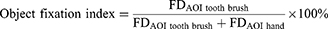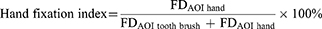Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Effect of Object on Kinesthetic Motor Imagery in Autism Spectrum Disorder: A Pilot Study Based on Eye-Tracking Methodology
Received 12 September 2023
Accepted for publication 17 January 2024
Published 24 January 2024 Volume 2024:20 Pages 167—183
DOI https://doi.org/10.2147/NDT.S435258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Ying Liu, Jinsheng Hu
Department of Psychology, Liaoning Normal University, Dalian, People’s Republic of China
Correspondence: Jinsheng Hu, Department of Psychology, Liaoning Normal University, No. 850 Huanghe Road Shahekou District, Dalian, Liaoning Province, People’s Republic of China, Email [email protected]
Introduction: Social disturbance is a significant autism spectrum disorder (ASD) symptom. Action representation, which is a fundamental component of social interaction, can be investigated through kinesthetic motor imagery (KMI). KMI has been commonly studied with the well-developed laterality judgment paradigm, wherein participants are required to discriminate the laterality of a hand rotated by different angles along one or more axes. Here, we investigated the KMI processing in individuals with ASD by hand laterality judgment paradigm with eye-tracking methodology.
Methods: The current study included 22 participants with ASD and 22 typical developing (TD) peers matched for age, gender, and intelligence. Participants were asked to judge the laterality of hand-with-tooth brush images.
Results: Compared to the TD controls, individuals with ASD performed KMI with lower accuracy and longer response time in both correct and incorrect action conditions. The incorrect action representation had greater effect on KMI for individuals with ASD. Differences in eye-movement patterns were also observed, characterized by individuals with ASD were more focused on the object area while TD peers were more focused on the hand area.
Conclusion: Results suggest that while altered KMI performance was observed, the incorrect action representation elicited more engagement of KMI in both groups. The object-centered eye-movement pattern may contribute to the refine of motor simulation intervention for individuals with ASD.
Keywords: autism spectrum disorder, kinesthetic motor imagery, action representation, eye-tracking
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder mainly characterized by deficits in social communication and repetitive stereotyped behaviors.1 Much of how we think about others’ actions, and in turn engage in social interaction, arises from the activation or simulation of our own action representations.2 Action representation, which is a fundamental component of conceptualizing one’s own and others’ actions, it can be activated through observation of others’ behaviour,3 or by imagining one’s own or others’ actions.4 Nonetheless, due to the lack of active manipulation of action representation in passive action observation tasks, kinesthetic motor imagery (KMI), a mental simulation of a body movement without actual motor output through internal embodiment of action representation, appears to be an appropriate alternative to study the internal embodiment of action representation.5
KMI shares similar neural mechanism and temporal dynamics with motor execution. The time course of KMI is highly correlated with the time taken to motor execute.6 Exercise imagination and exercise execution both appears to increase heart rate and breathing rate due to similar autonomic nerve activity.7 The main motor cortex (M1/S1), premotor area (PMA), supplementary motor area (SMA), and other brain areas work together to produce KMI and motor execution.8 Generally, KMI can induces higher activations in motor-related areas than passive action observation.9
KMI has been commonly studied with the well-developed hand laterality judgement paradigm, which requires participants to choose whether a stimulus presented from different angles is a left or a right hand, is typically used to test KMI.10 The engagement of KMI can be demonstrated by two key metrics. One is the angle effect of reaction time (RT) which states that as the stimulus rotation angle increases, so does the accuracy. The second is the biomechanical effects of the RTs. In the comfortable position, the hand rotates toward the body’s center line, whereas in the awkward position, the hand rotates away from the center line. Participants are faster and more accurate in judging hand in physiologically comfortable positions than hand in awkward positions.11
A number of studies have suggested dysfunctional KMI in ASD. Conson et al employed left or right hands portrayed from back at 0° (upright), 90° (clockwise), 180° (upside down) or 270° (90°anticlockwise) orientations as the stimulus materials for the hand laterality judgement test.12 The findings demonstrated that whereas the AS (Asperger syndrome) group did not exhibit biomechanical effects, the typical development participants did. The AS group performed worse on the test and took longer to respond, showing KMI impairments and had trouble imagining action representation.
