Back to Journals » OncoTargets and Therapy » Volume 16
Epithelioid Subtype Gastrointestinal Stromal Tumors of Stomach in an Endoscopic Biopsy: A Potential Diagnostic Pitfall
Authors Xu W , Tang H , Chen Y , Wang J, Chen Z, Xu Y, Guo D
Received 13 October 2023
Accepted for publication 30 November 2023
Published 12 December 2023 Volume 2023:16 Pages 1043—1049
DOI https://doi.org/10.2147/OTT.S444532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Wenfeng Xu, Hao Tang, Ying Chen, Jiashuang Wang, Zhongjiao Chen, Yujuan Xu, Deyu Guo
Department of Pathology, Guiqian International General Hospital, Guiyang City, Guizhou Province, People’s Republic of China
Correspondence: Deyu Guo, Email [email protected]
Background: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract with a broad morphological spectrum. Although epithelioid GISTs account for 20% of GISTs, their morphological features may pose a diagnostic pitfall for pathologists due to their morphological similarities to poorly differentiated adenocarcinoma and lymphoma.
Case Presentation: Herein, we report a 65-year-old male patient with gastric epithelioid GIST misdiagnosed as adenocarcinoma for four years. During this period, he was treated with chemotherapy combined with PD-L1 immunotherapy. The clinicians thought the treatments were effective. However, there was no significant change in tumor size. The patient’s clinical symptoms did not improve significantly as well. Finally, an endoscopic biopsy was performed again and gastric epithelioid GIST was confirmed in our hospital through morphology, immunohistochemistry, and whole-genome sequencing.
Conclusion: A broad morphological spectrum and diverse immunophenotypic changes of GISTs could represent a pitfall for pathologists. However, predisposed anatomical sites, morphology, and corresponding immunohistochemical markers are of great significance for the diagnosis of GISTs and the differential diagnosis from other diseases. On the other hand, clinicians should diagnose and comprehensively evaluate treatment effects based on the patient’s clinical symptoms and relevant laboratory examinations, instead of over-reliance on pathological diagnosis.
Keywords: gastrointestinal stromal tumors, misdiagnosis, poorly differentiated adenocarcinoma, stomach
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the gastrointestinal tract, which represent approximately 80% of gastrointestinal mesenchymal tumors.1 They mainly affect populations that are older than 50 years old, with the median age being their sixth decade of life, and the incidence rate is slightly higher in males than in females.1–3 GISTs mainly occur in the gastrointestinal tract, with most presenting in the stomach (50%–60%) and small intestine (30%–35%).4 Histologically, 70% of GISTs are spindle cell tumors, approximately 20% are composed of epithelioid cells, and the remainder are mixed morphology type.5 GISTs with epithelioid appearance are typically composed of large rounded epithelioid cells with abundant eosinophilic or sometimes ring-like clear cytoplasm and rounded nuclei. That makes it easy to misdiagnose as poorly differentiated adenocarcinoma or lymphoma. Herein, we present a case of gastric epithelioid GIST misdiagnosed as adenocarcinoma, and summarize some diagnostic pitfalls of GISTs to reduce misdiagnosis.
Case Report
A 65-year-old male patient was transferred to our hospital due to liver metastasis of gastric adenocarcinoma for more than 4 years and epigastric pain for 1 month. This patient was initially admitted to another hospital with abdominal pain for 1 day in December 2008. Related auxiliary examinations such as gastroscopy, gastroscopic biopsy, and computed tomography (CT) examination of the chest and abdomen were performed. Gastroscopy examination showed a tumor with a size of 30×40 mm protruding to the gastric cavity in the upper part of the gastric body near the posterior wall of the lesser curvature. The pathological diagnosis of gastric adenocarcinoma was confirmed by the method of endoscopic biopsy. In addition, CT of the abdomen revealed multiple-liver metastases of gastric adenocarcinoma.
