Back to Journals » Infection and Drug Resistance » Volume 17
Effectiveness of Drip Infusion of Lascufloxacin, a Novel Fluoroquinolone Antibiotic, for Patients with Pneumonia Including Chronic Lung Disease Exacerbations and Lung Abscesses
Received 20 December 2023
Accepted for publication 29 February 2024
Published 8 March 2024 Volume 2024:17 Pages 911—918
DOI https://doi.org/10.2147/IDR.S453634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Daishi Shimada,1 Masafumi Seki1,2
1Division of Infectious Diseases, Tohoku Medical and Pharmaceutical University Hospital, Sendai City, Japan; 2Division of Infectious Diseases and Infection Control, Saitama Medical University International Medical Center, Hidaka City, Japan
Correspondence: Masafumi Seki, Division of Infectious Diseases and Infection Control, Saitama Medical University International Medical Center, Yamane 1397-1, Hidaka City, Saitama, 350-1298, Japan, Tel +81-42-984-4392, Fax +81-42-984-0280, Email [email protected]
Background: Lascufloxacin (LSFX), a novel fluoroquinolone antibacterial agent, has recently been used as a drip infusion for treating pneumonia, apparently with good effectiveness against various bacteria, including anaerobes, and good intrapulmonary penetration.
Methods: The clinical effectiveness of LSFX was retrospectively investigated for the 55 patients admitted to our hospital with pneumonia, including chronic lung disease exacerbations and lung abscesses, from May 2021 to July 2023.
Results: The median age of the 55 patients was 76.1 (34.1– 93.1) years, 45 (81.8%) were male, and 48 (87.5%) patients had underlying disease. Community-acquired pneumonia was seen in 47 (85.5%) patients, including 9 (16.4%) with lung abscess, and the other 8 (14.5%) had nursing and healthcare-associated pneumonia/hospital-acquired pneumonia. Moderate pneumonia was present in 33 (61.8%) of 55 patients, and LSFX was used as a second-line treatment for 28 (50.9%) patients in whom first-line antibiotics were ineffective. The median duration of intravenous LSFX administration was 9 (2.0– 49) days. Streptococcus pneumoniae and methicillin-susceptible Staphylococcus aureus were isolated from 3 (7.1%) and 2 (4.8%) patients, respectively. Of the 55 patients, 45 (81.5%) improved clinically with intravenous LSFX administration; 20 (95.2%) of 21 community-acquired pneumonia cases, including 9 (100.0%) of 9 bacterial pneumonia cases, were improved by LSFX as first-line treatment, and 8 (88.9%) of 9 lung abscess patients also showed clinical improvement with LSFX as a second-line treatment. There were no severe adverse effects in any of the 55 patients.
Conclusion: Based on these data, intravenous administration of LSFX seems effective for bacterial pneumonia, including chronic lung disease exacerbations and lung abscesses, and it appears to have broad antimicrobial activity and good tissue penetration into the lung.