In addition to using hand images as materials, some researchers also used images of the whole human body in the laterality judgement task, asking ASD participants to judge the lateralization of the marked hand. In this kind of experimental task, pictures of human bodies were usually divided into backside images and frontal images. Researchers holds that the backside pictures can encourage people to execute KMI with the aid of their own body representation.13 Additionally, ASD participants had more KMI errors as well as longer RTs in the back-facing condition, while the typical developing participants also had an advantage in judging the back image compared to the frontal image.
Recently, Chen et al used pictures of hands gripping spoons as a stimulus by laterality judgement task.14 The results indicated that individuals with ASD were capable of performing KMI and reacted faster to hand-without-spoon images. It seems to indicate an inhibitory role of objects in hand laterality judgement. Indeed, there is clear evidence that ASD individuals failed to simulate non-object gestures while performing normally when there were objects present.15 In other words, it has been demonstrated that objects play a crucial role in the effectiveness of simulation, while Chen et al, did not address the potential mechanisms of the difference.
Viewing an object can activate the operational action representation, which is a representation of operational action information related to the object and engaged in the object recognition process.16 The operational action information enables individuals to use objects in meaningful ways,17 have been referred to actional knowledge (eg knowing that a toothbrush should be grasped at the side of the handle and not at the brush side) and functional knowledge (eg knowing that a toothbrush is typically brought towards the mouth and used with a swinging movement).18 The operational action representation can active the sensorimotor brain areas and influence manual response times.19 According to the TRoPICALS Model (Two Route, Prefrontal Instruction, Competition of Affordances, Language Simulation Model), the neuron clusters in the cortex that encode various types of information compete with one another.20 The competing processes would terminate the competition for processing resources when the object representation communicated by the visual channel is compatible with the original representation in the brain. This model suggesting that the existence of objects may facilitate cognition processing.
The Present Study
In this study, we set up correct and incorrect operational action conditions to explore the role of objects in KMI on the basis of Chen et al. According to the TRoPICALS model, we assume that RTs in the correct action condition should be faster if objects can serve as facilitators; on the other hand, there might not be difference between individuals’ RTs in the correct and incorrect action conditions. Additionally, the presence of biomechanical effects throughout the response suggests that the participants processed their own body’s representations in order to complete the KMI. If the adolescents with ASD can spontaneously use KMI, their performance would likely be affected by biomechanical constraints, reflecting their spared ability to activate action representation in laterality judgement task. In contrast, if individuals with ASD cannot use KMI automatically, resulting in no biomechanical effect, just as when processing the non-corporeal stimuli.
Eye movement technology has also been applied to action representation processing. Federico and Brandimonte used hammer images as materials to explore the attention allocation to objects in visual scenes by eye-tracking techniques.21 The researchers divided areas of interest (AOI) into the “structural” part (hammer handle) and the “functional” part (hammer head) of the object. The analyses of fixation duration showed that the visual encoding started with a focus on the functional AOI at the first 500 ms, while the fixation pattern towards to focus on the structural AOI at the 1750ms time window. The eye movement pattern suggested that the object’s function is more important than its use. It is the first study of specific visual attention patterns for object-related structural and functional features. However, the eye movement pattern of individuals with ASD in the action representation processing is still relatively unexplored.
Eye-movement measurements provide a complementary approach to capture cognitive processes with high resolution. Eye-tracking technology offers the possibility of capturing visual behaviour information in real time and obtaining gaze position on specific stimuli.22 The obvious advantage of collecting fixation information is that the behaviour during each trial can potentially be deconstructed into various processing states whose durations can be directly measured. There is evidence that visual attention is typically drawn to the most task-relevant aspects of a display in situations where visual attention is not directed explicitly.23
Eye tracking is suited for studying cognitive processes and processing demands at a detailed level.24 In the present study, we selected fixation duration reflects the difficulty of retrieving information from the task and the depth of processing. Longer fixation duration indicates higher cognitive load.25 The stronger consistency between mental representation of material and correct object-related action representation, the more information connected to the action readiness evoked by the object.21 Thus, the difficulty of extracting information will be reduced, indicating shorter fixation duration. Second, gaze metric parameters could be used as an objective unobtrusive approach to assess engagement in KMI task.26 Participants observed index finger abduction-adduction movements while imagining the same action, eye tracking data showed that visual attention was focused on the index finger. Eye-movement metrics provided insight into the underlying attentional and cognitive mechanisms.27 Thus, we calculated the proportion of the individual’s gaze on the object as the object fixation index, to assessed how much attention was focused on the hand area versus the object area. With this indication, we could compare the variations in eye movement patterns to analyze how an object affected KMI. We proposed that the object should have an impact on the object fixation index, possibly resulting from the differences of object-related action representation.