The patient was treated with lobaplatin and TS-1 chemotherapy combined with PD-L1 immunotherapy and targeted therapy with apatinib for 8 courses, then TS-1 and apatinib maintenance therapy for 1.5 years, and finally apatinib maintenance therapy. Due to headache and nausea, the dose of apatinib was reduced to 1 tablet per day and this patient took it regularly for half a month, with a one-month break in between, until March 2023. During treatment, the patient’s clinical symptoms had not improved significantly, and imaging studies and gastroscopy showed no significant reduction in the mass. After chemotherapy, the clinician thought that the chemotherapy regimen was effective because the condition of the patient had not progressed further.
However, the patient suffered from epigastric pain again and was transferred to our hospital for further treatment in April 2023. After hospitalization, this patient underwent a re-examination of the gastroscopy and CT scan of the abdomen.
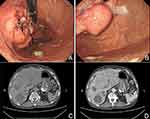
Gastroscopy showed a huge mass with an uneven surface and ulcer formation on the posterior wall of the upper part of the gastric body and another huge submucosal bulge on the posterior wall of the gastric fundus. CT scan of the abdomen revealed multiple hepatic metastatic lesions, which increased compared with previous ones (Figure 1).
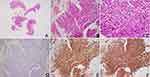
Then, the endoscopic biopsy was performed again. Microscopically, gastroscopic biopsy showed sheet-like atypical cells composed of round and oval cells, with eosinophilic cytoplasm, and a few vacuoles can be seen in the cytoplasm (Figure 2A-C).
As in routine diagnostic thinking, poorly differentiated adenocarcinoma was initially considered due to its clinical history of gastric adenocarcinoma with liver metastases, and corresponding immunochemistry was performed, including pan-cytokeratin (CK-pan), LCA, and Ki67. However, what puzzled us was that the tumor cells were all negative for CK-pan and LCA. To rule out the conditions that either false-negative immunohistochemical results or the loss of CK-pan marker secondary to long-term chemotherapy. The second phalanx of CK-pan and CK8-18 immunostaining was detected again. The immunohistochemical results showed negative staining for CK-pan and CK8-18 as well. Subsequently, we considered mesenchymal tumors with epithelioid morphology. Based on the location of the tumor, GISTs were first considered, and relevant immunohistochemical examinations, including CD117, DOG1, and CD34 were detected. Finally, positive immunohistochemical results for CD117, DOG1, and CD34 supported a diagnosis of epithelioid GIST (Figure 2D-F).
However, this diagnosis was inconsistent with the previous result of gastric adenocarcinoma, and the possibility of two kinds of tumors could not be ruled out. Therefore, we suggested the patient borrow biopsy slices from the first-visit hospital where he was first diagnosed to our hospital for further pathological consultation.
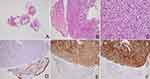
The consultation sections showed sheets of epithelioid cells in the submucosa, with abundant eosinophilic or sometimes ring-like clear cytoplasm. The pathological diagnosis of gastric adenocarcinoma was made by the first-visit hospital, without performing relevant immunohistochemistry. Epithelioid morphologic features did warrant consideration of poorly differentiated adenocarcinoma and epithelioid GISTs, thus a series of immunohistochemical examinations were performed. Immunohistochemical results showed the tumor cells were positive for CD117, DOG1, and SDHB, while were negative for CK-pan (Figure 3). Then, whole-genome sequencing of tumors was performed and found a mutation in KIT Exon11 (KIT p.I563_P577del Exon11), resulting in the deletion of isoleucine at position 563 to proline at position 577. Therefore, the results of the consultation sections and whole-genome sequencing approved of the diagnosis of epithelioid GIST. Epithelioid histological features are indeed easily misdiagnosed as poorly differentiated adenocarcinoma, especially in the absence of immunohistochemistry. Finally, this patient was given targeted therapy with Imatinib mesylate for 400 mg daily.
