Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials
Authors Mahzari MM , Alqirnas MQ , Alhamadh MS , Alrasheed F, Alhabeeb AY , Al Madani W, Aldera H
Received 10 November 2023
Accepted for publication 16 March 2024
Published 23 March 2024 Volume 2024:17 Pages 1425—1440
DOI https://doi.org/10.2147/DMSO.S445114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Moeber M Mahzari,1– 3,* Muhannad Qirnas Alqirnas,1,2,4,* Moustafa S Alhamadh,1– 3 Faisal Alrasheed,1,2 Abdulrahman Yousef Alhabeeb,1,2 Wedad Al Madani,5 Hussain Aldera1,2
1College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 2King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia; 3Department of Medicine, Ministry of the National Guard-Health Affairs, Riyadh, Saudi Arabia; 4Department of Family Medicine, Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia; 5General Authority of Statistics, Riyadh, 11481, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Moeber M Mahzari, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Ministry of the National Guard-Health Affairs, Riyadh, 11426, Saudi Arabia, Email [email protected]
Aim: To assess the efficacy and safety of Dipeptidyl Peptidase IV (DPP-4) inhibitors in patients with Type-2 Diabetes Mellitus (T2DM) and chronic kidney disease (CKD) using level 1 evidence.
Methods: The Cochrane and PubMed databases were searched from inception until January 1, 2022. RCTs that studied the efficacy and safety of DPP-4 inhibitors in diabetic patients with CKD were included. The primary efficacy outcome was assessed as the mean difference between HbA1c at the beginning and the end of each study for each arm, and the primary safety outcome was assessed as the incidence of adverse events and severe adverse events in each study.
Results: Twenty-one studies satisfied the pre-defined eligibility criteria. In assessing the efficacy of DPP-4 inhibitors in the treatment of T2DM and CKD, a total of 2917 patients under the DPP-4 inhibitors group and 2377 patients under the control group were included; The mean difference between the HbA1c of DPP-4 Inhibitor and the control group was − 0.5295 with a 95% CI of − 0.5337 to − 0.5252. The included studies had high heterogeneity p < 0.00001 and I2 = 99%. In assessing the safety outcome and tolerability of DPP-4 inhibitors, a total of 8138 patients under the DPP-4 inhibitors group and 7517 patients under the control group were included; the odds ratio of adverse events between both groups was 0.9967 with a 95% CI of 0.9967 to 1.1047. The included studies had low heterogeneity p = 0.25 and I2 = 15%. The overall effect, Z = 0.06 (p = 0.95), was insignificant.
Conclusion: Patients suffering from both T2DM and CKD exhibited a significantly enhanced glycemic control when treated with DPP-4 inhibitors in comparison to the control group. Furthermore, no significant difference in the incidence of adverse events was observed between the DPP-4 inhibitors and the control group.
Keywords: diabetes, dipeptidyl peptidase IV, efficacy, safety, chronic kidney disease
Introduction
The past few years have seen a tremendous increase in type 2 diabetes mellitus (T2DM) cases and chronic kidney disease (CKD). Diabetic kidney disease (DKD), which is a major complication of long-lasting diabetes mellitus, is one of the main factors leading to end-stage renal diseases (ESRD) affecting approximately 30% of the diabetic population.1,2 It has been proven that maintaining good glycemic control plays an important role in preventing the progression of deteriorating kidney function.3 Nevertheless, the use of anti-hyperglycemic drugs in patients with T2DM with CKD is still controversial pertaining to their efficacy and safety.
Metformin, a well-known anti-hyperglycemic drug, for instance, might no longer be the first choice for CKD patients because of the risk of lactic acidosis.4 Dipeptidyl peptidase-4 (DPP-4) inhibitors are a class of oral anti-diabetic agents that work by decreasing the inactivation of incretins such as glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide to stimulate the release of insulin in a glucose-dependent manner.5,6 By inhibiting DPP-4, these medications can help to lower blood sugar levels and improve glycemic control in people with T2DM.7 Most of the DDP-4 inhibitors are excreted by the kidney; thus, a decrease in the dose given is essential for patients with ESRD, with the exception of Linagliptin due to its relatively low renal metabolism.8
DPP-4 inhibitors have been used safely in CKD patients; however, their impact on kidney outcomes is still questionable. Preclinical studies have implied that the pleiotropic effects of DPP-4 inhibition might actually be beneficial for the kidney, while clinical trials results were inconsistent.9–11 Giorda et al12 conducted a systematic review assessing the efficacy and safety of DPP-4 inhibitors in patients with T2DM with renal impairment, and their results suggest that it is safe and appropriate to use. Nevertheless, their study did not include enough clinical trials, and accordingly further meta-analysis is needed. The aim of our study is to conduct a meta-analysis of randomized controlled trials to investigate the efficacy and safety of Dipeptidyl peptidase-4 inhibitors in diabetic patients with CKD. This review provides a more comprehensive and reliable overview of the evidence than any single study7 and could help identify areas where further research is needed.13 The finding of the review could also inform clinical practice and policy,7 helping to guide treatment decisions for patients with T2DM (American Diabetes Association).