Summary
Here, the present study focuses on two main issues. First, can ASD individuals use KMI spontaneously? Second, Does the existence of object have effect on KMI in individuals with ASD? If true, how do those with ASD and TD people vary from one other in terms of the cognitive processing mechanisms that are represented in their eye movement patterns? We also expected different eye-movement patterns between the individuals with ASD and TD individuals, in order to provide fresh concepts for the future motor simulation deficiencies treatments for the ASD group.
Method
Participants
Twenty-two ASD participants were recruited for this study (3 females and 19 males, range from 16 to 38, mean age = 20.96, SD = 6.50). They were both recruited from the Aina autism service center in Dalian. All were diagnosed with autism without other major physical diseases or neurological or mental disorders by the qualified hospitals (eg, Peking University Sixth Hospital). The diagnosis was also validated using the Autism Spectrum Quotient (AQ) conducted by qualified psychiatrist.28 The control group consisted of 22 students through bill-posting (5 females and 17 males, range from 16 to 25, mean age = 19.59, SD = 2.30). Three of them were recruited from a high school and the other were recruited from a university. In all participants, the IQ was measured by the Chinese version of Wechsler Adult Intelligence Scale – Third Edition (WAIS-III).29 We adopted the minimum criteria of 80. There were no significantly differences between the ASD participants and control groups in chronological age and full-scale IQ. All participants were right-handed with normal or corrected-to-normal vision. Participant characteristics for ASD and TD groups can be found in Table 1.
 |
Table 1 Demographic Data of Participants of the ASD and TD Group |
The studies involving human participants were reviewed and approved by the Human Ethics Committees of Liaoning Normal University (Ethical Number: LL2023058). Every individual was informed about the aims of the study and provided written consent before participation. The university health participants signed the consent form independently; The health participants in high school and all of the ASD participants were signed by their guardians. In addition to the guardians, the ASD adults also signed the consent form by themselves. Through the interview, we determined in advance whether the ASD participants could understand the experimental requirements. Participants received gifts after participation, equivalent to approximately 60 RMB.
Materials and Procedures
This study explored the effect of object on KMI and eye-movement processing model in the individual with ASD. We set two basic templates: in the correct condition, the picture is a hand gripping a tooth brush handle with the brush close to the person’s mouth; in the incorrect condition, the picture is a hand gripping the handle area with the brush seems to removed far away from person’s mouth. The stimuli were divided into left and right hands, rotating 0°, 60°, 120°, 180°, 240°, and 300°, respectively, in a total of 24 different combinations. Both pictures had a pixel size of 810 × 810 and were processed using Photoshop in grayscale (see Figure 1).
 |
Figure 1 Stimulus examples: here we show stimulus examples for the right hand with correct action condition (left-side) and with incorrect action condition (right-side) in laterality judgement task. |
The materials were presented on a 22″ CRT monitor with a 60 Hz refresh rate and 1024 × 768 pixel resolution, and were viewed from a distance of approximately 60 cm in a dimly lit room. Eye movements were recorded by Tobii T120 Eye Tracker with a sampling rate of 120 Hz. Participants were asked to keep their chin fixed on the rest, holding head stationary to avoid blinking as much as possible. Participants’ hands were covered by a black plastic board above the keyboard to avoid visual cues from hand-viewing.
Before the experiment, participants completed a five-point calibration phase (the angle of deviation was less than 1°). After the successful calibration, participants were told to observe the stimuli displayed on the screen and judge whether the stimulus displayed was a left or right hand. Their responses were indicated by pressing the “F” or “J” keys as quickly and accurately as possible with their left or right index finger. Each trial began with a fixation dot (500 ms duration) followed by a test stimulus, which remained on screen until the participants made a response (see Figure 2). The test phase took about 20–40 minutes, including 2 blocks with 240 trails in total, there were 10 trials in each condition.
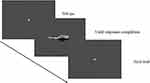 |
Figure 2 Schematic illustration of a single trial in the experiment. |
Data Analysis
We made a priori power analysis of samples by using G-Power 3.1.9.2. The result showed that 96% power (effect size = 0.25) was achieved with the sample size of 24 participants. Thus, the sample size of 44 in the present study would ensure the representativeness. The dependent variables are accuracy and RT. The data were only calculated for correct trials. The α level was set at 0.05 for all analyses.
