Back to Journals » Journal of Asthma and Allergy » Volume 16
Errors in Metered Dose Inhaler Use Amongst Pediatric Asthma Patients
Received 13 August 2023
Accepted for publication 13 November 2023
Published 20 November 2023 Volume 2023:16 Pages 1259—1265
DOI https://doi.org/10.2147/JAA.S435197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Eeshta Bhatt, Robert A Malkin
Pratt School of Engineering, Duke University, Durham, NC, USA
Correspondence: Robert A Malkin, Duke University, 100 Science Drive, Durham, NC, 27708, USA, Tel +1-919-660-8266, Email [email protected]
Purpose: The aim of this paper is to use easily accessible smartphones as a straightforward means for physicians to objectively check Medical Device Inhaler (MDI) technique, without the need for additional devices. Additionally, we seek to assess the frequency of inhaler technique errors and their impact on asthma control.
Patients and Methods: Thirty-two children between the ages of 5 and 18 receiving asthma therapy through MDIs were included. Three surveys were administered to all participants to gauge device history, asthma control, and patient characteristics. Patient technique was scored using inhaler audio signals recorded with a smartphone. For subjects that were able, forced oscillation technique (FOT) was performed during tidal breathing conditions before and after corticosteroid administration.
Results: 81% (25/31) of participants used their MDIs incorrectly with the most common errors being rapid shallow breathing, inadequate breath-holding, and excessive actuations. Poor inhaler technique correlated with poorly controlled asthma symptoms.
Conclusion: The use of smartphone recordings can a convenient way to evaluate technique errors and could allow patients to demonstrate and refine their technique and usage without a doctor’s visit, ensuring proper technique and enhancing treatment effectiveness.
Keywords: asthma control, inhaler technique, children, non-compliance, asthma management
Introduction
Asthma is one of the most prevalent chronic respiratory diseases worldwide with more than 404 million sufferers.1 About 13% of cases are seen in India, due to environmental allergens like dust, pollen, air pollutants, and insects.2 Children are particularly vulnerable due to their smaller airways that get constricted when exposed to triggers. Prevalence is ten to fifteen percent in five-to-eleven-year-old’s. While around fifty percent of children that experience wheezing below the age of five will outgrow it, asthma remains the most common chronic disorder amongst children.2
Inhaler devices are the principal route for delivering aerosolized bronchodilators and corticosteroids crucial for the treatment of asthma in children. Metered dose inhalers (MDI) are widely prescribed for their affordability, convenience, portability, quick action, and lower inspiratory force required to dispense medication.3 Children below the age of eight are generally unable to generate enough force to activate breath actuated dry powder inhalers (DPIs). Further, unlike DPIs, MDI’s can be used even during exacerbations (acute asthma attacks) and are available in a wider range of bronchodilators.4
Although technical aspects of inhaler device design have improved, correct inhalation technique plays a crucial role in effective asthma treatment.4 Sub-optimal usage leads to insufficient medical delivery, poor clinical outcomes, increased asthma attacks, decreased quality of life and increased expenditure associated with the management of asthma.3 Despite a steady increase in cases, there is a severe lack of research conducted in pediatric populations concerning their ability to use MDIs especially in an Indian setting. Most studies conducted are qualitative in nature, classifying usage errors according to steps described in the manufacturer inserts.4 Hence, there is a need for quantitative assessment of patient technique. Further, there is a lack of standardization amongst studies evaluating the efficacy of training programs for patients and healthcare professionals. The most prevalent method involves the physical demonstration of inhaler usage. In recent years, researchers have sought to quantitatively evaluate patient inhaler technique using audio-based systems, such as add-ons to inhaler mouthpieces and sensor-based technologies.5 Currently, only one device (Flo-Tone Trainer), is available for purchase. However, these studies focus on the development and performance of the flo-tone device rather than evaluating patient asthma outcomes.5–7 Hence, there is a need for research that quantitatively assesses patient technique in the context of asthma treatment efficacy and patient symptoms.
The aim of this paper is to use easily accessible smartphones as a straightforward means for physicians to objectively check Medical Device Inhaler (MDI) technique, without the need for additional devices. Additionally, we seek to assess the frequency of inhaler technique errors and their impact on asthma control.