Discussion
GISTs are the most common mesenchymal tumors of the gastrointestinal tract, usually involving various parts of the gastrointestinal tract, with the stomach being the most common, followed by the small intestine, and rarely occurring in other anatomical sites outside the gastrointestinal tract – also known as extra-gastrointestinal stromal tumors (EGISTs), such as omentum, mesentery, retroperitoneal, bladder, hepatic, etc.4,6,7 The annual incidence of GISTs is estimated to be 10–15 people per million.1,8 The clinical manifestations of GISTs are variable, mostly depending on the locations and the sizes of the tumors. Some patients with larger tumors even suffer from digestive tract obstruction, dysphagia or obstructive jaundice, while some patients with smaller tumors usually are asymptomatic.9 Most GISTs are submucosal tumors, however, submucosal gastric cancer can mimic GISTs, and conversely, GISTs can also present with nodular tumors and deep ulcers, mimicking gastric cancer.10,11 All these increase the difficulty for clinicians to differentiate between GISTs and gastric cancer.
Diagnosis of GISTs is confirmed by histopathology and immunohistochemistry. Histologically, GISTs are composed of 3 different subtypes, spindle cell type (70%), epithelioid type (20%), and mixed type (10%).5 Neoplasms with spindle cell morphology in the gastrointestinal tract are reminiscent of GISTs, but they need to be differentiated from smooth muscle tumors.12 Some rare GISTs even exhibit multiple myxoid nodules composed of spindle cells with histological features similar to plexiform fibromyxoma.13 Epithelioid GISTs were composed of round or polygonal cells with a prominent plasmacytoid morphology characterized by abundant clear or eosinophilic cytoplasm and indistinct cellular borders.14 GISTs with epithelioid features have been reported to occur more frequently in the stomach.15 Most GISTs are submucosal tumors covered by normal mucosa; however, submucosal signet ring cell gastric cancer that mimics GISTs has recently been reported.10 Thus, GISTs with epithelioid features usually make pathological diagnosis challenging and easily misdiagnosed as poorly differentiated carcinoma.16 At present, four distinct subtypes of epithelioid gastric GISTs have been identified: sclerosing, hypercellular, heterogeneous, and sarcomatoid, adding to the difficulty of diagnosis.17 As the wide morphological spectrum of GISTs, immunohistochemistry is a vital means of diagnosis and differential diagnosis of GISTs.
It is well known that approximately 95% of GISTs stain positive for CD117 (KIT), 75%–100% for DOG1, and 60%–70% for CD34.18 CD117 is the most sensitive and highly specific marker for GISTs, while approximately 3%–5% of GISTs are focally positive or negative, especially in the epithelioid subtype.19 CD117 is not only expressed in GISTs, but also in other tumors such as melanoma, germ cell tumors, and perivascular epithelioid cell tumor (PEComa), as well as normal cells, such as melanocytes, mast cells, germ cells, and interstitial cells of Cajal. In this condition, the combined use of DOG1 and CD34 can reduce the misdiagnosis.20 However, it is reported that nearly 50% of the gastrointestinal intramural leiomyomas focally express DOG1. A primary mesenteric leiomyosarcoma even diffusely expresses DOG1, while lacking of CD117 immunopositivity.21 GISTs usually focally express SMA and desmin. Diffuse expression of SMA and desmin can help to differentiate DOG1 positive smooth muscle tumors from CD117 negative GISTs.
In our case, the first clue that made us consider GISTs was negative staining for CK-pan. However, keratin expression was reported in 1%–2% of GISTs, which is a pitfall to easily misdiagnosed as poorly differentiated carcinoma.5,16 In addition, some rarest sarcomatoid epithelioid GISTs could be positive for CKAE1, while being weakly positive for CD117.22
It is reported that approximately 85% of GISTs harbor exclusive mutations involving the KIT or the PDFGRA.23,24 KIT p.I563_P577del Exon11 mutation was detected in our case. KIT is a proto-oncogene located on chromosome 4q11-q12 and encodes receptor tyrosine kinase (RTK), a transmembrane receptor with tyrosine kinase (TK) activity. Mutation in KIT leads to the activation of MAPK and PI3K-AKT pathways and results in cell proliferation and inhibition of apoptosis. At present, different gene mutation regions have been reported, including exons 8, 9, 11, 13, 14, 15, and 17. Mutations in exon 11 are the most common, with a frequency of 60%.25
Management of GISTs largely depends on the extent of the disease and whether it has metastasized. Complete surgical resection is still the first-line treatment for localized lesions.26 Furthermore, the risk of recurrence after surgery can be assessed by the modified NIH classification systems. The modified NIH classification systems determine the risk of recurrence by looking at the size, mitotic activity, and location of tumor to classify patients into very low, low, intermediate, and high-risk groups. This classification systems indicate that tumor rupture during surgery has a significantly adverse prognosis.27
However, GISTs are tumors with multiple malignant potential. About 40% of GISTs metastasize at the first presentation, and about 10%–20% of GISTs even have distant metastases.4,28,29 For this case that we reported with unresectable primary tumors, liver metastases, and KIT gene mutation, the tyrosine kinase inhibitors (TKI) imatinib was the first-line agent, but could not benefit from conventional adjuvant chemotherapy and radiotherapy. While our patient received traditional chemotherapy and PD-L1 immunotherapy. Although clinicians thought the treatment regimen was effective, imaging studies and gastroscopy showed no significant reduction in the mass. Therefore, this is also a lesson for clinicians: clinicians should diagnose and comprehensively evaluate treatment effect based on the patient’s clinical symptoms, relevant imaging examinations, laboratory examinations, and pathological examinations, instead of over-reliance on pathological diagnosis.