Methods
This review was performed in accordance with the checklist and guidelines provided by Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Data Sources and Searches
Our aim was to find randomized controlled trials (RCTs) assessing the efficacy and safety of DPP-4 therapy in patients with T2DM and chronic kidney disease (CKD). KDIGO (Kidney Disease Improving Global Outcomes) criteria were used to define CKD, which defines it as an eGFR <60 mL/min/1.73 m2 for 3 months or more, regardless of the attributed cause. The search strategy was developed by (M.Q., M.H., and W.M.) and conducted by (M.Q. and M.H.). The Cochrane and PubMed databases were searched using this strategy from inception until January 1, 2022. The following mesh terms were used “chronic kidney disease” AND “hypoglycemic agents” to screen the databases (PubMed and Cochrane). A limitation to RCTs only was applied to the search engines.
Study Selection
Two independent reviewers (M.Q. and M.H.) screened articles as abstracts and then in full text. Disagreements were resolved with a third reviewer (W.M.). Studies were included if they were RCTs studying the efficacy and safety of DPP-4 inhibitors in T2DM patients with CKD. Studies that either did not report Hba1c or used fasting/random blood glucose instead were excluded. Also, studies that did not report the mean difference in Hba1c at the beginning and at the end for both arms were excluded. Preliminary RCTs that were extended were excluded and the extension period publication was included if it met the exclusion/inclusion criteria. If data on the CKD subgroup of the RCT were inadequately reported, the authors of the RCT were contacted. Studies that met all selection criteria had their data abstracted.
Data Extraction and Quality Assessment
Information on study characteristics collected from eligible RCTs included basic study information (author identification, year of publication, National Clinical Trial number, sample size for each group, duration of intervention); participants’ baseline characteristics (mean age, mean Hemoglobin A1C [HbA1C] at baseline, and pre-specified outcomes of efficacy and safety). Our primary outcome was glycemic control efficacy as measured by the difference in HbA1C from baseline to the end point of the intervention. Safety outcomes extracted included hypoglycemia, death, occurrence of any adverse event, and discontinuation rate from adverse events.
Risk of Bias Assessment and Grading the Quality of the Evidence
We synthesized selected articles and then assessed them using a modified version of the Quality Assessment Checklist for Prevalence Studies Risk of Bias tool. The analysis was done using the Cochrane tool also known as RoB 2. The RoB 2 tool offers a framework for evaluating the risk of bias in a single result from any form of randomized trial (an estimate of the impact of an experimental intervention compared with a comparator intervention on a specific outcome).14 Randomized intervention studies were evaluated for risk of bias. The majority of the articles15 had an overall low judgment; 2 articles had some concerns as an overall judgment and 3 articles had a high overall judgment. Summary plot [Figure 1] and Traffic light [Figure 2] were generated.
 |
Figure 1 A summary plot that summarizes the risk of bias for all the included studies. |
 |
Figure 2 Traffic Plot figure that illustrates the risk of bias in all the domains in each included study. |
Data Synthesis and Statistical Analysis
A meta-analysis was performed to determine whether the difference in HbA1C between the baseline and the end of the intervention differed between groups using DPP-4-inhibitors comparing both placebo and controls, and whether the adverse events differed in the DPP-4-inhibitor arm. Aggregate estimates were presented both as a fixed effects model and as a random effects model. For each comparison, a Cochran’s Q test was conducted to assess heterogeneity, and an I2 statistic was calculated to estimate the percentage of total between study variations. All analyses were conducted using review manager software version 5.4.1 from the Cochrane collaboration.
