appropriateness for preventer treatment.
Outcome Measures
Feasibility and Acceptability Outcome Measures
To assess the feasibility of community pharmacists delivering the RRT intervention, data were collected on participant recruitment and retention rates, intervention fidelity, the appropriateness of, and procedures of outcome measures pre- and post-intervention. To determine the acceptability of the intervention in the community pharmacy setting, feedback from participants and pharmacists was obtained. Participants in the intervention group were invited to complete an acceptability questionnaire directly after their first pharmacist consultation at enrolment. This questionnaire was developed using the Theoretical Framework of Acceptability,22 to assess the acceptability of the intervention’s content and pharmacist delivery of the intervention. Participants rated their agreement with statements on a 5-point Likert-type scale, with higher scores indicating higher levels of acceptability. Pharmacy staff were invited to provide feedback on the intervention after patient recruitment through a structured feedback session with a researcher, covering the intervention training, intervention content, research design issues, intervention delivery, barriers to recruitment and the potential for future implementation (Supplementary File 1).
Exploratory Analyses on Treatment Beliefs, Asthma Control and Medication Adherence
As this was a feasibility study, we aimed to identify any signals that may demonstrate that the RRT could be effective at changing behaviour. This was done to help inform future studies and gauge the appropriateness of the measures used.23 Differences in beliefs about SABA (measured by the SRQ), ACT and MARS-5 scores between the intervention group at baseline compared to the study time points were investigated. SABA refill data were also collected and compared. Data on SABA use were obtained from participants’ electronic dispensing records. Dispensing information on the number of SABA inhalers was obtained for the 90-day period prior to enrolment (baseline), and compared with the dispensing rate in the 90 and 180 days after enrolment (ie, during the study duration).
Sample Size
The primary aim of the study was to obtain estimates of feasibility and acceptability, as well as outcome variability to inform planning of a larger, sufficiently powered randomised controlled study.24 Previous research suggests a sample size of 12 per group would allow for sufficiently precise estimates of the variance of the SRQ change to use in future studies.25 The study aimed to recruit a sample of 120 participants (60 per group).
Data Analysis
Recruitment and attrition rates, quality of data collection, and number of contacts and dropouts were reported descriptively. Acceptability feedback from pharmacists was analysed qualitatively using the general inductive approach (GIA).26 Emerging themes from the feedback sessions with pharmacists on intervention acceptability were developed by systematically studying interview notes under pre-arranged topics. Any similarities and differences were also explored and documented.
Changes in continuous outcomes (SRQ, MARS-5 and ACT scores) at baseline, immediately after enrolment, over 30- and 90-days follow-up between and within intervention arms were compared using independent t-tests and paired samples t-test, respectively. To assess differences in SABA refill rates between the intervention and control over time, a between-subject design repeated measures ANOVA was performed.
Results
Feasibility
Recruitment and Retention Rates
In total, 44 participants were eligible and consented to be involved in the study from four different pharmacies across Auckland, New Zealand. Nine participants contributed no or little data and were excluded (did not complete any of the questionnaires after providing consent [n = 6], only completed the first two questions [n = 2] and one participant completed the questionnaire twice [the first questionnaire was used only]). This left 19 participants recruited into the control group and 16 participants into the intervention group. At the 90-day follow-up, 10 control participants and 5 intervention participants completed the final survey (Figure 1).
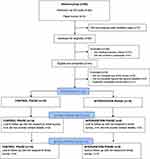 |
Figure 1 Participant flow diagram at enrolment, 30-day and 90-day study follow-up. |
Most participants were female (control = 10 [55.6%], intervention = 10 [71.4%]) with a median (IQR) age of 45.5 (23.3) and 36.0 (23.5) years in the control and intervention groups, respectively. Most reported having asthma for more than 10 years (control = 16 [88.9%]; intervention = 12 [85.7%]). Five (14.3%) participants (1 control, 4 intervention; p = 0.16) self-reported not being prescribed a preventer inhaler. Of the remaining 29 participants who reported using a preventer, there was no difference in MARS-5 score between the two groups (p = 0.08). There were 20 (57.1%) patients in the study that scored high (≥18) on the SRQ indicating high reliever reliance. Table 1 describes the full baseline characteristics of the cohort.
 |
Table 1 Baseline Characteristics of Participants (n = 32*) |
Unfortunately, it was not possible to assess intervention fidelity through pharmacist observation, due to COVID lockdowns during the intervention period and inability for the researcher to be present in the pharmacy. Instead, where possible, this was discussed during the structured pharmacist feedback session.
