Back to Journals » Patient Preference and Adherence » Volume 18
Evaluating Patient and Provider Preferences for a Once-Weekly Basal Insulin in Adults with Type 2 Diabetes
Authors Kerr D, Rajpura JR, Namvar T
Received 16 November 2023
Accepted for publication 28 January 2024
Published 14 February 2024 Volume 2024:18 Pages 411—424
DOI https://doi.org/10.2147/PPA.S436540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Video abstract presented by Kerr.
Views: 99
David Kerr,1 Jigar Ramesh Rajpura,2 Tarlan Namvar3
1Center for Health Systems Research, Sutter Health, Santa Barbara, CA, USA; 2Department of US Health Economic and Outcomes Research – Rare Disease Portfolio, Novo Nordisk Inc, Plainsboro, NJ, USA; 3Department of Evidence Synthesis and Value Assessment, Novo Nordisk Inc, Plainsboro, NJ, USA
Correspondence: David Kerr, Diabetes Research and Digital Health Equity, Sutter Center for Health Systems Research, Santa Barbara, CA, USA, Tel +1 (805) 624-8688, Email [email protected]
Purpose: The global burden of disease of type 2 diabetes (T2D) is significant, and insulin currently plays a central role in T2D management. This study sought to assess the preferences of patients with T2D and healthcare providers (HCPs) involved in T2D care regarding a hypothetical once-weekly basal insulin in comparison to current basal insulin options.
Patients and Methods: In a survey-based study in the United States that included a discrete choice experiment (DCE), patients with T2D (insulin naïve and current insulin users) and providers who treat individuals with T2D were asked to evaluate current basal insulins and identify attributes of importance regarding a hypothetical once-weekly basal insulin. A regression analysis was conducted to identify drivers of preference by relevant demographics, attitudes, and behaviors.
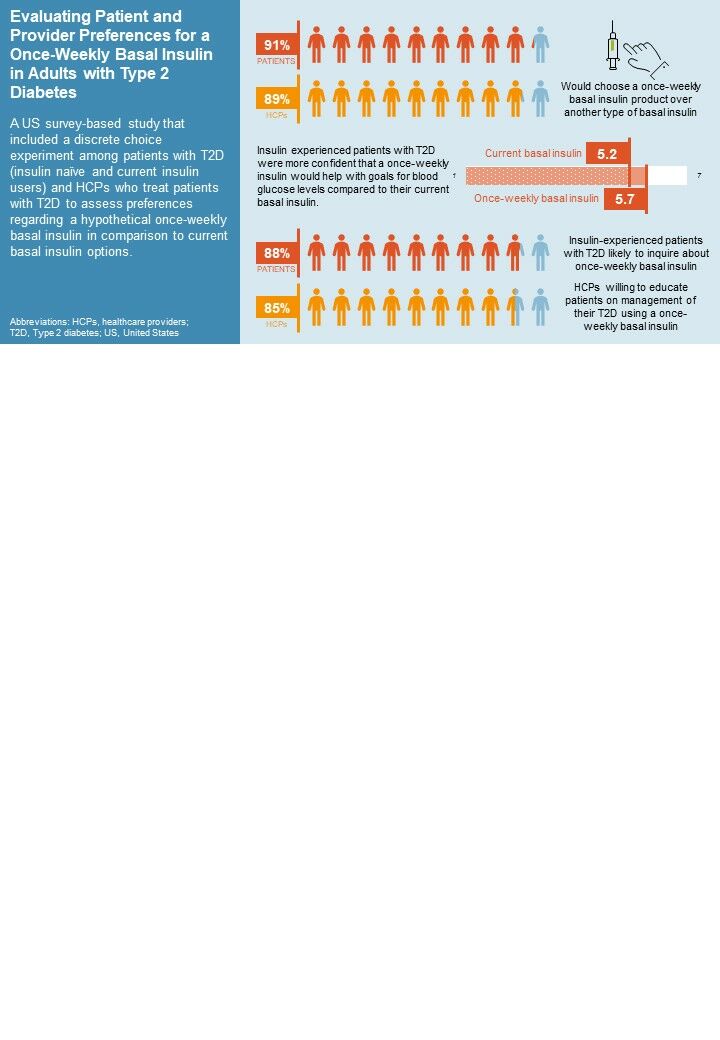
Results: Most respondents (91% of patients with T2D and 89% of HCPs in the base case scenario) would choose a once-weekly basal insulin product over another type of basal insulin. Both patients with T2D and HCPs rated insulin type and delivery method to be attributes of highest importance in the discrete choice exercise. Current basal insulin users (“insulin experienced”) reported higher levels of confidence that a once-weekly insulin would help them to achieve their desired blood sugar levels compared to their current basal insulin (5.7 vs 5.2 on a 7-point Likert scale). Most insulin-experienced respondents (88%) were likely to inquire about once-weekly basal insulin, and most HCPs (85%) indicated willingness to educate patients on management of their T2D using a once-weekly basal insulin.
Conclusion: Discussing preferences for T2D medication management is important for patients and HCPs to ensure treatments are offered for patients based on their preferences. This study showed that patient and provider preferences are similar towards a once-weekly basal insulin over current basal insulin preparations.