Results
Search Outcomes
The database search yielded 317 studies. A total of 214 articles were retrieved from PubMed, and 103 from Cochrane Library. Thirty-two studies were duplicates and were eliminated. The remaining 285 studies were subjected to title and abstract screening where 259 studies were eliminated. The remaining 26 studies were assessed in full, where 21 satisfied the predefined eligibility criteria.
Figure 3 shows the PRISMA flow diagram summarizing the selection criteria outlined above.
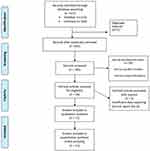 |
Figure 3 PRISMA flow chart showing how the studies were identified. Abbreviations: CKD: Chronic Kidney Disease, DPP-4 Inhibitors: Dipeptidyl peptidase-4 inhibitor, SD: Standard Deviation. |
Study Characteristics
Table 1 shows the description of each study that was included in this meta-analysis and it gives an insight of each included paper.
DPP-4 inhibitors play a significant role in the stimulation of insulin secretion and inhibition of glucagon secretion by elevating endogenous GLP-1 concentrations. DPP-4 inhibitors explored in the included studies are trelagliptin, omarigliptin, tenegliptin, sitagliptin, saxagliptin, linagliptin, and vildagliptin.
 |
Table 1 Study Descriptor Table |
This meta-analysis aims to find out the Efficacy and Safety of DPP-4 inhibitors in the treatment of T2DM and renal insufficiency. It included 21 RCTs. The patients were categorized into two groups: DPP-4 inhibitors group and the control group. Three different variables were meta-analyzed, which are efficacy (changes from baseline in HbA1c), Adverse Events (AE), and Severe Adverse Events (SAE).
Efficacy (Changes from Baseline in HbA1c): Meta-Analysis Results
The efficacy of DPP-4 inhibitors was evaluated by measuring the changes from baseline in HbA1c in the included studies [Figure 4]. In assessing the outcome of the efficacy of DPP-4 in the treatment of Type Two Diabetes (T2D) and renal insufficiency, a total of 2917 patients under the DPP-4 inhibitors group and 2377 patients under the control group were included. All studies did not cross the line of zero effect, and therefore all studies were individually significant (p< 0.05). A study by Yki-Jarvinem et at, 2013 had the highest precision (58.8%), while Shira Perl et al, 2016 had the lowest precision (0%). A fixed effect model was used, and the pooled summary effect was −0.5295 and the 95% CIs were −0.5337 and −0.5252. The included studies had high heterogeneity p < 0.00001 and I2 = 99%. Since the CI did not cross the line of no effect, the overall effect was significant. Overall effect Z = 242.02 (p < 0.00001). The results favour patients in the DPP-4 group. Therefore, patients in the DPP-4 inhibitors group had improved glycemic control.
 |
Figure 4 Forest plot of comparison: DPP-4 inhibitors vs control group (efficacy). |
Adverse Events (AE): Meta-Analysis Results
The safety and tolerability of DPP-4 inhibitors in the treatment of T2DM and renal insufficiency were evaluated by assessing the occurrence of Adverse Events [Figure 5]. In assessing the outcome of the safety and tolerability of DPP-4 inhibitors, a total of 8138 patients under the DPP-4 inhibitors group and 7517 patients under the control group were included. Only two studies (Udell et al and Shira et al) did not cross the line of zero effect and therefore were individually significant (p < 0.05). The remaining studies crossed the line of no effect and were individually not significant. A study by Rosenstock 2016 had the highest precision (24.2%), while Chan 2008, Kaku 2020, and McGill 2014 Severe had the lowest precision (0.2%). A random effect model was used, and the pooled summary effect was 0.9967 and the 95% CIs were 0.9967 and 1.1047. The included studies had relatively low heterogeneity p = 0.25 and I2 = 15%. Since the CI crossed the line of no effect, the overall effect was not significant. Overall effect Z = 0.06 (p = 0.95). The results show that there is no significant difference in the number of patients who suffered adverse events between the DPP-4 and the control group. Therefore, DPP-4 inhibitors are safe and well tolerated by patients.