Materials and Methods
MDI technique was evaluated amongst asthmatic children from a specialized respiratory clinic in Mumbai, India. This study was approved by Solutions IRB as a minimal risk study under the registration #IORG0007116 (FWA #IRB00008523). This study was conducted in accordance with the principles of the Declaration of Helsinki. A convenience sample of children within the ages of five to eighteen receiving current asthma therapy via an MDI were enrolled. Participants were required to speak, read, and understand English, provide informed consent, and comply with study demands and procedures. For patients under the age of 18, a parent or legal guardian was required to provide signed informed consent. Patients were excluded if they were undergoing long term systemic treatment for asthma including nebulizer therapy either at home, at a hospital, or a clinic or suffering from active tuberculosis, pneumonia, chronic pulmonary disease, other comorbid diseases including connective tissue disease, congestive heart failure, myocardial infarction, tumors, peripheral vascular disease, ulcer diagnosis, leukemia, dementia, liver disease, lymphoma, diabetes mellitus, hemiplegia, cerebrovascular disease, moderate or severe renal disease. No monetary compensation was provided for participation.
All subjects and their parents had received prior instruction on how to use their device from clinic staff. Specifically, upon receiving their prescribed inhaler for the first time, patients were given standardized verbal instructions by the doctor who demonstrated each step of correct metered dose inhaler use. The specific inhalation steps were taken from manufacturer inserts and the NIH.8 The steps included shaking the inhaler, positioning the inhaler vertically upside down, exhaling to functional residual capacity, inhaling, and actuating the device once, breath holding for ten counts, and then exhaling slowly, followed by a second actuation and inspiration. Subjects were then allowed to practice under the observation of the clinic nurse. Patients were allowed to leave, once the nurse had deemed their technique was appropriate.
At least 6 months after initial training, an audio recording of the patients using their MDI was made via smartphone. This was done at the clinic by study staff. Subjects were instructed to demonstrate how they use their inhalers with or without a spacer. Audio was collected approximately 5 cm from the inhaler mouthpiece. The actuation count and number of inhalations per actuation were extracted from the recording. Audio signal processing was carried out using MATLAB. After down sampling, peaks in the envelope of the wave were used to differentiate between inhales, exhales, and actuations. Peaks for actuation and inhalation were differentiated based on frequency content and duration. The highest 10% of frequencies with a duration of less than 1 second were classified as actuations. Total breaths as well the number of actuations was then be counted and plotted.
In additional to recording of their MDI usage, Pulmonary Function Tests was recorded using the Forced Oscillation Technique (FOT) based system via the Antlia™ Pulmonary Diagnostic Device (Caltech Innovations) during tidal breathing conditions, documenting reactance measured at specific frequencies. R5 20 Pre and R5 20 Post were chosen for clinical significance.
Finally, three surveys consisting of a 5–6 multiple choice questions were completed. The first survey focused on patient characteristics, to better understand current health status, judge asthma control and exacerbation rate, checking for associations between poor technique and increased symptoms. The second survey ascertained MDI device history. A third instrument was adapted from the Asthma Control Test (ACT) developed by the Global Initiative for Asthma (GINA). It focused on asthma control and exacerbation to gauge the effectiveness of the treatment the patient has been receiving and if symptoms are well controlled.
Based on the ideal inhaler technique identified above (and delivered to the patient by the doctor), we considered an actuation count of two as ideal, 1 as appropriate and above 2 as a high actuation count error. For overall analysis, subjects with greater than one error were classified as having incorrect technique. The numbers and percentages of patients were calculated and cross-tabulated with GINA-based asthma control categories (controlled, uncontrolled) using results from the ACT.
For the purpose of this study, the effect of bronchodilation on easing airway blockage and flexibility was categorized on the basis of the percentage change in overall reactance collected from impedance data in the range of 5–20Hz. A decrease of 35% or greater was established as the cut off for defining a positive response to medication in asthmatic subjects, in agreement with previous studies assessing inhaled bronchodilator response in clinical treatment and management of pediatric asthma.9–11 In summary, patients aged 5–18 were prescribed MDIs. After initial training and at least 6 months of device use, they were eligible to be enrolled in the study. Subjects participated in the study by completing three surveys, allowing clinic staff to record audio samples of inhaler use, and if feasible undergoing pulmonary function testing through the forced oscillation technique.