First, to investigate general performance in the two groups, we subjected measures of accuracy and RTs to a three-way mixed-design analysis of variance (ANOVA): the between-subject factor of GROUP (TD, ASD), the within-subject factor of CONDITION (correct action, incorrect action) and ANGLE (0°, 60°, 120°, and 180°). The 60° angle included stimuli with 60° and 300° angle of the hand, the 120° angle included stimuli with 120° and 240°angle of the hand.
Second, as the biomechanical effect can prove the implementation of KMI, we investigated the biomechanical effect on RTs. We subjected RT measures to a three-way mixed-design ANOVA with CONDITION (correct action, incorrect action) and ORIENTATION (lateral rotation, medial rotation) as the within-subjects factor, and GROUP (TD, ASD) as the between-subjects factor. The medial rotation orientation included stimuli with 60° and 120° rotation of the hand. The lateral rotation orientation included stimuli with 240° and 300° rotation of the hand.
Third, we introduced a biomechanical index to compare the size of biomechanical effects between two conditions in both groups. Therefore, the index represents the percentage of additional processing effort for the lateral rotation compared to the medial rotation condition, which was defined as the following formula (Chen et al, 2018):
The RTlateral rotation referred to the average of both the 240° and 300° rotation stimuli, the RTmedial rotation referred to the average of 60° and 120° rotation stimuli, the RTall angles was the average of all stimuli. We subjected measures of RTs to paired sample t test.
Fourth, we investigated the eye-tracking performance in the two groups. We only analyzed those participants whose eye-movement sampling rate exceeds 30% (four ASD participants were excluded).30 Finally, we collected the eye-movement data of 18 ASD participants and 22 TD participants. Area of interests (AOI) were selected for the “hand” area and the “tooth brush” area.
Fixation duration reflects the difficulty of information exploration of the materials, including both the AOI of “hand” and “tooth brush” area. Object fixation index is the objects’ ability to attract attention, which represents the influence of the action representation activated by objects on the overall stimulus processing, and can reflect the individual’s internal processing mechanism.
We calculated the object fixation index using the following formula:
Similarly, we calculated the hand fixation index using the following formula:
FDAOI tooth brush is the fixation duration of “tooth brush”, and FDAOI hand is the fixation duration of “hand”. Based on these AOIs, we subjected measures of fixation duration, object fixation index and hand fixation index to a two-way mixed-design ANOVA: the between-subject factor of GROUP (TD, ASD), the within-subject factor of CONDITION (correct action, incorrect action).
Result
Behavioral Data
Accuracy
The accuracy under different conditions and angles for the two groups are shown in Table 2 and Figure 3. The three-way mixed ANOVA on accuracy revealed significant main effects of ANGLE (F(3, 126)=28.381, p<0.001, ηp2=0.403) and GROUP (F(1, 42)=17.288, p<0.001, ηp2=0.292). There were significant two-way interactions between CONDITION and GROUP (F(1, 42)=6.459, p=0.015, ηp2=0.133), between ANGLE and GROUP (F(3, 126)=7.848, p=0.003, ηp2=0.157).
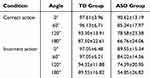 |
Table 2 The Accuracy of Participants of the ASD and TD Group (Mean ± SD) |
The three-way interaction between CONDITION, ANGLE and GROUP was also significant (F(3, 126)=3.282, p=0.034, ηp2=0.072), further analyses showed that for the ASD group, the accuracy were higher in the correct action condition than in the incorrect action condition at 180° (p=0.001, Bonferroni correction). The accuracy has no significant difference at all angle pairs for the TD group (p>0.05). In both conditions, the accuracy were higher for the TD group than that for the ASD group at all angles (p<0.05, Bonferroni correction). In the ASD group, the accuracy has no significant difference between 60° and 120° for the correct action condition (p>0.05), but there were significant differences between the rest of angle pairs in both conditions (p<0.05 for all comparisons).