Keywords: aspiration pneumonia, empyema, fluoroquinolone, lascufloxacin, lung abscess
Introduction
Pneumonia, especially aspiration pneumonia, is a significant issue in a high-age society, including Japan, because pneumonia is associated with higher morbidity and mortality in elderly patients than in younger patients.1,2 The clinical importance of pneumonia in elderly persons relates to age-dependent and pathological changes in the immune system, as well as lung functions. In fact, pneumonia is a major infection that has been in the top three to five of the leading death causes in Japan, and aspiration pneumonia is known to be one of the important types of nursing- and healthcare-associated pneumonia (NHCAP).3,4
Aspiration pneumonia could be a life-threatening condition in elderly persons, such as those with severe hemoptysis, and mortality rates might be as high as 15–20%.1,4 The initial step in the pathogenesis of these diseases is usually aspiration of infectious material from the oropharynx or stomach. Therefore, a mixed spectrum, including anaerobic, microaerobic, and aerobic microorganisms makes up the expected microbiological flora,1,4 and penicillin G had been the first choice antibiotic for a long time, until it was outperformed by ampicillin/sulbactam (ABPC/SBT) or clindamycin.5 Furthermore, third- and fourth-generation cephalosporins, piperacillin, and fluoroquinolones (FQs) have recently been recommended.6,7
In addition, as a complication of aspiration pneumonia, lung abscesses are indolent at onset, and they complicate acute mono- or poly-microbial infections with pyogenic bacteria, including Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and anaerobic bacteria.4,6,8 Lung abscesses can also result from secondary infection of pre-existing lung cavities, bronchial obstruction, septic embolization, or direct extension from local infections such as empyema. Inflamed tissue usually surrounds the pus-filled cavity in lung abscesses and shows the typical round-shape formation on chest X-ray photographs and computed tomography (CT).6,9 Therefore, lung abscesses are difficult to treat by common antibiotics, such as cephalosporins and penicillins, because they frequently show less penetration through thick, inflamed capsules than FQs, although levofloxacin (LVFX), a representative FQ, did not show effectiveness against anaerobic bacteria.5,10
Lascufloxacin (LSFX) became available in Japan in 2021, and this agent shows good penetration to lung tissues, and a wide range of pathogenic bacteria, including anaerobes, are susceptible to it.11,12 These data suggested its clinical effectiveness for adult pneumonia, especially aspiration pneumonia and lung abscess in elderly persons, and the safety and efficacy of LSFX against NHCAP have actually been shown.13
In this study, the effectiveness in the clinical setting of intravenous administration of LSFX for adult hospitalized patients with respiratory infectious diseases, including community pneumonia, secondary exacerbations in those with chronic lung diseases, lung abscess, empyema, and NHCAP, was retrospectively investigated.
Methods
Patients and the Definition of Pneumonia
Data on patients who were hospitalized and treated by LSFX drip infusion for pneumonia/respiratory infectious diseases at Tohoku Medical and Pharmaceutical University Hospital from May 1, 2021, to July 31, 2023, were investigated. Pneumonia patients were defined as patients who had lower respiratory tract infections based on new infiltrate shadows on chest X-ray and did not have other emerging alternative diagnoses. Bacterial pneumonia was diagnosed as probable in patients with sputum cultures positive for the bacteria, and these patients did not have chronic lung diseases in the present study. In addition, chronic lung disease exacerbation was defined as pneumonia in patients with underlying chronic pulmonary diseases, such as chronic obstructive pulmonary disease, old tuberculosis, and so on.
Cases of suspected atypical pneumonia, such as young patients, and those who had interstitial shadows and dry cough were excluded. Furthermore, mycoplasma or legionella antigen kit tests (Ribotest, AsahiKasei, Tokyo, Japan) were performed and confirmed, and then the patients with positive results on these tests were excluded.
LSFX was administered intravenously, with a loading dose of 300 mg one-time on the first day, and followed by 150 mg once a day from the second day.
Community-acquired pneumonia (CAP), NHCAP, and hospital-acquired pneumonia (HAP) were classified according to the definition of the Japanese Respiratory Society guideline for adults.3,14,15 In brief, HAP is defined as a pneumonia that occurs 48 hr or more after admission in patients with some severe underlying disease including cancers, and CAP is a pneumonia that occurs outside the hospital in patients who usually do not have severe underlying diseases. NHCAP is a pneumonia that matched at least one of the following: 1) elderly or physically disabled people who require care; 2) admission to a long-term care or nursing home; 3) discharge from hospital in the preceding 90 days; and 4) outpatients who regularly receive infusion therapy (including antibiotics, anticancer agents, dialysis, immunosuppressant drugs, etc.) or hemodialysis.3 This type of pneumonia was recently combined with HAP and is now considered NHCAP/HAP in Japan.