Results
Thirty-two subjects met the eligibility criteria for entry. One subject was excluded, and all corresponding data was removed from analysis due to problems with the audio signal and having FOT data several standard deviations above the mean. Subject demographics, lung function, asthma control score and inhaler usage are shown in Table 1. Pulmonary lung function was tested for twenty-one patients during tidal breathing. For seventeen patients, forced oscillometry (FOT) was performed before and after bronchodilation to determine persistent bronchodilator response (BDR). Only 6 of 31 (19%) subjects reported well controlled asthma. All subjects were prescribed both inhaled corticosteroids and a long acting β-agonists to be taken using an MDI with an attached spacer for asthma treatment.
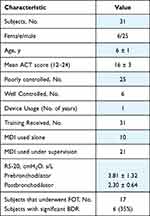 |
Table 1 Subject Characteristics |
MDI Technique Errors and Asthma Control
Only six subjects (19%) performed all the essential steps for proper use of their MDI, despite all 31 subjects having received comprehensive inhalation instructions with checks by the clinic staff. The most common errors were related to device actuation and inhalation technique (Table 2). Specifically, rapid shallow breathing, failing to hold their breath for ten seconds after inhalation following actuation, and frequent actuation. The recorded mean total breaths per actuation was 11 (±12) with a maximum of 44 breaths after a single actuation, well above the recommended 1–2 breaths per medication release into the spacer chamber. The mean actuation was 2 (±1) with a maximum of 6 actuations.
 |
Table 2 Quantification of Measured Inhalation Technique Errors |
Breath count per actuation was statistically observed to be the most clinically relevant indicator of incorrect patient technique. Hence, patients were classified into two quanta (controlled and uncontrolled) as governed by their respective ACT scores. When categorized according to asthma control scores, patients with poor symptom control (<20) were grouped together. All members within this group exhibited rapid breathing taking greater than 4 breaths per single actuation. The second group consisted of patients with well controlled asthma (ACT Score >22), each performing the advised 1–2 breaths per actuation. A t-test was then applied to compare the two groups, and a p-value of p < 0.001 was obtained as presented in Figure 1. This indicates that poor inhalation technique does play a significant role in determining health-related outcomes in asthmatic children, leading to disruption of normal routines (inability to sleep through the night, coughing, wheezing) as reported in the asthma control survey.
BDR and Asthma Control
The effect of inhaler technique on health outcomes was quantitatively determined for seventeen patients (55%) via persistent bronchodilator responses assessed using impulse oscillometry. Seven subjects (41%) exhibited a substantial decrease in reactance after bronchodilation via MDI. Of these seven, six exhibited correct inhalation maneuvers and one subject was nearly correct (4 actuations). Ten patients did not exhibit a significant decrease in reactance after MDI use and all of them exhibited poor MDI use. Furthermore, the seven patients with good or nearly correct MDI use had ACT scores >19 indicating partially to well controlled asthma.
Discussion
Despite in-person instruction provided by the clinic, most children in the study did not know how to use their inhaler correctly. Furthermore, the results from this study show that poor inhaler technique has a clinically relevant effect on patient health, identified through poor asthma control scores. Given, the high proportion of subjects with incorrect technique among a cohort who had received extensive training, it is evident that in-person instruction is not sufficient to ensure proper usage.
Our results indicate a possible dependence of bronchodilator response on reactance. A substantial decrease in reactance was observed when inhalation maneuvers were correctly performed.
Importantly, we have shown for the first time that a simple phone recording of audio during inhaler use, can be used to judge errors in breathing technique. The benefit of a simple system which monitors and provides feedback to patients can serve as a vital method to ensure improvement in asthma treatment and outcomes. Sub optimal treatment in the studied age group can lead to an exacerbation of asthma symptoms, negatively affecting development.
Our results demonstrate a strong correlation between patient usage of prescribed MDIs and poor asthma control. This is consistent with prior studies surveying MDI technique in asthmatic children, with inadequate usage being associated with poor asthma outcomes.12–14 The majority of children failed to wait 10 seconds between successive inhalations, and as a result were unable to receive the appropriate dosage of medication due to shallow breathing. Previous researchers have suggested frequent training interventions involving multimedia-delivered training and physician supervised checks to improve patient technique, with physical demonstrations being the most effective at reducing errors.15 Although the impact of feedback devices on improving MDI technique has been extensively studied in adults, research on its effects in children, particularly those below the age of 10, is still limited. It must be acknowledged that patient outcomes, mainly bronchodilator response and asthma control scores, are influenced by the quality and efficacy of prescribed medication. Varying inhaled corticosteroid therapy has been previously shown to improve airflow limitation.16 To mitigate potential biases, all enrolled study participants received the same medication through their MDIs, ensuring uniform treatment across the cohort.