Reaction Times
The RTs under different conditions and angles for the two groups were shown in Table 3 and Figure 4. The three-way mixed ANOVA revealed significant main effects of CONDITION (F(1, 42)=14.616, p<0.001, ηp2=0.258), GROUP (F(1, 42)=27.546, p<0.001, ηp2=0.396), and ANGLE (F(3, 126)=26.154, p<0.001, ηp2=0.384). There were significant two-way interactions between CONDITION and GROUP, F(1, 42)=12.284, p=0.001, ηp2=0.226, between CONDITION and ANGLE, F(3, 126)=4.425, p=0.012, ηp2=0.095.
 |
Table 3 The Reaction Time of Participants of the ASD and TD Group (Mean ± SD) |
Further analyses on the significant two-way interaction between CONDITION and GROUP revealed that the RT was slower in the incorrect condition for the ASD group (p<0.001, Bonferroni correction) and has no significant difference in the TD group (p=0.823). The RTs of the ASD group were significant slower in both conditions than the TD group (p<0.001, Bonferroni correction). In terms of the significant two-way interaction between CONDITION and ANGLE revealed significant angle effects in both conditions (p<0.05, except the RTs in the correct action condition between 60° and 120° has no significantly difference, p=0.136; except the RTs in the incorrect action condition between 0° and 60° has no significantly difference, p>0.9). The RTs in the correct action condition at 0° (p=0.004), 120° (p=0.036) and 180° (p<0.001) were significantly slower than that in the incorrect action condition.
All other two and three-way interactions were non-significant (p>0.05).
The three-way mixed ANOVA of biomechanical effect revealed significant main effects of GROUP, F(1, 42) = 27.393, p < 0.001, ηp2= 0.395, CONDITION, F(1, 42) = 11.017, p = 0.002, ηp2=0.208, and ORIENTATION, F(1, 42) = 39.181, p < 0.001, ηp2= 0.483 (see Figure 5). The significant two-way interactions were found between CONDITION and GROUP, F(1, 42) = 10.261, p = 0.003, ηp2= 0.196, between CONDITION and ORIENTATION, F(1, 42) = 14.121, p = 0.001, ηp2= 0.252.
A significant three-way interaction was also found between GROUP, CONDITION and ORIENTATION, F(1, 42) = 4.636, p=0.037, ηp2= 0.099. In the ASD group, the RTs for the correct action condition were significantly quicker than that for the incorrect action condition at both orientations (medial rotation orientation: p = 0.012; lateral rotation orientation: p<0.001, Bonferroni correction). In two conditions, the RTs for the ASD group were significantly slower than that for the TD group at both orientations (p < 0.001, Bonferroni correction). In the ASD group, the RTs for the medial rotation orientation were significantly quicker than that for the lateral rotation orientation in both conditions (the correct action condition: p = 0.012; the incorrect action condition: p = 0.002, Bonferroni correction), while the TD group had the same trend in both conditions (the correct action condition: p = 0.006; the incorrect action condition: p < 0.001, Bonferroni correction).
The biomechanical index in the two conditions are shown in Table 4. By paired samples t test analyses, the biomechanical index in the correct condition was significantly smaller than that in the incorrect condition for both groups (ASD: t = −2.712, p = 0.013, Cohen’d = 0.58; TD: t = −2.843, p = 0.010, Cohen’d = 0.61).
 |
Table 4 The Biomechanical Index (Mean ± SD) in Correct/Incorrect Action Condition (%) |
Eye Movement Data
Fixation Duration
In the correct condition, the fixation duration of the TD group was 829.65 ± 215.61 ms, the fixation duration of the ASD group was 1296.24 ± 353.57 ms. In the incorrect condition, the fixation duration of the TD group was 903.96 ± 262.03 ms, the fixation duration of the ASD group was 1545.33 ± 477.39 ms.
The two-way mixed ANOVA of fixation duration revealed significant main effects of GROUP, F(1, 38) = 30.026, p < 0.001, ηp2= 0.441, CONDITION, F(1, 38) = 27.039, p < 0.001, ηp2= 0.416 (see Figure 6). The significant two-way interactions were found between CONDITION and GROUP, F(1, 38) = 7.897, p = 0.008, ηp2= 0.172. In the ASD group, the fixation duration in the correct condition was shorter than in the incorrect condition (p < 0.001, Bonferroni correction). In both conditions, the fixation duration of the ASD group was longer than the TD group (p < 0.001, Bonferroni correction).
Object Fixation Index
In the correct condition, the object fixation index of the TD group was 17.13 ± 5.59 (%), the object fixation index of the ASD group was 26.44 ± 12.25 (%). In the incorrect condition, the object fixation index of the TD group was 39.28 ± 8.46 (%), the object fixation index of the ASD group was 45.33 ± 9.12 (%).