Ethics
The Committee for Clinical Scientific Research of Tohoku Medical and Pharmaceutical University Hospital approved this study on March 15, 2021 (No. ID2021-2-156) as a trial of treatment for bacterial infectious diseases. All patients whose specimens were used and who participated in this study provided written informed consent as part of the comprehensive consent obtained at admission to have any accompanying images and their case details published. Patients in particular were provided the means to opt out of these clinical studies. This study adhered to the Declaration of Helsinki.
Assessment of Severity
The A-DROP system was used according to the Japanese Respiratory Society guideline.15,16 In brief, the A-DROP system is based on five clinical items: age (A), dehydration (D), respiration (R), orientation (O), and blood pressure (P). The cases in this study were regarded as “mild” with none of the five items, as “moderate” with one or two of the items, as “severe” with three of the items, and as “extremely severe” with four or five of the items.
Clinical Efficacy Judgment and Analysis
The attending physician evaluated the clinical effectiveness of the treatment in individual cases using a three-category scale: improved (absence or decrease in fever (temperature >37.5 °C), chest pain, chills, cough, and dyspnea), stable (fever, symptoms such as chest pain, chills, cough, and persistence of sputum), and exacerbated/death (all other cases) based on the time course of the clinical symptoms from the start of dosing to the end of treatment.
Adverse Events (AEs)
The date of onset, nature, severity, treatment provided, and outcome were recorded when a symptom or laboratory abnormality appeared during the treatment period. Causal relationships to the study drugs were rated as follows: related, possibly related, or not related. Serious AEs were defined as life-threatening and/or related to prolonged hospitalization. Nephrotoxicity was defined as a serum creatinine increase of 0.5 mg/dL or 50% from baseline.17,18 Hepatotoxicity was defined as AST or ALT that was three-fold the upper limit of normal (ULN; AST: 13–33 IU/L, ALT: 8–42 IU/L).17,18 If the AST or ALT baseline was abnormal, hepatotoxicity was defined as AST or ALT three times increased from the baseline value.
Statistical Analysis
The Mann–Whitney test and the chi-squared test were used to compare continuous variables between two groups. A p-value of less than 0.05 was defined as indicating a significant difference. All analyses were performed using Stat View software (Abacus Concepts, Cary, NC, USA).
Results
Patients’ Characteristics and Treatments
A total of 55 pneumonia and respiratory infectious disease patients were analyzed (Table 1). The median age was 76.1 (34.1–93.1) years, and 45 (81.8%) of the 55 patients were male. Of the 55 patients, 39 (70.9%) and 48 (87.5%) had a history of smoking or underlying diseases, respectively, and 47 (85.5%) were admitted as CAP, including 14 (25.5%) with bacterial pneumonia and 23 (41.8%) with an exacerbation of a chronic lung disease. Furthermore, 9 (16.4%) patients had lung abscesses, and 1 (1.8%) patient had empyema. Of the 55 patients, 8 (14.5%) were defined as having NHCAP/HAP. In more than half of the patients, pneumonia was graded as moderate (33, 61.8%), with the number of severe, mild, and extremely severe at 12 (21.8%), 6 (10.9%), and 4 (7.3%), respectively.
 |
Table 1 Patients’ Characteristics (n=55) |
LSFX was used as a second-line treatment after treatment with other antibiotics, such as sulbactam/ampicillin, ceftriaxone, tazobactam/piperacillin, and meropenem in 28 (50.9%) of 55 patients. The patients received intravenous administration of LSFX for 9 (2.0–49) days.
Bacteria Isolated from Cultures
Enterococcus faecalis was isolated in 1 (3.1) of 32 blood cultures from 55 pneumonia patients (Table 2). In the 42 sputum cultures from 55 patients, S. pneumoniae, MSSA, MRSA, H. influenzae, K. pneumoniae, K. oxytoca, E. coli, S. marcescens, and P. aeruginosa were isolated.
 |
Table 2 Bacteria Isolated from Cultures |
Clinical Outcomes
Of the 55 patients, 52 (94.5%) survived, 45 (81.8%) improved clinically, 6 (10.9%) were stable (Figure 1), and 4 (7.3%) were ultimately exacerbated or died.