Plain Language Summary: Type 2 diabetes (T2D) is a medical condition where the body has difficulty managing insulin, a hormone used to control glucose, or sugar, that is broken down in the bloodstream. Many people with T2D have to take insulin to help their bodies manage their blood sugar effectively. We aimed to find out what people with T2D and healthcare providers (HCPs) think about a new type of basal insulin that only needs to be taken once a week, as compared to current basal insulins that are taken every day. We found that most people with T2D (91%) and HCPs (89%) would prefer a once-weekly insulin rather than another type of basal insulin. People with T2D currently taking insulin were also more confident that a once-weekly insulin could help control their blood sugar. Many people who are already using basal insulin (88%) said they would ask their HCP about a once-weekly insulin. HCPs said they were willing to educate patients about a once-weekly insulin (85%). This study shows that it is important for people with T2D and their HCPs to discuss what type of insulin is best for them, including the type of insulin and how often it is taken. Sharing preferences about medication options may be helpful in reducing the overall impact of the condition.
Keywords: diabetes mellitus, type 2, insulin, long-acting, surveys and questionnaires, patient preference, provider preference
Graphical Abstract:
Introduction
The global prevalence of type 2 diabetes (T2D) is significant, affecting roughly 462 million people, or 6% of the world’s population, with varying rates across age groups.1 In 2022, the Diabetes Surveillance System of the United States Centers for Disease Control and Prevention estimated that 37.3 million adults, representing 11.3% of the United States (US) adult population, were diagnosed with diabetes, primarily T2D.2,3 Many people with T2D eventually need insulin to help reduce their risk of serious complications.4 Although insulin is considered highly effective in managing T2D,5 various studies have reported a reluctance by individuals to start or continue insulin therapy6,7 and a delay in healthcare providers (HCPs) prescribing insulin therapy.8,9 Physicians may also overestimate patients’ concerns or lack of experience in the initiation and timing of insulin.10 Other important factors when considering starting or intensifying insulin in T2D include fear of hypoglycemia,11,12 health beliefs based on culture and previous experiences, and provider biases regarding the abilities of individuals to safely initiate or intensify insulin.13 Further, the adjustment of insulin doses is often led by HCPs, but timely access to high-quality care to support effective self-titration remains challenging.14 Low adherence rates and lack of continuity in insulin therapy have been identified as impediments to achieving effective glycemic control and contributors to escalated healthcare expenditures. Conversely, evidence suggests that consistent adherence to basal insulin therapy is associated with reduced healthcare resource utilization, diminished healthcare expenses, and fewer diabetes-related complications.15
Currently available subcutaneous basal insulin formulations are dosed once or twice a day.16 Furthermore, missed or mistimed doses of basal insulin are common17 and can negatively impact blood glucose control.18 Insulin analogs with longer duration of action and less variability in pharmacokinetics may offer greater dosing flexibility and convenience, which has the potential to improve adherence to insulin therapy.19 Newer formulations of longer acting basal insulin regimens that can maintain consistent blood glucose levels throughout the week may have the potential to offer flexibility in injection timing, reduce the burden of daily injections, enhance a person with diabetes’s treatment experience, attenuate injection-related complications, and potentially decrease glucose variability. The aim of this study was to examine the perspective and perceptions of HCPs and of adults with T2D (currently taking basal insulin and those who are insulin naïve) regarding once-weekly basal insulin relative to the once-a-day dosing standard of care (SOC). The study also sought to understand similarities and differences between patients’ treatment preferences and HCPs’ prescribing decisions.
Materials and Methods
Study Design and Ethical Approval
A cross-sectional study consisting of an online survey was conducted in the US among adults with T2D and HCPs who treat people with T2D. Data were collected from November 18, 2022, to January 5, 2023. All respondents were recruited via email from online panel companies (ClinicalVoice Community, Dynata, LLC, and Schlesinger Group) to which respondents had previously provided permission to be contacted for research purposes. Upon entering the survey, participants provided consent and were required to qualify for the survey by meeting screening criteria. Eligible respondents completing the entire survey received a modest stipend for their participation. Sample sizes were determined based on prior research from a targeted literature review, which reported sample sizes varying between 100–600 participants.20–24 Quotas were set for key patient and HCP subgroups.
The study protocol submitted to the WCG Institutional Review Board was determined to be exempt because the research included survey procedures with adequate provisions to protect the privacy of participants and maintain data confidentiality. Respondents provided consent to participate and were made aware that they could discontinue their participation at any time.
Survey Design
Separate quantitative surveys (Appendix 1) were used for patients and HCPs to measure preferences, perspectives, and attitudes toward once-weekly basal insulin compared to SOC.5 The patient and HCP surveys were developed based on the targeted literature review. The patient survey was pretested among six patients (three insulin naïve and three current insulin users [“insulin experienced”]) using web-assisted telephone interviews to assess face validity of the survey questions. Minor revisions were made for clarity and relevance based on feedback from the pretests. The HCP survey was not pretested.