The two-way mixed ANOVA of fixation duration revealed significant main effects of GROUP, F(1, 38) = 11.247, p = 0.002, ηp2= 0.228, CONDITION, F(1, 38) = 145.767, p < 0.001, ηp2= 0.793 (see Figure 7).
The object fixation index for the correct action condition were significantly smaller than that for the incorrect action condition in both groups, and the object fixation index for the ASD group was significantly higher than that for the TD group in both conditions.
Hand Fixation Index
In the correct condition, the hand fixation index of the TD group was 82.87 ± 5.45 (%), the hand fixation index of the ASD group was 73.56 ± 11.90 (%). In the incorrect condition, the hand fixation index of the TD group was 60.73 ± 8.21 (%), the hand fixation index of the ASD group was 54.72 ± 8.81 (%).
The two-way mixed ANOVA of fixation duration revealed significant main effects of GROUP, F(1, 38) = 14.508, p < 0.001, ηp2= 0.160, CONDITION, F(1, 38) = 103.772, p < 0.001, ηp2= 0.577 (see Figure 8).
The hand fixation index for the correct action condition were significantly bigger than that for the incorrect action condition in both groups, and the hand fixation index for the TD group was significantly higher than that for the ASD group in both conditions.
Discussion and Implications
This study used hand laterality judgement paradigm to compare the KMI performance of the individuals with and without ASD, and an eye-tracking approach to explore the influence of object on cognitive processing during KMI.
Kinesthetic Motor Imagery Disorder in ASD
The angle effect and biomechanical effect on RTs were found in ASD individuals in the present study. The RTs increased as the angle of rotation increased, suggesting that both groups indeed performed KMI. They compared their mental representations with the object operational representation, rather than directly rotating the stimulus representation. However, longer RTs and fixation duration indicates difficulties in performing KMI for participants with ASD.31
Consistent with the present study results, Pearson et al (2014) used 3D body images as stimulus and asked participants to judge the laterality of the extended arm.32 Result showed that individuals with ASD performed worse than individuals with TD in speed and accuracy. Embodied cognition theory holds that our experiences are stored in a manner that maps onto the motor, visual, auditory, and olfactory brain systems that originally encoded them.33 The sensorimotor systems involved in the initial representation of the objects are reactivated when they are retrieved from memory to support action goals. Because of noisier synchronization of neural assemblies,34 or because of reduced cortical connectivity,35 sensorimotor information may be poorly integrated across modalities. This leads to more difficult to access and reproduce object operational representation. In this account, individuals with ASD have difficulty with “embody” their experiments along with objects, which leads to obstacles in KMI.
However, a recent study by Conson et al indicated that individuals with ASD did not show a significant biomechanical effect in hand laterality judgement task.12 The difference between Conson et al’s study and the present findings could be accounted for the animacy of materials. Animacy is termed as the process that individuals identify object as living being with self-awareness interactive behavior.36 Obstacles to animacy perception in individuals with ASD are mainly manifested in higher thresholds than TD group. Burnett and Jellema explored animacy perception in individual with ASD by morphed sequences.37 The morphed sequences started with an unidentifiable black contour drawing, consisting of 50% animate object (A) and 50% inanimate object (B). The unidentifiable object morphed into object A or B gradually, each morphed sequences consisted with 5% change for one frame. Participants could make unlimited guesses about what the object was morphing into. The experimenter recorded the frame number at which the correct answer was given. The result showed that ASD group required significantly more frames than the TD group. This indicated the difficulty for individuals with ASD to identify materials as animate objects.
Importantly, the stimuli of Conson et al were hand-drawn images, while the stimuli of this study were real hands images. Therefore, during the observation in Conson et al, ASD participants might have recognized the hand-drawn images as artifact and only processed the stimuli shape without understanding its animacy implications. This could lead to the rotation of stimuli representation during the judgement process, which had no biomechanical effect. In the present study, the real hand images have more animacy, leading the association with stimuli and their own hands representation for the ASD participants, resulting in the existence of biomechanical effect.