Last but not least, the morphology and immunophenotype of GISTs can be transformed after TKI treatment. Koufopoulos et al reported a patient with spindle-shaped GIST showed epithelioid GIST transition with focal dedifferentiated chondrosarcoma after a period of treatment with TKI. This report also summarized 18 cases of patients with GISTs who received TKI treatment. Dedifferentiated high-grade malignancies appeared in the specimens after treatment, among which rhabdomyosarcomatous was the most common. The dedifferentiated components partially or completely lost the originally expressed immunohistochemical markers, such as CD117, DOG1, and CD34.30 In addition to morphological changes, some patients with GISTs even lost the expression of CD117 and gained the expression of CK-pan after treatment with TKI.31
In conclusion, we describe a case of epithelioid GIST misdiagnosed as adenocarcinoma. GISTs harbor a broad morphological spectrum, in which rare morphological changes could represent a pitfall for pathologists. However, predisposed anatomical sites, morphology, and corresponding immunohistochemical markers are of great significance for the diagnosis of GISTs and the differential diagnosis from other diseases. Finally, we summarize some rare immunophenotypic changes of GISTs for a better and more detailed understanding of GISTs as well.
Abbreviations
GISTs, Gastrointestinal stromal tumors; CK-pan, pan-cytokeratin; EGISTs, extra-gastrointestinal stromal tumors; PEComa, perivascular epithelioid cell tumor; RTK, receptor tyrosine kinase; TK, tyrosine kinase; TKI, tyrosine kinase inhibitors.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Guiqian International General Hospital. Our institution approved the publication of the case details. Written informed consent has been obtained from the patient for the release of relevant clinical and imaging data from their cases, included consent for the publication of the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was available to support this research.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi:10.1016/j.canep.2015.10.031
2. Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104(8):865–873. doi:10.1002/jso.21945
3. Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33. doi:10.1016/j.annonc.2021.09.005
4. Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi:10.1016/S1470-2045(11)70299-6
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi:10.1053/hupa.2002.123545
6. Cheng X, Chen D, Chen W, Sheng Q. Primary gastrointestinal stromal tumor of the liver: a case report and review of the literature. Oncol Lett. 2016;12(4):2772–2776. doi:10.3892/ol.2016.4981
7. Gheorghe G, Bacalbasa N, Ceobanu G, et al. Gastrointestinal Stromal Tumors-A Mini Review. J Pers Med. 2021;11(8):694. doi:10.3390/jpm11080694
8. Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456(2):111–127. doi:10.1007/s00428-010-0891-y
9. Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10(1):144–154. doi:10.21037/jgo.2018.08.20
10. Lee J, Oh SJ. Signet Ring Cell Carcinoma Mimicking Gastric Gastrointestinal Stromal Tumor: a Case Report. Case Rep Oncol. 2020;13(2):538–543. doi:10.1159/000506448
11. Li Y, Ye L, Hu B. Gastric neoplasm: gastrointestinal stromal tumor mimicking gastric carcinoma. Dig Liver Dis. 2021;53(5):648–649. doi:10.1016/j.dld.2020.06.035
12. Manxhuka-Kerliu S, Sahatciu-Meka V, Kerliu I, et al. Small intestinal gastrointestinal stromal tumor in a young adult woman: a case report and review of the literature. J Med Case Rep. 2014;8:321. doi:10.1186/1752-1947-8-321
13. Li B, Zhang QF, Han YN, Ouyang L. Plexiform myxoid gastrointestinal stromal tumor: a potential diagnostic pitfall in pathological findings. Int J Clin Exp Pathol. 2015;8(10):13613–13618.