Participant Acceptability
Nine (56%) participants from the intervention group completed the feedback survey after receiving the intervention (Table 2). Participants were positive regarding their interaction with the pharmacist, with most agreeing the information received from the pharmacist was easy to understand (78%). However, 78% still believed they need their SABA inhaler and only 33% stating they will reduce how often they use their SABA. Based on their information provided by pharmacists, five (55.6%) participants stated they intend to have a consultation with their GP to discuss their asthma.
 |
Table 2 Participant Feedback on the RRT |
Pharmacy Staff Feedback
Two pharmacists, two pharmacy interns and one pharmacy technician individually participated in the structured feedback session. Prior to the introduction to the research and intervention, four pharmacy staff reported that they had no specific approach for supporting patients with asthma who they had identified as non-adherent. Most asthma-related counselling was for new patients only, and this focused on the correct inhaler technique. Regarding the training received for this study, all pharmacy staff reported it met their needs. Two pharmacy staff stated it would be helpful to provide training on specific patient scenarios, particularly when patients are in a rush, and provide pre-reading prior to the in-person training. Three pharmacy staff noted the main barrier to implementing this RRT intervention widely would be time/current workload.
Regarding the consultation with patients and RRT delivery, pharmacists reported having more resources available to them useful, and one pharmacist specifically stating
The study showed [me] more exposure of patients with issues. Finding more patients were overusing [their reliever] (Ph2).
Two pharmacy staff stated that people often did not show them their SRQ score, either due to time or they believed that patients did not want them to know their score was high. One pharmacist stated that patients would often provide reasons their score was high, for example acute sickness. Three pharmacy staff believed patients found the intervention resources useful, and one was unsure.
All pharmacy staff clearly articulated that their main challenge with the study was the research components (recruitment, ensuring data was collected), rather than intervention feasibility (eg, providing the intervention, patient interaction). Specifically, all pharmacy staff reported that time for pharmacy staff to invite patients into the study and for patients to go through the participant information and consent were the main barriers to recruitment of patients.
Overall, all pharmacy staff stated the experience in the study changed their practice with examples of providing more knowledge, resources and strategies to address SABA over-reliance. Pharmacist 1 stated
Intervention by itself [could be effectively delivered] yes, easy to understand. Best way would be for pharmacist to go through SRQ with patient and then calculate score right there and provide intervention on the spot.
Similarly, Pharmacist 3 noted, “Yes [it was useful], if time available it was helpful to give strategies for intervening on SABA and seeing if asthma well controlled”. All agreed the intervention could be effectively delivered in the future and would have a positive impact on asthma care in NZ. It was noted that future implementation needs to take into account pharmacy time pressures and the potential for compensation to deliver the service.
Exploratory Outcome Analyses
In patients who received the intervention, there was a trend towards decreased SRQ scores and increased ACT and ICS MARS-5 scores, although these changes were not statistically significant (Supplementary File 2). Due to lower than expected recruitment, we were unable to explore differences in GP visits for participants at high-risk of SABA overreliance, as described in outcome analysis.
Figure 2 describes the mean SABA refill rates in each group from 90 days prior to enrolment (baseline) to 90 and 180 days later. There was also a non-significant reduction in SABA use over time in the intervention group, and this was of a greater magnitude in the intervention than the control group F(1, 23)=0.278, p = 0.603.