The surveys consisted of two parts: 1) a variety of yes/no, multiple-choice, and Likert-scale questions on a seven-point scale (where one was strongly disagree / extremely unsatisfied / restrictive / not at all influential and seven was strongly agree / extremely satisfied / convenient / extremely influential) or an 11-point scale (where zero was not at all likely and ten was extremely likely); and 2) a discrete choice exercise (DCE), a research technique used to elicit preferences based on hypothetical choice sets.25 In the DCE, patients selected which basal insulin they would most prefer to use to self-manage their T2D. Patients were instructed to assume the following as they made their decisions in the DCE: a) “you can afford all medication options”, b) “the cost to you is the same for all medications”, c) “all medications are available at your preferred pharmacy”, d) “all medications have been approved for use in patients with type 2 diabetes by the FDA”, and e) “all medications have been approved by your doctor.” HCPs indicated how many of their next ten patients with T2D (who were eligible for basal insulin) they would recommend/prescribe for each medication shown. HCPs were instructed to assume the following as they make their decisions: a) “all of your patients can afford all medication options”, b) “the cost is within the same range for all medications”, c) “all medications are available at patients’ preferred pharmacies”, and d) “all medications have been approved for the management of type 2 diabetes by the FDA.”
Attributes selected for the DCE and their tested levels were also chosen based on the targeted literature review26–28 and the ONWARDS clinical trials investigating once-weekly insulin icodec in diabetes.29–32 Attributes identified and presented to insulin-experienced patients and HCPs are presented in Supplemental Figure 1 and Supplemental Figure 2, respectively. The following attributes were identified: 1) insulin type, 2) dose timing (patients) or administration timing (HCPs), 3) insulin delivery method, 4) glycemic control (phrased as “change in HbA1c” in the exercise), and 5) low blood sugar risk (patients) or hypoglycemic risk (HCPs). Attributes and their tested levels did not change for patients or HCPs.
An analysis of the DCE was conducted to measure preferences to use (patients) and prescribe/recommend (HCPs) a once-weekly insulin product under a variety of different scenarios. Additional regression modeling was used to further delineate patient and HCP drivers of overall preference for a once-weekly basal insulin. Prior to modeling, specified variables were analyzed for correlations and relative importance in predicting once-weekly insulin preference. Variables with low correlations/importance or insufficient variability were removed.
Participants
The study samples were independent; patients and HCPs surveyed were not matched pairs. Patients were included in the study if they were US residents, aged ≥18 years, diagnosed with T2D by an HCP, currently treated with a medication prescribed by an HCP for T2D management, and not using an insulin pump. HCPs qualified for the study if they: were employed in US facilities (except Vermont to comply with Sunshine Act reporting requirements); were a physician, nurse practitioner, physician assistant, diabetes educator, registered dietician, or registered nurse; worked in endocrinology or primary care (internal medicine, family medicine, or general practice); spent at least 50% (if diabetes educator, registered dietician, registered nurse) or 70% of time in direct patient care (if a physician, nurse practitioner, physician assistant); were in practice between three and 35 years; and primarily practiced in a private practice, a private or academic hospital, acute care/outpatient center, long-term care/nursing home, or diabetes center/clinic. Additionally, HCPs had to manage a specific number of patients with T2D per month (≥10 for diabetes educators/nurse practitioners/physician assistants and ≥20 and ≥50 for physicians practicing in primary care and endocrinology, respectively), and at least 20% of their patients with T2D had to be taking basal insulin. HCPs also had to self-report being at least somewhat confident in managing a patient with T2D taking basal insulin (ie, selecting a five, six, or seven on a seven-point scale of “not at all confident” to “extremely confident”). Diabetes educators were also required to provide medication counseling for patients with T2D.
For the purposes of this study, primary care providers (PCPs) were considered a physician, nurse practitioner, or physician assistant working in internal medicine, general practice, or family practice. Specialists were considered a physician, nurse practitioner, or physician assistant working in endocrinology or diabetes educators, registered nurses, or registered dieticians working in primary care, internal medicine, general practice, family practice, or endocrinology.
Statistical Analyses
De-identified survey data were collected and utilized in this study. Descriptive statistical analyses (means, frequencies) of the aggregated data using Q Research Software for Windows (a Division of Displayr, Inc., New South Wales, Australia) were performed. Categorical data were expressed as frequencies and proportions. Continuous and count variables are presented using mean and standard deviation (SD). Continuous variables were categorized into intervals as relevant. Tests of differences (chi square, t-tests) within respondent types were performed using Q Research Software tables; additional analyses were performed using Microsoft Excel, R Statistical Software (R Core Team 2021), and CBC Hierarchical Bayes (v5.5.6, Sawtooth Software, Inc). Statistical significance was set at p<0.05, using 2-tailed tests.
Subgroup analyses were conducted to compare patients who were 1) insulin naïve, defined as those taking some form of medication for T2D but not insulin, and 2) insulin experienced, defined as those currently taking basal insulin for T2D but not via an insulin pump. Comparisons were also made between 1) patients who were injection naïve, defined as not taking an injectable medication for their T2D or another condition, and 2) injection user (“injection experienced”), defined as an insulin user or those taking an injectable medication for their T2D or another condition that is injected at home without the help of an HCP. Additional analyses conducted were stratified by duration of T2D: 1–10 years, 11–20 years, and ≥21 years. Analyses were also performed to compare PCPs and specialists.