 |
Figure 5 Forest plot of comparison: DPP-4 inhibitors vs control (Adverse Events). |
Severe Adverse Effects (SAE)
The safety and tolerability of DPP-4 inhibitors in the treatment of T2DM and renal insufficiency were also evaluated by assessing the occurrence of severe adverse events [Figure 6]. In assessing the outcome of the safety and tolerability of DPP-4 inhibitors, a total of 8138 patients under the DPP-4 inhibitors group and 7517 patients under the control group were included. All studies crossed the line of no effect and were individually not significant p > 0.05. A study by Rosenstock et al had the highest precision (57.9%) (reference), while Groop et al had the lowest precision (0.1%) (reference). A fixed effect model was used, and the pooled summary effect was 0.9739 and the 95% CIs were 0.9054 and 1.0475. The included studies had low heterogeneity p = 1 and I2 = 0%. Since the CI crossed the line of no effect, the overall effect was not significant. Overall effect Z = 0.71 (p = 0.48). The results show that there is no significant difference in the number of patients who suffered severe adverse events between the DPP-4 and the control group. Therefore, DPP-4 inhibitors are safe and well tolerated by patients.
 |
Figure 6 Forest plot of comparison: DPP-4 inhibitors vs control (Severe Adverse Effects). |
Discussion
This meta-analysis aimed to assess the efficacy and safety of Dipeptidyl peptidase-4 inhibitors in patients with T2DM who had renal impairment. Patients with T2DM frequently have impaired renal function, particularly those older than 65, and these conditions are independently linked to higher death rates, cardiovascular events, and hospitalizations.32
Few treatment alternatives are available for managing hyperglycemia in patients with type 2 diabetes mellitus with ESRD receiving dialysis.21 In order to gather more knowledge on DPP-4 inhibitors in patients with ESRD, the current study was built on a prior clinical study. It indicated that treatment with several DPP-4 inhibitors led to a reasonable improvement in glycemic control compared to a placebo. Antihyperglycemic therapy options are scarce and frequently linked with adverse side effects in patients with type 2 diabetes and CKD. Newer antihyperglycemic medications like DPP-4 inhibitors and incretin mimics may be used more frequently in people with type 2 diabetes with CKD.15 Patients with T2DMoften have CKD, which is linked to a higher risk of hypoglycemia. Impaired renal glucose release and abnormal medication metabolism are additional risk factors for hypoglycemia brought on by CKD. Due to their lower risk of hypoglycemia, given their mechanism of action, DPP-4 inhibitors are thought to have the potential to solve several issues with hypoglycemic medications in patients with CKD.27
Patients in this research who received saxagliptin 2.5 mg compared favorably to those who received a placebo for the adjusted mean change in HbA1c from baseline to week 12. In individuals with moderate or severe renal impairment, analyses by baseline renal impairment category demonstrated that saxagliptin produced statistically higher adjusted mean decreases in HbA1c than placebo. The adjusted mean reduction in HbA1c caused by saxagliptin in the ESRD subgroup was equivalent to gains made in the subgroups of patients with other forms of renal impairment. Still, it was comparable to increases made by placebo.30 This investigation’s efficacy findings align with further research in which linagliptin, combined with popular OADs, dramatically improved HbA1c levels in patients with T2DM and normal renal function. The placebo-adjusted mean decreases in HbA1c from baseline in the current group are comparable to glycaemic improvements seen in studies of other DPP-4 inhibitors in renal impairment patients.19 The present investigation, however, is the first to precisely evaluate the use of exogenous insulin and DPP-4 inhibitor in patients with T2DM and CKD. Therefore, we think this research has therapeutically applicable applications given that most CKD patients require insulin therapy.