Discussion
This study was the first to explore whether a brief community pharmacy-delivered intervention aimed at changing patients’ beliefs about SABA is feasible and acceptable to patients and pharmacy staff. Our findings show that the intervention was feasible and acceptable, that the RRT highlighted SABA-related issues for patients, and made pharmacists aware that many patients were overusing SABA. These findings are important as there have been no previous studies that have focused on reducing SABA reliance via a community pharmacy setting.
Since the introduction of AIR therapy and fundamental changes to asthma treatment introduced in 2019 guidelines, there has been a move for patients to come off SABA in favour of AIR therapy.1 This responsibility has been placed on healthcare professionals, including pharmacists, to initiate and motivate patients to change. Patients are often attached to their SABA and also have concerns about steroid-containing inhalers.8,27,28 Our study revealed that prior to our study, pharmacists had no specific technique for identifying or addressing SABA overuse. However, we found that reliever reliance is common, with 50% of the study cohort reporting high levels of reliever reliance. After receiving brief training on the intervention, pharmacists were able to provide patients with the meaning of their RRT score and provide behaviour change messages aimed at addressing SABA over-reliance in their patients. Pharmacists found the RRT useful and appreciated the resources and strategies available to them. Participants reported that the information they received was easy to understand, and over half reported that they liked the information they were provided and a pharmacist providing the intervention. Around one-third of patients reported not liking a pharmacist providing the intervention. This is not completely unexpected as this would have been a new experience for patients, suggesting a need for an adjustment period, where patients would need to become comfortable with this extra service provided by community pharmacists. As pharmacist roles are extended further such as providing clinical consultations and vaccinations, it will be critical that patients are aware of the new services offered and the skills pharmacist hold to be able to provide these services.29
Whilst there were challenges with patient recruitment and retention, the barriers identified from pharmacist feedback were related to the research rather than the intervention delivery itself. The changes in the outcome and process measures suggest that the outcome measures are appropriate. Whilst there were no significant differences between SRQ scores at enrolment and at any follow-up timepoints, the intervention group had a greater reduction in their SRQ score and a higher ACT score at study end. The findings provide important initial data to inform future clinical studies and that the SRQ appears an appropriate outcome to measure as it is directly related to the RRT intervention.
This feasibility study had several strengths. We included multiple pharmacy sites to evaluate whether the intervention delivery was feasible in different types of pharmacies. We also examined acceptability from the perspective of the patient and pharmacist. The timing of the study, however, may have underestimated recruitment potential as the study was conducted during the COVID-19 pandemic in NZ, which included several lockdowns, with various levels of restrictions. This led to patients being very cautious about spending extra time in community pharmacies and pharmacists having extra workload. Feedback from pharmacists indicated that pharmacist lack of time and patients time to read the participant information and consent form were the main barriers to patient recruitment, which is commonly reported in community pharmacy research.30,31 To overcome this in future studies, a cluster design is recommended, where randomisation occurs at the level of the pharmacy. However, measuring outcome measures at the patient level (eg reduction in SABA use) would still be most appropriate given that the intervention is individualised to the patient. It is also important to note that if the RRT was to be implemented in community pharmacies it would become part of standard practice, rather than a research study, and so this barrier would not exist.
Further research into how to improve the recruitment process and better support community pharmacy is needed. Our study explored the use of a digital component for the sign up and consent process; however, further automation or digital delivery of the intervention may have further facilitated the process, for example by using pharmacy dispensing data to flag high-risk patients for intervention delivery.
Conclusion
This study is the first to show that a brief community pharmacy-delivered behaviour change intervention to shift patients’ beliefs about SABA is feasible and is mostly acceptable to patients and pharmacists. These results provide the basis to inform the design of future larger studies to assess the effect of the intervention of patient’s beliefs about SABA and actual SABA use.
Funding
This work is supported by the University of Auckland Faculty Research Development Fund.