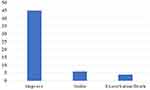 |
Figure 1 Clinical outcomes of the patients treated by lascufloxacin drip infusion. |
In detail, 39 (83.0%) of 47 CAP patients improved (Table 3); 20 (95.2%) of 21 were improved when LSFX was used as first-line treatment, and 19 (73.1%) of 26 were also improved after other antibiotics were used and were ineffective. CAP and bacterial pneumonia improved significantly when LSFX were used as first-line treatment in 20 (95.2%) of 21 patients and 9 (100%) of 9 patients, respectively, and 11 of 12 (91.7%) chronic lung exacerbations also improved with first-line use. Furthermore, in both lung abscess and empyema patients, LSFX was used as second-line treatment, and 8 (88.9%) of 9 lung abscess patients, and the 1 (100%) empyema patient improved.
 |
Table 3 Improvement by Diagnosis/Treatment Line |
In NHCAP/HAP, 6 (75%) of 8 patients improved, and 3 (100%) of 3 patients improved with LSFX used as second-line treatment.
According to severity, 5 (83.3%) of 6 mild and 28 (84.8%) of 33 moderate patients improved, more than 80% overall, and LSFX treatment showed high effectiveness in moderate patients, although LSFX was used as second-line treatment in 14 (87.5%) of 16 patients. In severe and extremely severe patients, LSFX showed good effectiveness in the first-line treatment group, 5 (83.3%) of 6 and 2 (100%) of 2 patients, respectively.
There were no severe AEs, including allergic reactions, liver dysfunction, renal dysfunction, phlebitis, and abnormalities of the electrocardiogram, such as a prolonged QT (data not shown).
Discussion
In the present study, the clinical effectiveness of LSFX, one of the novel FQs, was investigated for adult pneumonia, including lung abscess. The overall effectiveness of LSFX was 81.3%, and in first-line treatment, 95.2% (100% in bacterial pneumonia and 91.2% in chronic lung disease exacerbations) of CAP cases were improved. These data suggested that LSFX might be a strong candidate as a first-choice antibiotic for adult pneumonia patients.
It has been reported that LSFX might have outstanding intrapulmonary penetration and antibacterial activity against major respiratory pathogens.11,19 In addition, selective toxicity to bacteria because of the strong inhibitory effects on both topoisomerase IV and DNA gyrase, a minimum reduction of inhibitory activity when mutant strains appear,20 and a lower tendency to select resistant mutants, have been reported compared to LVFX and garenoxacin (GRNX).21
In fact, the clinical effectiveness of LSFX for CAP was 81.3% overall and 95.2% as first-line treatment for elderly patients, which might be similar or slightly higher than ABPC/SBT at 91.4% and imipenem/cilastatin (IPM/CS) at 87.5%.22 Depending on pneumonia severity, 82.4% to 100% improvement was found in mild to extremely severe pneumonia when used as a first-line treatment; therefore, LSFX should be used immediately in elderly pneumonia patients.
In addition, as a second-line treatment, improvement was seen in 88.9% of lung abscess, 100% of empyema, and 100% of NHCAP/HAP cases. These data suggested very high lung penetration, including into inflammatory tissue capsules of lung abscesses, and strong bactericidal effects on pathogenic bacteria, including pathogenic anaerobes in lung abscess, empyema, and aspiration pneumonia, which are among the major factors related to NHCAP/HAP in Japan.1,3
It has been reported that LSFX might be distributed rapidly to the epithelial lining fluid with a time to maximum drug concentration (Tmax) of 1 h, similar to that in plasma.11 The values for the maximum concentration of drug (Cmax) in plasma, epithelial lining fluid, and alveolar macrophages were 0.576, 12.3, and 21.8 μg/mL, respectively, and these drug levels exceeded the MIC90 values for common respiratory pathogens in alveolar macrophages and epithelial lining fluid.11 Bactericidal effects on cell-internalized Group A Streptococci were also reported recently.23 Furthermore, not only in vitro, but also in vivo, LSFX has been reported to be effective for Streptococcus pneumoniae, Prevotella intermedia, anaerobes, and the Streptococcus anginosus group.12,20,21,24 These data also suggested that LSFX might be effective as a second-line treatment for lung abscess, empyema, and NHCAP/HAP cases, even if other antibiotics, including ABPC/SBT, CTRX, PIPC/TAZ, and MEPM had been used before and were ineffective as first-line treatments.