The DCE was analyzed separately using a statistical model to develop utility scores for the tested attributes and levels. The statistical model is a hierarchical Bayesian multinomial regression model that utilizes Markov Chain Monte Carlo (MCMC) methods for estimating parameters. Posterior estimates are taken from distribution of MCMC draws when the model shows appropriate convergence. Regression models were developed to isolate demographic, attitudinal, and behavioral differences with respect to preference for a once-weekly basal insulin product. Preferences for a once-weekly basal insulin product, derived from the analysis of the DCE, were used as a dependent variable, while independent variables were chosen based on hypothesized relationships. The set of hypothesized independent variables was reduced prior to modeling based on insufficient variation, bi-variate correlations, and relative importance in predicting once-weekly basal insulin preference. From there, a stepwise model was used to remove variables that resulted in worse overall model fit to the data. Beta-regression models were used as the dependent variable was on a probability scale between 0 and 1.
Results
Sample Characteristics
Of the 21,931 invitations sent to patients, 1216 patients entered the survey, and 401 qualified and completed the survey. The remaining respondents did not complete the survey, did not qualify, or were over the quota set for the survey. Of the qualified patients, n=200 were categorized as insulin naïve and n=201 as insulin experienced as per survey quotas. Approximately one-third (36%, n=143) of patients were injection naïve and 64% (n=258) were injection experienced. Of the 4209 invitations sent to HCPs, 3277 entered the survey, and 362 qualified and completed the survey. All other HCP respondents either did not complete the survey, were not qualified, or were over quota for the survey. Sample characteristics of patients and HCPs are shown in Table 1.
 |
Table 1 Sample Characteristics |
Patients’ Experiences and Perceptions of T2D Management by Insulin Use
Just over half (54%) of insulin-experienced patients were extremely satisfied with their current basal insulin medication. Most strongly agreed that they were able to take it as prescribed (88%), were confident in their ability to administer the dose (84%), and were comfortable administering the dose (83%). A similar proportion of patients who were insulin naïve (51%) were extremely satisfied with their current T2D medication.
Patients who had been diagnosed with T2D for 21 years or more were most likely to consider once-weekly basal insulin to be highly convenient (86% vs 70% of those with T2D for 1–10 years and 71% of those with T2D for 11–20 years, p<0.05). Insulin-experienced patients reported a significantly higher level of agreement (p<0.05) with almost all attributes related to use of a hypothetical once-weekly insulin product for T2D management compared with patients who were insulin naïve (Figure 1). However, patients who were insulin naïve agreed that a once-weekly basal insulin would be both convenient to take and easy to administer, that they would be both comfortable and confident in administering the dose, and that they would be able to take it as prescribed (Figure 1).
 |
Figure 1 Agreement with Once-Weekly Insulin Attributes for Management of T2D Among Patients who are Insulin Naïve and Insulin Experienced. Abbreviation: T2D, type 2 diabetes. |
Patients’ Experiences and Perceptions of T2D Management by Injection Experience
Less than half (41%) of injection-experienced patients were extremely satisfied with their current T2D medication, significantly less that that reported by patients who were injection naïve (53%, p<0.05). Injection experienced patients were equally satisfied with the method of administering their medication and how often they need to take their medication (mean of 5.7 for both), and they were most satisfied with the flexibility of their medication schedule (mean of 5.8). Injection experienced patients had significantly higher levels of agreement compared with patients who were insulin naïve on statements regarding comfort in changing (attaching/removing) the needle on a syringe or injection device (76% strongly agree vs 40%, p<0.05) and having no concerns about injecting themselves with a needle (69% strongly agree vs 30%, p<0.05).
HCPs’ Perceptions and Attitudes Toward T2D Management
Half of all HCPs (50% of PCPs and 50% of specialists) reported they were very satisfied with the current treatment options available for T2D (rating a six or seven on a seven-point scale where one was “extremely unsatisfied” and seven was “extremely satisfied”). PCPs were less satisfied than specialists with current basal insulin options (39% vs 50%, p<0.05). When asked to rate statements on a seven-point scale where one indicated “does not describe my beliefs at all” and seven indicated “describes my beliefs completely”, PCPs were more likely than specialists to report that prescribing basal insulin is a hassle because it takes a lot of time to train patients on managing their doses (3.3 vs 2.6, p<0.05), it takes a lot of time to manage patients’ disease progression (3.2 vs 2.5, p<0.05), and it takes a lot of time to train patients in how to inject their doses (3.2 vs 2.3, p<0.05). Specialists were slightly more likely than PCPs to agree that basal insulin is easy for their patients to administer and convenient for their patients to take (5.3 and 4.9 vs 4.9 and 4.6, respectively, p<0.05). When asked to indicate their level of agreement related to once-weekly basal insulin, HCPs mostly agreed that a once-weekly option would be convenient for their patients to take, easy for their patients to administer, and would have a minimal impact on their patients’ day-to-day lives (Figure 2).
 |
Figure 2 Healthcare Providers’ Level of Agreement with Once-Weekly Insulin Attributes for Management of type 2 diabetes. Abbreviation: HbA1c, hemoglobin A1C. |
DCE Analysis
A base case scenario was created for the analysis of the DCE in which attributes of the hypothetical once-weekly basal insulin product were established as 1) ultra long-acting insulin type, 2) administered any time of the day for each dose, 3) delivered using a multiple-dose disposable pen, 4) offering glycemic control of a 1.6%-point decrease of HbA1c (insulin naïve) or 0.9%-point decrease (insulin experienced), and 5) for patients, having hypoglycemic risk based on one event in three years (insulin naïve) or one event in two years (insulin experienced), and for HCPs, having hypoglycemic risk based on 0.3 events per patient per year (insulin naïve) or 0.7 events per patient per year (insulin experienced).