Disclosure
Dr Holly Foot: Freelance consultant for UCL-business company Spoonful of Sugar Ltd. Dr Kebede Beyene: Currently a guest editor for the Patient Preference and Adherence journal and reports grants from HRC 23/181, grants from HRC 21/867, and grants from HRC 21/056. Associate Professor Jeff Harrison: Personal fees and non-financial support from Enigma Solutions Ltd. and grants from Health Research Council of New Zealand, outside the submitted work. Dr James Fingleton: Grants, personal fees and non-financial support from AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim; grants from Genentech, outside the submitted work. Professor Rob Horne: Grants/research support AstraZeneca; National Institute for Health Research (NIHR), Collaboration for Leadership in Applied Health Research and Care (CLAHRC), North Thames at Bart’s Health NHS Trust and Asthma UK (AUKCAR); Honoraria/consultation fees: AbbVie, Amgen, Astellas, AstraZeneca, Biogen, Erasmus, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp Dohme, Novartis, Pfizer, Roche, Shire Pharmaceuticals, TEVA. Founder and shareholder of a UCL- Business company (Spoonful of Sugar Ltd) providing consultancy on supporting patients with medicines and treatment-related behaviours to healthcare policy makers, providers and industry. Professor Rob Horne is also supported by the National Institute for Health Research (NIHR, Collaboration for Leadership in Applied Health Research and Care (CLAHRC), North Thames at Bart’s Health NHS Trust and Asthma UK (AUKCAR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Dr Amy Chan: Director of AHYC Consultancy Ltd, providing freelance consultancy to UCL-business company Spoonful of Sugar Ltd; grants from Innovate UK, A+ charitable trust (Auckland District Health Board), Health Research Council, Oakley Mental Health Foundation and Maurice and Phyllis Paykel trust, outside the submitted work. She also received fellowship in 2023 to support research into digital technologies and asthma from Auckland Medical Research Foundation; Contractor in 2022 to advise a Masters student investigating digital technologies and organisations for AcademyeX; co-investigator on a grant to renew funding for the Asthma UK Centre of Applied Research which finishes in 2023; received a grant in 2021 to develop an asthma exacerbation risk prediction model and seed funding in 2023 to look at exhaled breath in asthma from University of Auckland; the recipient of a postdoctoral fellowship from Robert Irwin Pharmacy Foundation from 2019 to 2021; principal investigator on a research grant to look at the acceptability and effect of a digital mental health platform to Maori awarded in 2020 from Chorus Ltd; received research funding for a project in asthma from Life AI Corp; subcontract via UCL in 2021 to conduct qualitative evidence synthesis for TB antigen-based skin testing for World Health Organization; subcontract to conduct pharmacoepidemiology studies in COVID-19 for Hong Kong University. In addition, she is an unpaid board member of Asthma NZ Councillor for Pharmacy Council of New Zealand Member of Research, Policy and Implementation steering committee for ESPACOMP Member of Respiratory Effectiveness Group (REG). The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma; 2019.
2. Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:10.1183/13993003.01872-2019
3. FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–140. doi:10.1016/j.rmed.2017.08.014
4. Reddel HK, Ampon RD, Sawyer SM, Peters MJ. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-The-counter reliever use in a cross-sectional population survey. BMJ Open. 2017;7(9):e016688. doi:10.1136/bmjopen-2017-016688
5. Sadatsafavi M, Tavakoli H, Lynd L, FitzGerald JM. Has asthma medication use caught up with the evidence?: A 12-year population-based study of trends. Chest. 2017;151(3):612–618. doi:10.1016/j.chest.2016.10.028
6. Atlas of Healthcare Variation Asthma. Health quality & safety commission New Zealand; 2018. Available from: https://public.tableau.com/profile/hqi2803#!/vizhome/Asthmasinglemap2018/AtlasofHealthcareVariationAsthma?publish=yes.