No patients showed severe AEs, and suspected atypical pneumonia cases were excluded from the present study. However, about 10–20% patients did not improve with LSFX administration, especially the patients who received second-line treatments. Only in 13 of 55 (23.6%) patients, 1 from 32 blood cultures and 12 from 42 sputum cultures, were bacteria isolated cases by culture, but many pathogenic and LSFX-resistant bacteria might not have been detected in the present study. Two of 13 (15.4%) cases with bacteria isolated did not improve, because one was MRSA, and the other was P aeruginosa. Both MRSA and P aeruginosa are known to not be susceptible to LSFX,20 and we should be careful with these kinds of resistant bacteria and change the LSFX to either anti-MRSA agents or anti-pseudomonas agents immediately.
In addition, host condition was also an important factor, because the effectiveness of LSFX in the second-line treatment decreased to about 50% in severe and extremely severe patients, although it was 75–87.5% in mild-to-moderate patients, showing that LSFX as second-line treatment was effective in mild-to-moderate patients (Table 3). Pneumonia patients should be identified early, and antibiotic treatment with LSFX should be started as soon as possible.
In conclusion, the clinical effectiveness of the novel respiratory FQ, LSFX, for adult pneumonia and respiratory infectious diseases, including lung abscess and empyema, was investigated. Based on its good penetration into lung tissue and wide-ranging coverage of various bacteria, including anaerobes, LSFX showed excellent improvement not only for pneumonia, especially severe and extremely severe cases, in first-line treatment, but also for lung abscess, empyema, and NHCAP/HAP cases as second-line treatment when other antibiotics were ineffective.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors have no competing interests to disclose for this work.
References
1. Cunha BA. Pneumonia in the elderly. Clin Microbiol Infect. 2001;7(11):581–588. doi:10.1046/j.1198-743x.2001.00328.x
2. Pletz MW, Blasi F, Chalmers JD, et al. International perspective on the new 2019 American Thoracic Society/Infectious Diseases Society of America Community-Acquired Pneumonia Guideline: a critical appraisal by a global expert panel. Chest. 2020;158(5):1912–1918. doi:10.1016/j.chest.2020.07.089
3. Kohno S, Imamura Y, Shindo Y, et al. Clinical practice guidelines for nursing- and healthcare-associated pneumonia (NHCAP). Respir Investig. 2013;51(2):103–126. doi:10.1016/j.resinv.2012.11.001
4. DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30(1):40–48. doi:10.1016/j.jcrc.2014.07.011
5. Allewelt M, Schüler P, Bölcskei PL, Mauch H, Lode H; Study Group on Aspiration Pneumonia. Ampicillin + sulbactam vs clindamycin ± cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect. 2004;10(2):163–170. doi:10.1111/j.1469-0691.2004.00774.x
6. Nicolini A, Cilloniz C, Senarega R, Ferraioli G, Barlascini C. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82(3):276–285. doi:10.5603/PiAP.2014.0033
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23–30. doi:10.1007/s15010-007-7043-6
8. Lode H. Microbiological and clinical aspects of aspiration pneumonia. J Antimicrob Chemother. 1988;21:C83–C90. doi:10.1093/jac/21.suppl_C.83