Difficulties in exhibiting successful technique despite in-person instruction underscore an urgent need to instate measures to improve poor inhaler handling with a focus on three most incorrectly performed aspects of MDI technique: device actuation, breaths per actuation and synchronization between the two (waiting between each inhalation for a single actuation).
Development of Specialized Training Devices
Future research should focus on the development of intuitive tools that are able to autonomously assess patient technique, hence reducing the burden on physicians to opportunistically assess usage on repeated outpatient attendance and providing patients a method to evaluate their own technique against the established standard.
To help children understand how, when, and why they need to use their inhalers properly, the delivery of instructions could be revamped via a gamification of the approach. This study used a simple phone application to analyze inhaler use. Using our work as a basis, a new device could offer a simple instructional device with a child friendly digital screen, that measures inspiratory force, advises children on how to take their medication and scores their performance, either installed in clinics or used at home. This will serve a twofold purpose: provide children with an incentive to practice their technique, increasing the likelihood of them successfully using MDIs, and giving them an opportunity to practice their technique in a low-risk environment without the chance of heightened medicine intake. This will also greatly reduce human error in observing and monitoring patient technique. Our findings highlight that the main impediments to proper inhalation technique are inspiration force and time. Hence a new device that measures peak inspiratory force, monitors breathing rate, and warns against repeated actuations would be ideal in improving treatment outcomes.
Even if a complete training device is not desired, as shown in the study there exists a straightforward method to judge technique based on simple smartphone sound recordings. Devices with built in recording functions, or mobile apps that come with package inserts could be used to record patient usage and highlight errors such as too many inhalations. We suggest the creation of smart phone apps that can classify and monitor real time inhalation events while a child uses their inhaler. The app would be able to send a score report to a physician as well as highlight mistakes to the user.
Redesign of Existing Medication Administration Devices
Although efforts have been made to introduce new asthma delivery devices such as dry powder and soft mist devices, metered dose inhalers are still the most prescribed devices amongst children.17–19
This is also attributed to concerns regarding relatively high inspiratory efforts required for medication release from capsules in dry powder devices. Instead, adjustments could be made to currently prescribed metered dose inhalers and spacers. Spacers could be designed to provide visual or auditory cues to indicate the presence or absence of medication in valved holding champers, allowing users to ascertain whether medication delivery was successful.
Limitations
This study focused on a limited number of participants within a specific age range (5–10 years) who were attending a single outpatient clinic. Hence, results could have been biased by clinic specific treatments, and training. Few data points were collected for bronchodilator response to inhaled corticosteroids, which served as a clinical marker of physiological benefit; making it difficult to ascertain quantitative medical impact of inhaler misuse. Lastly, a single repeated actuation that resulted in improved outcomes was observed. This can most likely be attributed to a single actuation event with a successful deep inhalation, or the increased likelihood of inhaling a significant dosage of medication from the valved spacer chamber due to oversaturation.
Further research could explore these findings in a larger and more diverse population to strengthen the generalizability of the results. Additionally, collecting a broader range of data points would provide a more comprehensive understanding of the bronchodilator response to inhaled corticosteroids in this specific age group. Lastly, it is relevant to note that the evaluation of patient technique did not rely on direct observation, a method commonly employed in similar research for technique scoring. Different staff members assessed each patient, raising significant concerns regarding inter-staff variability and its potential to compromise data integrity. Hence, pulmonary function testing and the asthma control survey, which assess the clinical benefits derived from inhaler therapy, were employed to gauge the effectiveness of technique and medication adherence.