Across all patients, insulin delivery method (33%) and insulin type (31%) were rated as the most important attributes in the DCE whereas administration timing, achieved glycemic control, and hypoglycemic risk were relatively least important. Insulin type was more important to patients who were insulin naïve and injection naïve (35% each) than to insulin experienced (26%) and injection experienced (28%). HCPs also considered insulin delivery method and insulin type to be the most important attributes; insulin delivery method and delivery type were slightly more important to specialists than to PCPs (34% vs 32% and 33% vs 27%, respectively). Administration timing, hypoglycemic risk, and glycemic control were relatively least important.
When presented with the option for a hypothetical once-weekly basal insulin in the base case scenario, almost all patients chose the once-weekly option over other basal insulins (Figure 3). In another scenario, where patients were asked about their preferences for a once-weekly option, nearly three-quarters (71%) of patients chose once-weekly over all other options (20% for the current SOC and 9% for other basal insulin). Similarly, the majority of HCPs selected a once-weekly option in the base case scenario (Figure 4). Nearly two-thirds (64%) of HCPs indicated they would prefer a once-weekly basal insulin over current SOC long-acting basal insulins (28%) or other basal insulin (9%) for their patients with T2D. In evaluating the preference share changes for a hypothetical once-weekly basal insulin, decreasing the number of hypoglycemic events had the most positive impact. Specifically, the change of 0.7 events per patient year (as stated in the base case scenario) to 0.3 events per patient year had the most positive impact on preference share for HCPs (6% for PCPs and 4% for specialists), meaning their preference for a hypothetical product increased when the number of such events decreased in patients.
 |
Figure 3 Basal Insulin Preference among Patients in the Base Case Scenario. |
Likelihood of Patient Requests and HCP Recommendations
Although more insulin experienced than insulin naïve patients were highly likely to ask their doctor about using a once-weekly basal insulin once presented with a description of the hypothetical treatment, a minority of insulin naïve patients were not at all likely (Figure 5). Similar patterns were observed by injection experience (Figure 5). A similar proportion of patients who were insulin naïve and insulin experienced (67% and 71%, respectively) indicated it was extremely important that their doctor provide information about a once-weekly basal insulin as a factor in their decision-making for T2D. PCPs and specialists indicated an equal level of willingness (85% each) to educate patients on management of their T2D using a once-weekly basal insulin.
Most HCPs (90%) indicate patients willing to take an insulin or who use a continuous glucose monitor (CGM) device for blood glucose monitoring are the patients for whom they would recommend or prescribe a once-weekly option. The number of medications a patient is currently taking is also a consideration; 78% would prescribe a once-weekly product if a patient is taking one to two other medications as opposed to “no other medications” (33%) or “three or more medications” (63%).
Results of a regression analysis showed that both PCPs and specialists had a greater preference for a once-weekly insulin when they received a request to prescribe a once-weekly basal insulin (odds ratio [OR]=1.23, 95% confidence interval [CI]: 1.09–1.39 and OR=1.29, 95% CI: 1.12–1.49, respectively). PCPs who were more willing to educate their patients about a once-weekly insulin had a greater preference for a once-weekly insulin (OR=1.21 95% CI: 1.0–1.46). Patients’ preference for a once-weekly insulin increased as the importance of a doctor’s recommendation in the decision to take a once-weekly insulin also increased (OR=1.14, 95% CI: 1.06–1.22). However, as patients felt more equipped to cope with the effort it takes to manage T2D, they indicated less of a preference for once-weekly insulin (OR=0.86, 95% CI: 0.80–0.93).
Discussion
The results of this study suggest that both patients with T2D and providers managing T2D prefer a once-weekly basal insulin product over current SOC. Insulin delivery method and insulin type were of greatest relative importance to HCPs and insulin-experienced patients, who reported convenience and ease of administration to be improvements over current basal insulins. Conversely, glycemic control, hypoglycemic risk, and administration timing were considered by patients and HCPs to be relatively less important attributes.