9. Prather AD, Smith TR, Poletto DM, et al. Aspiration-related lung diseases. J Thorac Imaging. 2014;29(5):304–309. doi:10.1097/RTI.0000000000000092
10. Lin J, Zhang Y, Bao C, et al. The clinical features and management of empyema caused by Streptococcus constellatus. Infect Drug Resist. 2022;28(15):6267–6277. doi:10.2147/IDR.S382484
11. Furuie H, Tanioka S, Shimizu K, Manita S, Nishimura M, Yoshida H. Intrapulmonary pharmacokinetics of lascufloxacin in healthy adult volunteers. Antimicrob Agents Chemother. 2018;62(4):e2169–17. doi:10.1128/AAC.02169-17
12. Yamagishi Y, Matsukawa Y, Suematsu H, Mikamo H. In vitro activity of lascufloxacin, a novel fluoroquinolone antibacterial agent, against various clinical isolates of anaerobes and Streptococcus anginosus group. Anaerobe. 2018;54:61–64. doi:10.1016/j.anaerobe.2018.08.002
13. Hosogaya N, Takazono K, Ota K, et al. Evaluation of efficacy and safety of lascufloxacin for nursing and healthcare associated pneumonia: single-arm, open-label clinical trial: a study protocol. Medicines. 2023;102(8):e33092. doi:10.1097/MD.0000000000033092
14. Seki M, Watanabe A, Mikasa K, Kadota J, Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13:880–885. doi:10.1111/j.1440-1843.2008.01348.x
15. The committee for the Japanese Respiratory Society guidelines in the management of respiratory infections. The Japanese Respiratory Society guidelines for the management of community-acquired pneumonia in adults. Respirology. 2006;11:S1–S133. doi:10.1111/j.1440-1843.2006.00815.x
16. Kohno S, Seki M, Watanabe A; CAP Study Group. Evaluation of an assessment system for the JRS 2005: a-DROP for the management of CAP in adults. Intern Med. 2011;50(11):1183–1191. doi:10.2169/internalmedicine.50.4651
17. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29:1275–1279. doi:10.1592/phco.29.11.1275
18. Seki M, Yabuno K, Miyawaki K, Miwa Y, Tomono K. Loading regimen required to rapidly achieve therapeutic trough plasma concentration of teicoplanin and evaluation of clinical features. Clin Pharmacol. 2012;4:71–75. doi:10.2147/CPAA.S37528
19. Tanaka K, Vu H, Hayashi M. In vitro activities and spectrum of lascufloxacin (KRP-AM1977) against anaerobes. J Infect Chemother. 2021;27(8):1265–1269. doi:10.1016/j.jiac.2021.03.026
20. Kishii R, Yamaguchi Y, Takei M. In vitro activities and spectrum of the novel fluoroquinolone lascufloxacin (KRP-AM1977). Antimicrob Agents Chemother. 2017;61(6):e00120–17. doi:10.1128/AAC.00120-17
21. Murata M, Kosai K, Yamauchi S, et al. In vitro activity of lascufloxacin against Streptococcus pneumoniae with mutations in the quinolone resistance-determining regions. Antimicrob Agents Chemother. 2018;62(4):e01971–17. doi:10.1128/AAC.01971-17
22. Yanagihara K, Fukuda Y, Seki M, et al. Clinical comparative study of sulbactam/ampicillin and imipenem/cilastatin in elderly patients with community-acquired pneumonia. Intern Med. 2006;45(17):995–999. doi:10.2169/internalmedicine.45.1717
23. Kono M, Sakatani H, Kinoshita T, et al. Bactericidal effect of lascufloxacin on HEp-2 cell-internalized group A Streptococcus. J Infect Chemother. 2023;29(4):401–406. doi:10.1016/j.jiac.2023.01.008
24. Hagihara M, Kato H, Shibata Y, et al. In vivo pharmacodynamics of lascufloxacin and levofloxacin against Streptococcus pneumoniae and Prevotella intermedia in a pneumonia mixed-infection mouse model. Anaerobe. 2021;69:
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