Conclusion
This study has demonstrated that errors in MDI technique are common, even among individuals who have previously received instruction through physician demonstration. Inhaled medications are the preferred therapeutic approach for individuals with asthma. Nevertheless, the efficacy of these treatments is hindered by the patient’s ability to correctly operate the inhalation device, an aspect that is often disregarded during the prescription process. Regular reinforcement of inhalation instructions is necessary to establish and maintain correct inhalation technique in children with asthma. The only existing approach for evaluating patient technique errors relies on physician observation, which is susceptible to perceptual inaccuracies and variability among physicians in identifying and documenting errors. This study underscores the advantages of having clinical staff record audio samples to enable quantitative assessment of patient technique errors and to maintain a comprehensive record of patient performance. In the future, research could be conducted to explore the feasibility of assessing patient technique through self-recorded audio samples at their convenience.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. chronic respiratory diseases; 2022. Available from: https://www.who.int/health-topics/chronic-respiratory-diseases.
2. Trivedi M, Denton E. Asthma in children and adults-what are the differences and what can they tell us about asthma? Front Pediatr. 2019;7:256. doi:10.3389/fped.2019.00256
3. Gillette C, Rockich-Winston N, Kuhn JA, et al. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. 2016;16(7):605–615. doi:10.1016/j.acap.2016.04.006
4. Scotney E, Fleming L, Saglani S, et al. Advances in the pathogenesis and personalised treatment of paediatric asthma. BMJ Medicine. 2023;2(1):e000367. doi:10.1136/bmjmed-2022-000367
5. Taylor TE, Zigel Y, De Looze C, et al. Advances in audio-based systems to monitor patient adherence and inhaler drug delivery. Chest. 2018;153(3):710–722. doi:10.1016/j.chest.2017.08.1162
6. Taylor TE, Zigel Y, Egan C, et al. Objective assessment of patient inhaler user technique using an audio-based classification approach. Sci Rep. 2018;8(1):2164. doi:10.1038/s41598-018-20523-w
7. Ntalianis V, Fakotakis ND, Nousias S, et al. Deep CNN sparse coding for real time inhaler sounds classification. Sensors. 2020;20(8):2363. doi:10.3390/s20082363
8. Institute, N.H.L.a.B. How to use a metered-dose inhaler fact sheet; 2023. Available from: https://www.nhlbi.nih.gov/resources/how-use-metered-dose-inhaler-fact-sheet.
9. Alblooshi A, Alkalbani A, Albadi G, et al. Is forced oscillation technique the next respiratory function test of choice in childhood asthma. World J Methodol. 2017;7(4):129–138. doi:10.5662/wjm.v7.i4.129
10. Lauhkonen E, Kaltsakas G, Sivagnanasithiyar S, et al. Comparison of forced oscillation technique and spirometry in paediatric asthma. ERJ Open Res. 2021;7(1):00202–2020. doi:10.1183/23120541.00202-2020
11. Galant SP, Komarow HD, Shin H-W, et al. The case for impulse oscillometry in the management of asthma in children and adults. Anna Aller Asth Immunol. 2017;118(6):664–671. doi:10.1016/j.anai.2017.04.009
12. Roche N, Aggarwal B, Boucot I, et al. The impact of inhaler technique on clinical outcomes in adolescents and adults with asthma: a systematic review. Respir Med. 2022;202:106949. doi:10.1016/j.rmed.2022.106949
13. Trivedi D. Interventions to improve inhaler technique for people with asthma. Prim Health Care Res Dev. 2019;20:e72. doi:10.1017/S1463423619000501
14. Levy ML, Hardwell A, McKnight E, et al. Asthma patients’ inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the Global Initiative for Asthma (GINA) strategy: a retrospective analysis. Primary Care Respirat J. 2013;22(4):406–411. doi:10.4104/pcrj.2013.00084
15. Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. doi:10.1016/j.rmed.2012.09.017
16. Hirano T, Matsunaga K, Sugiura H, et al. Persistent elevation of exhaled nitric oxide and modification of corticosteroid therapy in asthma. Respir Investig. 2013;51(2):84–91. doi:10.1016/j.resinv.2013.01.002
17. Cataldo D, Hanon S, Peché RV, et al. How to choose the right inhaler using a patient-centric approach? Adv Ther. 2022;39(3):1149–1163. doi:10.1007/s12325-021-02034-9
18. Virchow JC, Crompton GK, Dal negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–19. doi:10.1016/j.rmed.2007.07.031
19. Pedersen S, Dubus JC, Crompton G, et al. The ADMIT series — issues in Inhalation Therapy. 5) Inhaler selection in children with asthma. Primary Care Respirat J. 2010;19(3):209–216. doi:10.4104/pcrj.2010.00043
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