14. Abrari A, Mukherjee U, Tandon R, Chandrashekhar M. Round cell epithelioid GIST (gastrointestinal stromal tumour) in an endoscopic biopsy is a diagnostic confounder. BMJ Case Rep. 2011;2011:bcr1020114996. doi:10.1136/bcr.10.2011.4996
15. Bülbül Doğusoy G; Turkish GIST Working Group. Gastrointestinal stromal tumors: a multicenter study of 1160 Turkish cases. Turk J Gastroenterol. 2012;23(3):203–211.
16. Mourra N, Dehni N. Cytokeratin expression in GIST: a diagnostic pitfall in gastric biopsy. Appl Immunohistochem Mol Morphol. 2010;18(5):486–488. doi:10.1097/PAI.0b013e3181e36221
17. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52–68. doi:10.1097/01.pas.0000146010.92933.de
18. Karakas C, Christensen P, Baek D, Jung M, Ro JY. Dedifferentiated gastrointestinal stromal tumor: recent advances. Ann Diagn Pathol. 2019;39:118–124. doi:10.1016/j.anndiagpath.2018.12.005
19. Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28(7):889–894. doi:10.1097/00000478-200407000-00007
20. Moldovan VT, Pătraşcu OM, Ali L, Costache M, Sajin M. Morphological and immunohistochemical diagnostic of extragastrointestinal stromal tumors - A 51 case series analysis. Rom J Morphol Embryol. 2021;62(4):1011–1016. doi:10.47162/RJME.62.4.13
21. Koufopoulos N, Damaskou V, Siozopoulou V, et al. DOG1-Positive Primary Mesenteric Leiomyosarcoma: report of a Case and Review of the Literature. Cureus. 2022;14(5):e25263. doi:10.7759/cureus.25263
22. Lech G, Korcz W, Kowalczyk E, Guzel T, Radoch M, Krasnodębski IW. Giant gastrointestinal stromal tumour of rare sarcomatoid epithelioid subtype: case study and literature review. World J Gastroenterol. 2015;21(11):3388–3393. doi:10.3748/wjg.v21.i11.3388
23. Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61(22):8118–8121.
24. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi:10.1126/science.1079666
25. Brčić I, Argyropoulos A, Liegl-Atzwanger B. Update on Molecular Genetics of Gastrointestinal Stromal Tumors. Diagnostics. 2021;11(2):194. doi:10.3390/diagnostics11020194
26. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19(1):3–14. doi:10.1007/s10120-015-0526-8.Lanke
27. Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol. 2017;8(2):135–144. doi:10.5306/wjco.v8.i2.135
28. Woodall CE, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg. 2009;144(7):670–678. doi:10.1001/archsurg.2009.108
29. Emile JF, Brahimi S, Coindre JM, et al. Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol. 2012;29(3):1765–1772. doi:10.1007/s12032-011-0074-y
30. Koufopoulos N, Zacharatou A, Athanasiadou S, et al. Gastrointestinal Stromal Tumor With Chondrosarcomatous Dedifferentiation Following Imatinib Therapy. Cureus. 2021;13(8):e17448. doi:10.7759/cureus.17448
31. Canzonieri V, Gasparotto D, Alessandrini L, et al. Morphologic shift associated with aberrant cytokeratin expression in a GIST patient after tyrosine kinase inhibitors therapy. A case report with a brief review of the literature. Pathol Res Pract. 2016;212(1):63–67. doi:10.1016/j.prp.2015.11.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.