Other studies have shown mixed results regarding the importance of glycemic control to patients with T2D when asked about their preferences for treatment, though the study designs varied from ours. Savarese et al found that patients placed more value on medication side-effect profiles and tolerability,33 and Ozdemir et al reported that patients with T2D placed less importance on weight loss and the effectiveness of the medications, instead preferring lower risks of hospitalization.34 Liu et al demonstrated that patients with T2D considered glycemic risk and efficacy to be more important than the mode of administration.35 Huang et al found that glycemic control followed by frequency of hypoglycemic events were most important to patients with T2D.36
Here, it appears that HCP recommendations strongly influenced patients’ decisions and that patient requests were a driver of preference for a once-weekly basal insulin among HCPs, suggesting there is potential for patients and HCPs to align on treatment options for the management of T2D based on stated preferences. By using a patient-centered approach to care, HCPs can involve patients in a discussion about aspects of a therapy that has the potential to improve pharmacoadherence.37 This approach also allows people with diabetes to have the opportunity to contribute actively in making informed decisions about their treatment. By utilizing this type of patient-centered care model, specifically in T2D management, HCPs can also help to facilitate effective engagement by adults with T2D in self-monitoring and self-management of diabetes.38
Further, utilizing a team-based care model has been shown to improve communication among specialists treating patients with T2D, leading to improvements in patient outcomes.39 Healthcare providers involved in team-based care may be able to utilize outputs from population segmentation strategies, which can help to identify patients who potentially benefit from different types of insulin, such as once-weekly therapies.40 Because patients are unique and their diabetes treatments may depend upon a number of clinical and sociodemographic factors, narrowing patient preferences based on subgroup analyses has the potential to help HCPs apply more precise treatment recommendations.41
The American Diabetes Association and Association of Diabetes Care and Education Specialists recently revised their standards for diabetes self-management and support.42 In the new guidelines, diabetes self-management education and support are considered paramount to improving clinical outcomes and quality of life.42 As such, healthcare teams who care for patients with T2D are encouraged to use a “person-centered approach” that emphasizes shared decision-making, goal setting and empowerment-based strategies, among others.42 The World Health Organization recently adopted global health targets for diabetes, which aim to set a standard that, by 2030, 80% of people with diabetes will have good glycemic control and that diabetes medications are provided equitably, comprehensively, and affordably.43
Persistence and adherence to insulin therapy also represent areas of concern for T2D. Persistence, defined as the period of time when an individual initiates therapy until discontinuation, and adherence, defined as the extent to which a patient acts within compliance of a stated treatment regimen, are notable factors in diabetes care.6,16 Consistency in insulin therapy administration and dosing is known to be associated with improved outcomes due to fewer episodes of insulin omission and reduced risks of adverse events.6,37,44 A once-weekly basal insulin regimen may potentially reduce the burden of daily injections, enhancing treatment experience and minimizing injection-related complications. Such a dosing approach also has the potential to result in improved clinical outcomes and reduced healthcare expenditures.
Limitations
This study has several limitations, many of which exist in cross-sectional studies with survey-based research. Patient responses included self-reported diagnoses of T2D that were not verified by medical records. Further, patients and HCPs did not represent matched pairs; therefore, differences in responses from patients and HCPs may represent real differences between the groups and not differences in perception. Sample sizes were calculated using assumptions based on prior experience;23,24 the lack of a power calculation may limit the overall generalizability of the study. Patients and HCPs who responded to the survey are not as demographically diverse as patients with T2D and HCPs who treat them, and respondents may not be entirely representative of patients with T2D or HCPs regarding their preferences toward a hypothetical medication, both of which limit overall generalizability. Selection bias was limited by having a randomized sample of participants screened for the main survey; however, this did not guarantee exact representation of the general population with T2D and providers who treat T2D. Patient respondents had to have access to and familiarity with online surveys, which may represent a more informed subset of the population and exclude those who lack the accessibility of online surveys. Responder bias may have been present if participants did not fully understand the questions or directions for the DCE and therefore adjusted their responses to something that did not accurately reflect their true preferences. Additionally, findings from this study may have varied between patients whose HbA1c levels were considered “in control” or “in range” and those who had inadequate glycemic control.
Although the attributes used in the DCE were identified through a targeted literature review and research of other patient preference studies, attribute selection may have simplified true real-world scenarios. Additionally, conjoint studies may differ from real-world situations because study respondents have equal information about the competing alternatives, there are no supply or budget constraints in our model, preference share often does not include adjustments for the proportion of the marketplace a respondent represents, and not all brand or supply options may be available to all consumers in the real-world market. Attitudes toward a once-weekly approach may be tempered by potential concern HCPs may have regarding patients’ ability to remember to take their medication. While perceptions of a once-weekly option were generally positive in this study, some reservations may exist among HCPs in prescribing or recommending a change in dosing regimen. Lastly, although a conjoint study assumes independence of choices by respondents, in reality, social desirability bias may have been inherent. However, this was largely mitigated by providing equal options and information about medication attributes across patient and HCP sample groups who were broadly generalizable.
Conclusion
With the growing rates of T2D and the subsequent demand for treatments, many opportunities exist to improve diabetes management. The results of this study showed that patients and HCPs may have similar goals in mind for basal insulin therapies, as both groups expressed more preference for a once-weekly basal insulin option and placed more relative importance on the same product attributes. Under certain circumstances, there is evidence that once-weekly basal insulin may be a preferred option compared to once-daily insulin as part of the management of T2D. When several basal insulin therapies are available to patients, HCPs should consider their patients’ treatment goals and impacts on lifestyle in their decision-making, especially if a therapy becomes available that has the potential to reduce the burden of treatment.
Abbreviations
CGM, continuous glucose monitor; DCE, discrete choice experiment; HCP, healthcare provider; HbA1c, glycated hemoglobin; MCMC, Markov Chain Monte Carlo; PCP, primary care provider; SD, standard deviation; SOC, standard of care; T2D, type 2 diabetes; US, United States.