Objects Can Serve as Facilitators of KMI in ASD Individuals
Shorter RT in the correct action condition means the objects can serve as facilitators in KMI. Consistent with our hypothesis, compared with incorrect action, the ASD and TD groups both had shorter RTs when an object was combined with a hand in the correct action mode. Most previous studies investigated the characteristics of action representation in individuals with ASD through simulation and perspective-taking tasks.38,39 However, the findings remain controversial. The presence of object can partly explain the distinction. Dowell et al, showed that children with ASD have worse gesture recognition on tasks not involving object (eg, waving goodbye) as compared to TD controls, but similar performance on gesture recognition involving object-use.40 Thus, our result can confirm the facilitating effect of object on forming action representation in individuals with ASD.
The present results were consisted with those of van Elk et al, in which researchers presented participants with images of a man holding an object in his hand.41 The images were classified into four categories based on whether the grip position and usage method of the object were correct. The task was to determine whether the object presented in the pictures would move toward faces generally. The results showed with facilitating effect that participants had quicker response to images in correct grip position and usage method condition. The facilitating effect is a phenomenon in which visual perceptual information automatically activates the most suitable interactive action with an object to improve the judgement response speed.42
The dual-route theory explains the facilitating effect, holding that there are two routes in the action system. In the direct route, individuals obtain action representations from the visual analysis to motor system, bypassing the long-term memory station and the short-term memory station.43 While in the indirect route, individuals obtain action representation from the visual analysis, by passing the short-term memory to the motor system. The direct route can only process incorrect action representations while correct action representations can be processed by both routes simultaneously. Due to the impairment of direct pathway, individuals with ASD have difficulty in processing incorrect action representation.15 However, the correct action representation can be processed normally through indirect pathway, and finally lead to facilitating effect.
As literate above, the ASD individuals had trouble in performing object-related action, while they had typical processing of action representation, suggesting changes in brain functional connectivity for action representation and action execution.44 KMI can evoke sensory input from peripheral receptors and brain output, accelerate brain functional reorganization by activating dormant synapses ultimately.45 The functional reorganization of the KMI network suggests a potential role of KMI in motor rehabilitation in individuals with ASD.
Greater Effect of Incorrect Action Representation on KMI in ASD
The result of the biomechanical index revealed a greater effect of incorrect action representation on KMI in individuals with ASD. The biomechanical index represents the ability to overcome biomechanical constraints in KMI, which can reflect the processing level. The action representation is related to information stored in long-term memory, requires more cognitive resources.46 In the present study, the biomechanical index was higher in the incorrect action condition than in the correct condition. It indicates more biomechanical constraint in the incorrect action condition, requiring higher processing level of KMI to finish the experimental task. That is, it was more difficult for the ASD participants to imagine the incorrect action representation rotating away from the mid-sagittal plane of the body relative to rotating toward it. Although the reaction speed became slower in the incorrect action condition, it can elicit more KMI engagement, which is benefit to individuals with ASD.
In addition, a result of MEG study was consisted with the idea of the present study.47 Researchers found a stronger activation of premotor areas for incorrect actions processing compared with correct actions. In the experimental task, participants were shown pictures of actors pressing buttons which were correct or incorrect according to the cues. Time-frequency representations of the MEG data demonstrated a stronger activity in the beta rebound in the observation of incorrect than correct actions, this would suggest involvement with higher forms of action understanding.
In sum, motor cortical areas may play the role of error monitoring in action correctness.48 The ability to classify the results of others’ actions as correct or incorrect is a crucial element of action learning, in which the action correctness need to be taken into account in the motor intervention for individuals with ASD.
Object-Centered Eye-Movement Pattern in ASD
Eye movement is spontaneous and can objectively reflect cognitive processing characteristics of individuals, which plays an important role in the study of individual differences. The object fixation index can reflect the effect of action representation on KMI in individual with and without ASD. Results of the eye-movement patterns showed that the ASD participants were object-centered, whereas the TD participants were hand-centered relatively. The result was consisted with the performance in point detection task, that ASD had a longer fixation duration for objects than body -related stimuli.49
The Enactive Mind theory holds that cognition is embedded in experiences associated with a particular form of adaptive action with environment. Under this situation, social stimuli are seen as having a higher degree of salience than inanimate stimuli. However, the relative salience of social stimuli might be diminished in autism. Thus, individuals with ASD might failing to accrue social experiences based on social cognition like typical development individuals, social behavior becomes truncated, slow, and inefficient.50 This theory explains the reduced ability to regard body as signs with high social value, which corresponds to the characteristics of avoiding social materials in individuals with ASD. In the present study, the hand was more likely to be associated with social situations than the tooth brush, the avoidance of social stimuli led to the ASD focus more on the tooth brush area, rather than the hand area.