7. Boulet LP, Vervloet D, Magar Y, Foster JM. Adherence: the goal to control asthma. Clinics Chest Med. 2012;33(3):405–417. doi:10.1016/j.ccm.2012.06.002
8. Cole S, Seale C, Griffiths C. ‘The blue one takes a battering’ why do young adults with asthma overuse bronchodilator inhalers? A qualitative study. BMJ Open. 2013;3(2):e002247. doi:10.1136/bmjopen-2012-002247
9. Kelly MP, Barker M. Why is changing health-related behaviour so difficult? Public Health. 2016;136:109–116. doi:10.1016/j.puhe.2016.03.030
10. Østrem A, Horne R. Reducing asthma attacks: consider patients’ beliefs. Npj Primary Care Respiratory Medicine. 2015;25(1):15021. doi:10.1038/npjpcrm.2015.21
11. Lycett H, Wildman E, Raebel EM, Sherlock JP, Kenny T, Chan AHY. Treatment perceptions in patients with asthma: synthesis of factors influencing adherence. Respir Med. 2018;141:180–189. doi:10.1016/j.rmed.2018.06.032
12. Chan AHY, Katzer C, Kaplan A, et al. SABA Reliance Questionnaire (SRQ): identifying patient beliefs underpinning reliever over-reliance in asthma. J All Clin Immunol in Practice. 2020;8:3482–3489.e1. doi:10.1016/j.jaip.2020.07.014
13. Chan AHY, Horne R, Hankins M, Chisari C. The medication adherence report scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281–1288. doi:10.1111/bcp.14193
14. Horne R, Chan A, Haughney J, Correia De Sousa J, Williams S, Kaplan A. Late breaking abstract - identifying and addressing patient beliefs driving SABA use and over-reliance. Eur Respir J. 2019;54(suppl 63):OA5333.
15. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
16. Foot H, Beyene K, Horne R, Fingleton J, Harrison J, Chan A. A study protocol for a feasibility trial in community pharmacy of a personalised behaviour change intervention to reduce reliever reliance and overuse in individuals with asthma. Protoc Exch. 2021;2021:1.
17. Thomas M, Kay S, Pike J, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Primary Care Respirat J. 2009;18(1):41–49. doi:10.4104/pcrj.2009.00010
18. International Primary Care Respiratory Group. Blue reliever reliance test; 2020. Available from: https://www.ipcrg.org/resources/search-resources/reliever-reliance-test-english.
19. Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633
20. Foot H, La Caze A, Gujral G, Cottrell N. The necessity-concerns framework predicts adherence to medication in multiple illness conditions: a meta-analysis. Patient Educ Couns. 2016;99(5):706–717. doi:10.1016/j.pec.2015.11.004
21. Horne R, Cooper V, Wileman V, Chan A. Supporting adherence to medicines for long-term conditions: a perceptions and practicalities approach based on an extended common-sense model. Eur Psychol. 2019;24(1):82–96. doi:10.1027/1016-9040/a000353
22. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88. doi:10.1186/s12913-017-2031-8
23. Orsmond GI, Cohn ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR. 2015;35(3):169–177. doi:10.1177/1539449215578649
24. Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research network database. BMC Med Res Method. 2013;13:104. doi:10.1186/1471-2288-13-104
25. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291. doi:10.1002/pst.185
26. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–246. doi:10.1177/1098214005283748
27. Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15026. doi:10.1038/npjpcrm.2015.26
28. Blakeston S, Harper G, Zabala Mancebo J. Identifying the drivers of patients’ reliance on short-acting β2-agonists in asthma. J Asthma. 2020;2020:1–8.
29. Khayyat S, Walters P, Whittlesea C, Nazar H. Patient and public perception and experience of community pharmacy services post-discharge in the UK: a rapid review and qualitative study. BMJ Open. 2021;11(3):e043344. doi:10.1136/bmjopen-2020-043344
30. Bertilsson E, Serhal S, Emmerton L, et al. Pharmacists experience of and perspectives about recruiting patients into a community pharmacy asthma service trial. Res Social Administrative Pharm. 2021;17(3):595–605. doi:10.1016/j.sapharm.2020.05.012
31. Hossain LN, Tudball J, Franco-Trigo L, Durks D, Benrimoj SI, Sabater-Hernández D. A multilevel stakeholder approach for identifying the determinants of implementation of government-funded community pharmacy services at the primary care level. Res Social Administrative Pharm. 2018;14(8):765–775. doi:10.1016/j.sapharm.2017.10.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