Data Sharing Statement
Data are available upon reasonable request to the corresponding author.
Ethical Approval and Informed Consent
WCG Institutional Review Board prospectively reviewed the study and considered it exempt because it is an online survey with adequate protections in place to protect the privacy of subjects and to maintain the confidentiality of data. The study and data accumulation were in conformity with all country, federal, or state laws, informed consent was obtained from participants, and the study was in adherence to the tenets of the Declaration of Helsinki.
Acknowledgments
The authors thank Stephanie Burkhead, MPH, Rebecca Hahn, MPH, and Ladan Panahi, PharmD, of KJT Group, Inc. Rochester, NY, for medical writing support, which was funded by Novo Nordisk Inc.
Funding
Novo Nordisk Inc. funded the study and had a role in the study design, data collection, analysis, and interpretation of data, as well as writing support of the manuscript.
Disclosure
DK is an employee of Sutter Health, has received consultancy fees from Sanofi, Abbott Rapid Diagnostics, Proteomics and Better Therapeutics and research support from Novo Nordisk and Abbott Diabetes Care. JRR is an employee of and shareholder in Novo Nordisk Inc. At the time of the study, TN was an employee of Novo Nordisk Inc. The authors report no other conflicts of interest in this work.
References
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi:10.2991/jegh.k.191028.001
2. National Diabetes Statistics Report website; 2002. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
3. Fang M, Wang D, Coresh J, Selvin E. Undiagnosed Diabetes in U.S. Adults: prevalence and Trends. Diabetes Care. 2022;45(9):1994–2002. doi:10.2337/dc22-0242
4. Laiteerapong N, Ham SA, Gao Y, et al. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care. 2019;42(3):416–426. doi:10.2337/dc17-1144
5. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140–s157. doi:10.2337/dc23-S009
6. Yavuz DG, Ozcan S, Deyneli O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient Prefer Adherence. 2015;9:1225–1231. doi:10.2147/ppa.S87935
7. United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310(6972):83–88.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (Dawn) study. Diabetes Care. 2005;28(11):2673–2679. doi:10.2337/diacare.28.11.2673
9. Polonsky WH, Jackson RA. What’s so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes. 2004;22(3):147–151.
10. Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488–496. doi:10.1111/dom.13132
11. Blumer I, Clement M. Type 2 Diabetes, Hypoglycemia, and Basal Insulins: Ongoing Challenges. Clin. Ther. 2017;39(8, Supplement 2):S1–S11. doi:10.1016/j.clinthera.2016.09.020
12. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418–24.
13. Kerr D, Warshaw H. Clouds and Silver Linings: COVID-19 Pandemic Is an Opportune Moment to Democratize Diabetes Care Through Telehealth. J Diabetes Sci Technol. 2020;14(6):1107–1110. doi:10.1177/1932296820963630
14. Kerr D, Edelman S, Vespasiani G, Khunti K. New Digital Health Technologies for Insulin Initiation and Optimization for People With Type 2 Diabetes. Endocr Pract. 2022;28(8):811–821. doi:10.1016/j.eprac.2022.04.006
15. Perez-Nieves M, Boye KS, Kiljanski J, Cao D, Lage MJ. Adherence to Basal Insulin Therapy Among People with Type 2 Diabetes: A Retrospective Cohort Study of Costs and Patient Outcomes. Diabetes Ther. 2018;9(3):1099–1111. doi:10.1007/s13300-018-0421-5
16. Sarbacker GB, Urteaga EM. Adherence to Insulin Therapy. Diabetes Spectr. 2016;29(3):166–170. doi:10.2337/diaspect.29.3.166
17. Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28(12):1933–1946. doi:10.1185/03007995.2012.743458
18. Robinson S, Newson RS, Liao B, Kennedy-Martin T, Battelino T. Missed and mistimed insulin doses in people with diabetes: a systematic literature review. Diabetes Technol. Ther. 2021;23(12):844–856.