Without raising the risk of hypoglycemia or weight gain, linagliptin 5 mg once daily helped with glycemic management, was well-tolerated, had a favorable safety profile, and when combined with background therapy already in situ, reduced HbA1c by a factor adjusted for placebo.23 The concurrent use of OADs, the kind of basal insulin, the patient’s age, or the degree of renal impairment had no impact on these benefits. Low rates of hypoglycemia were observed when linagliptin was added to basal insulin, suggesting that a basal insulin dosage decrease may not be required to prevent hypoglycemia when linagliptin is also administered. The extension period offered new data on linagliptin’s use in clinical practice, even though its primary goal was to provide long-term safety data for the drug. Linagliptin has a largely nonrenal route of elimination, like other DPP-4 inhibitors; hence, individuals with compromised renal function can change their dosage.25
Teneligliptin, a DPP-4 inhibitor, dramatically enhances glycemic control in diabetic patients with ESRD. Teneligliptin-related hypoglycemia or significant adverse effects were not observed during the research. Teneligliptin, a novel DPP-4 inhibitor, is effective, safe, and well tolerated in diabetic and hemodialysis patients. Teneligliptin significantly lowers blood glucose levels in diabetic ESRD patients.17 Since hypoglycemia is closely monitored, vildagliptin is safe for treatment in diabetic patients undergoing HD.29
DPP-4 inhibitors have a low frequency of AEs and a minimal risk of hypoglycemia.23 In patients with moderate-to-severe RI or ESRD, the usual clinical dose of saxagliptin, sitagliptin, and vildagliptin must be lowered. Lower doses of these DPP-4 inhibitors enhanced glycemic control in these participants. With the exception of a lower rate of hypoglycemia seen for patients in the DDP-4 inhibitors group compared with those in the placebo group, the incidences of adverse experiences overall, specific clinical adverse experiences, laboratory adverse experiences, and discontinuations because of a negative experience were comparable between the DDP-4 inhibitors and placebo groups. Patients on DDP-4 inhibitors who had type 2 diabetes and normal renal function had a low incidence of hypoglycemia in the past.15 In general, saxagliptin was well tolerated. Individuals with moderate and severe renal impairment experienced more adverse events (AEs) when treated with saxagliptin than patients in the placebo group. Still, equal AE rates were observed in patients with end-stage renal disease (ESRD).30 However, the total AE incidence was comparable across the two treatment groups when examined by therapy and background insulin use. There may be a misconception that saxagliptin medication is linked to more frequent AEs because insulin use was more prevalent in the saxagliptin group, despite the possibility that the imbalance is due to insulin use. Notably, all individuals taking background insulin therapy had their study drug discontinued due to AEs.
This long-term study found no brand-new safety signal or unexpected risk. There was generally no indication of hepatic, cutaneous, or pancreatitis-related safety associated with DPP-4 inhibitors when used for a year in patients with moderate or severe renal impairment, regardless of the specific focus areas for these drugs. Patients with severe renal impairment who received DPP-4 inhibitors experienced more adverse events (AEs) than those who received a placebo, primarily due to an increase in the number of mild instances of influenza.26 Hypoglycemia and constipation are the two main side effects of teneligliptin, according to the pharmaceutical manufacturer that supplied details on domestic clinical studies.17 Both in patients with moderate renal impairment (RI) and those with severe RI, the frequencies of any AE, any SAE, discontinuations due to AEs, and deaths with vildagliptin were comparable to those happening in patients receiving placebo.20 Additionally, linagliptin therapy did not significantly increase body weight in patients with mild, moderate, and severe RI. This conclusion is relevant since taking insulin has been linked to extreme weight gain.19
This study had some limitations. Although sitagliptin and saxagliptin have been studied in patients with T2DM and moderate-to-severe RI, and no safety signals have been found for either drug, the relatively small sample size and brief (12 weeks) duration of the placebo-controlled study period of the prior studies limit conclusions about the safety of DPP-4 inhibitors in patients with T2DM and moderate or severe RI. Second, there was heterogeneity between the parent studies, and some analyses were post hoc. Third, observations during the extension are hypothesis-generating only due to the study design. Third, the trial’s duration, which might have been too brief to affect kidney-related clinical outcomes like ESRD, could be a possible obstacle to correctly interpreting any renal effects. Fourth, although information on the introduction of treatments after the baseline period was available, it was not stated if linagliptin was associated with patients using fewer additional therapies. The timing of the blood sample is another limitation. Three days after the previous hemodialysis, a blood sample was collected when the dialysis procedure began. The interval following meals varied depending on the patient.
Conclusion
Patients with T2DM and CKD are among the most at risk for failure to achieve glycemic goals. The findings of this meta-analysis reveal that individuals with comorbid T2DM and CKD demonstrated significantly improved glycemic control when administered with DPP-4 inhibitors compared to the control group. Additionally, no significant disparity in the occurrence of adverse events was noted between the DPP-4 inhibitor-treated group and the control group.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in the United States population, 2009–2014. Clin J Am Soc Nephrol. 2017;12:1984–1990. doi:10.2215/CJN.03700417
2. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12:73–81. doi:10.1038/nrneph.2015.173
3. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517–523. doi:10.1038/ki.2012.401
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150.
5. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi:10.1016/S0140-6736(06)69705-5
6. Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46:1453–1469. doi:10.1345/aph.1R041
7. Wu Y, Li Z, Liu Y. The efficacy and safety of dipeptidyl peptidase-4 inhibitor in diabetic patients with chronic kidney disease: a systematic review and meta-analysis. Diabet Metab Synd Obes. 2020;13:3303–3314. doi:10.2147/DMSO.S263539
8. Ramirez G, Morrison AD, Bittle PA. Clinical practice considerations and review of the literature for the use of DPP-4 inhibitors in patients with type 2 diabetes and chronic kidney disease. Endocr Pract. 2013;19:1025–1034. doi:10.4158/EP12306.RA
9. Mulvihill EE, Drucker DJ, Bar-Tana J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:35. doi:10.1210/er.2013-1006
10. Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10:88–103. doi:10.1038/nrneph.2013.272
11. Kang YM, Jung CH. Effects of incretin-based therapies on diabetic microvascular complications. Endocrinol Metab. 2017;32:316–325. doi:10.3803/EnM.2017.32.3.316
12. Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine. 2014.46:406-–419
13. Lin Y, Liu Y, Y. C, Chen YJ, Liu N, Chen M. The efficacy and safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Medicine. 2017;96(50):e9170. doi:10.1097/MD.0000000000009170
14. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al., editors.Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022).Cochrane;2022.
15. Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obesity Metab. 2008;10(7):545–555. doi:10.1111/j.1463-1326.2008.00914.x
16. Udell JA, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38(4):696–705. doi:10.2337/dc14-1850
17. Otsuki H, Kosaka T, Nakamura K, Shimomura F, Kuwahara Y, Tsukamoto T. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol. 2014;46(2):427–432. doi:10.1007/s11255-013-0552-6
18. Chacra A, Gantz I, Mendizabal G, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int J Clin Pract. 2017;71(6):e12955. doi:10.1111/ijcp.12955
19. McGill JB, Yki-Järvinen H, Crowe S, Woerle HJ, von Eynatten M. Combination of the dipeptidyl peptidase-4 inhibitor linagliptin with insulin-based regimens in type 2 diabetes and chronic kidney disease. Diabetes Vasc Dis Res. 2015;12(4):249–257. doi:10.1177/1479164115579001
20. Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obesity Metab. 2011;13(10):947–954. doi:10.1111/j.1463-1326.2011.01467.x
21. Arjona Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kid Dis. 2013;61(4):579–587. doi:10.1053/j.ajkd.2012.11.043
22. Rosenstock J, Perkovic V, Johansen OE, et al.; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi:10.1001/jama.2018.18269
23. Groop PH, Del Prato S, Taskinen MR, et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairment. Diabetes Obesity Metab. 2014;16(6):560–568. doi:10.1111/dom.12281
24. Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care. 2015;38(2):e15–e17. doi:10.2337/dc14-1684
25. Yki-Järvinen H, Rosenstock J, Durán-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36(12):3875–3881. PMID: 24062327; PMCID: PMC3836100. doi:10.2337/dc12-2718
26. Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obesity Metab. 2012;14(11):1032–1039. doi:10.1111/j.1463-1326.2012.01634.x
27. Kaku K, Ishida K, Shimizu K, Achira M, Umeda Y. Efficacy and safety of trelagliptin in Japanese patients with type 2 diabetes with severe renal impairment or end-stage renal disease: results from a randomized, Phase 3 study. J Diabetes Invest. 2020;11(2):373–381. doi:10.1111/jdi.13126
28. Cornel JH, Bakris GL, Stevens SR, et al., TECOS Study Group. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304–2310. doi:10.2337/dc16-1415
29. Ito M, Abe M, Okada K, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocr J. 2011;58(11):979–987. doi:10.1507/endocrj.ej11-0025
30. Nowicki M, Rychlik I, Haller H, Warren ML, Suchower L, Gause-Nilsson I. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obesity Metab. 2011;13(6):523–532. doi:10.1111/j.1463-1326.2011.01382.x
31. Leiter LA, Carr MC, Stewart M, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized Phase III study. Diabetes Care. 2014;37(10):2723–2730. doi:10.2337/dc13-2855
32. Perl S, Cook W, Wei C, Iqbal N, Hirshberg B. Saxagliptin efficacy and safety in patients with type 2 diabetes and moderate renal impairment. Diabetes Ther. 2016;7(3):527–535. PMID: 27402391; PMCID: PMC5014790. doi:10.1007/s13300-016-0184-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