19. Rolla A. Pharmacokinetic and pharmacodynamic advantages of insulin analogues and premixed insulin analogues over human insulins: impact on efficacy and safety. Am J Med. 2008;121(6 Suppl):S9–s19. doi:10.1016/j.amjmed.2008.03.022
20. Boye K, Ross M, Mody R, Konig M, Gelhorn H. Patients’ preferences for once-daily oral versus once-weekly injectable diabetes medications: the REVISE study. Diabetes Obes Metab. 2021;23(2):508–519. doi:10.1111/dom.14244
21. Boye KS, Jordan JB, Malik RE, Currie BM, Matza LS. Patient Perceptions of and Preferences Between Characteristics of Injectable Diabetes Treatments. Diabetes Therapy. 2021;12(9):2387–2403. doi:10.1007/s13300-021-01097-9
22. Flood EM, Bell KF, de la Cruz MC, Ginchereau-Sowell FM. Patient preferences for diabetes treatment attributes and drug classes. Curr Med Res Opin. 2017;33(2):261–268. doi:10.1080/03007995.2016.1253553
23. King A, Rajpura J, Liang Y, Paprocki Y, Uzoigwe C. Impact of cardiovascular disease on health care economic burden and resource utilization: a retrospective cohort study in adults in the United States with type 2 diabetes with or without stroke, myocardial infarction, and peripheral arterial disease. Curr. Med. Res. Opin. 2022;38(11):1831–1840. doi:10.1080/03007995.2022.2125259
24. Tomaszewski KJ, Allen A, Mocarski M, et al. Divergence in perceptions of diabetes control among patients with type 2 diabetes mellitus treated with basal insulin and health care professionals: results from the US Perceptions of Control (POC-US) study. Patient Prefer Adherence. 2019;13:761–773. doi:10.2147/ppa.S194598
25. Noben CYG, Stammen LA, Vaassen S, et al. Discrete choice experiment on educating value-based healthcare. Postgraduate Medi J. 2020;97(1150):515–520. doi:10.1136/postgradmedj-2019-137190
26. Garcia-Dominguez JM, Muñoz D, Comellas M, Gonzalbo I, Lizán L, Polanco Sánchez C. Patient preferences for treatment of multiple sclerosis with disease-modifying therapies: a discrete choice experiment. Patient Prefer Adherence. 2016;10:1945–1956. doi:10.2147/ppa.S114619
27. Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. doi:10.2147/ppa.S101821
28. van Heuckelum M, Mathijssen EG, Vervloet M, et al. Preferences of patients with rheumatoid arthritis regarding disease-modifying antirheumatic drugs: a discrete choice experiment. Patient Prefer Adherence. 2019;13:1199–1211. doi:10.2147/ppa.S204111
29. Lingvay I, Asong M, Desouza C, et al. Once-Weekly Insulin Icodec vs Once-Daily Insulin Degludec in Adults With Insulin-Naive Type 2 Diabetes: the ONWARDS 3 Randomized Clinical Trial. JAMA. 2023;330(3):228–237. doi:10.1001/jama.2023.11313
30. Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023;11(6):414–425. doi:10.1016/s2213-8587(23)00093-1
31. Philis-Tsimikas A, Bajaj HS, Begtrup K, et al. Rationale and design of the phase 3a development programme (ONWARDS 1-6 trials) investigating once-weekly insulin icodec in diabetes. Diabetes Obes Metab. 2023;25(2):331–341. doi:10.1111/dom.14871
32. Singh AK, Singh A, Singh R, Misra A. Once-weekly basal insulin icodec: Looking ONWARDS from pharmacology to clinical trials. Diabetes Metab Syndr. 2022;16(9):102615. doi:10.1016/j.dsx.2022.102615
33. Savarese G, Sharma A, Pang C, Wood R, Soleymanlou N. Patient preferences for newer oral therapies in type 2 diabetes. Int J Cardiol. 2023;371:526–532. doi:10.1016/j.ijcard.2022.09.009
34. Ozdemir S, Baid D, Verghese NR, et al. Patient Preferences for Medications in Managing Type 2 Diabetes Mellitus: A Discrete Choice Experiment. Value Health. 2020;23(7):842–850. doi:10.1016/j.jval.2020.01.023
35. Liu S, Liu J, Si L, et al. Patient preferences for anti-hyperglycaemic medication for type 2 diabetes mellitus in China: findings from a national survey. BMJ Glob Health. 2023;8(4):56.
36. Huang Y, Huang Q, Xu A, Lu M, Xi X. Patient Preferences for Diabetes Treatment Among People With Type 2 Diabetes Mellitus in China: A Discrete Choice Experiment. Front Public Health. 2021;9:782964. doi:10.3389/fpubh.2021.782964
37. Klonoff DC, Zhang JY, Shang T, Mehta C, Kerr D. Pharmacoadherence: An Opportunity for Digital Health to Inform the Third Dimension of Pharmacotherapy for Diabetes. J Diabetes Sci Technol. 2021;15(1):177–183. doi:10.1177/1932296820973185
38. Rutten GEHM, Vugt HV, Koning E. Person-centered diabetes care and patient activation in people with type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(2):e001926. doi:10.1136/bmjdrc-2020-001926
39. Pagidipati NJ, Nelson AJ, Kaltenbach LA, et al. Coordinated Care to Optimize Cardiovascular Preventive Therapies in Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 2023;329(15):1261–1270. doi:10.1001/jama.2023.2854
40. Seng JJB, Monteiro AY, Kwan YH, et al. Population segmentation of type 2 diabetes mellitus patients and its clinical applications - A scoping review. BMC Med. Res. Method. 2021;21(1):49. doi:10.1186/s12874-021-01209-w
41. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi:10.1016/S2213-8587(18)30051-2
42. Davis J, Fischl AH, Beck J, et al. 2022 National Standards for Diabetes Self-Management Education and Support. Diabetes Care. 2022;45(2):484–494. doi:10.2337/dc21-2396
43. World Health Organization. First-ever global coverage targets for diabetes adopted at the 75th World Health Assembly. Available from: https://www.who.int/news-room/feature-stories/detail/first-ever-global-coverage-targets-for-diabetes-adopted-at-the-75-th-world-health-assembly.
44. Wei W, Zhao S, Fu SL, et al. The Association of Hypoglycemia Assessed by Continuous Glucose Monitoring With Cardiovascular Outcomes and Mortality in Patients With Type 2 Diabetes. Front Endocrinol. 2019;10:536. doi:10.3389/fendo.2019.00536
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