Except for the visual preference of non-social stimuli, individuals with ASD also had aberrant attentional disengagement.51 Thus, individuals with ASD prefer to focus on object area and had fewer or slower attentional disengagement. In the present study, object area attracted attention of individuals with ASD, they would take a long time to complete the local processing with fewer attention on hand area, resulting in object-centered eye-movement pattern.
Implications for Intervention
The ultimate goal of autism research is to combine theory with practice. This study provide empirical support for improving the motor simulation disorder intervention for individuals with ASD. Initially, most of the previous interventions for ASD’s motor simulation disorder were designed based on action observation (AO). AO is a perceptually driven cognitive process that is externally guided by an external stimulus.52 To our best knowledge, AO interventions are time-consuming and boring,53 which can also reduce the initiative of individuals with ASD.
Recently, McNeill et al, formally proposed the “AO+MI” (Action Observation+ Motor Imagery) intervention, in which individuals carried out action observation and motor imagery training at the same time.54 The “AO+MI” intervention has been proved to be effective in improving motor performance in young healthy adults.55 The potential mechanism through “AO+MI” exerts its effect have been posited.54 The first mechanism supports that AO acts as guide, reducing the priority of individual’s demand for visual stimulation during MI. AO frees up attentional space allowing participants to focus on the kinesthetic aspects of action exclusively. The second mechanism suggested that MI is responsible for kinesthetic representation processing, while AO is responsible for visual representation processing. Both processes are controlled by similar neural processes; thus, AO and MI could be held simultaneously and lead to mutual promotion.
According to the results of this study, we can refine the “AO+MI” intervention from the following aspects. First, the use of teaching aids can promote the processing of action representation in individuals with ASD. However, attention should be paid to the selection of high-animacy teaching aids. Second, pay attention to the aids’ functionality. Brain imaging studies have shown that tools (eg hammers) are more closely linked to actions than graspable objects, triggering greater activation of the brain’s motor area.56 Thus, choosing more functional tools during intervention is beneficial to the improvement of individual motor simulation skills. Finally, strengthening multidimensional feedback on intervention effect with instruments. Typically, the decrease in electromyogram (EMG) activity is associated with increased motor efficiency. The EMG data in Romano-Smith et al showed that the mean EMG activity in “AO+MI” groups significantly decreased than the “AO” group and “MI” group.57 Based on the evidence cited here, the simultaneous use of kinematic and biological instruments would contribute to examine the interventions efficiency. What’s more, it can also further clarify the potential mechanism of “AO+MI” intervention from the perspective of specific processing of individuals with ASD.
Limitation
This study had several limitations that should be addressed in the future. First, the present study is the small size. Although our sample has sufficient testing power, it is still essential to replicate the result in a larger sample to ensure the credibility. In addition, as ASD is more prevalent in males, making the limitation of the females we could find. Nonetheless, it is difficult to consider the gender ratio of participants, the similar male- to -female ratios is important for better sample representativeness.
Second, for the purpose to obtain processing mechanism of object’s effect in KMI, we chose a non-conflict object as material to keep consistence of hand action. Research had declared that stroop interference may occurs between object-related structural and functional actions.58 However, the present study did not analyze whether structural and functional action representation may be competitive versus facilitative. Considering of the greater discrimination between structural and functional actions in conflict objects, future studies should consider using conflict objects (such as calculators) as materials to investigate the representation processing priority in individuals with ASD.
Third, the present study did not check the baseline eye movement characteristics in both ASD and TD participants. Baseline eye movement performance can be tested in two ways. First, before the test starts, collecting approximately 5 minutes of eye movement performance while the participants are sitting still.59 Second, collecting eye movement performance while the screen presenting empty screens or stimuli unrelated to the task between trials.60 Banire et al, examined the effects of social and nonsocial stimuli on on-task attention in children with ASD and age-matched TD children.61 The ASD group has a significantly lower performance score than the TD group at baseline with static social and nonsocial stimuli. The results showed that the on-task attention deficit in the ASD group was not due to social and non-social stimuli, but to their impaired ability to maintain attention on target stimuli. Therefore, detecting baseline eye movement characteristics can more clearly indicate whether the eye movement data reflect the differences in cognitive processing directly.
In conclusion, our study investigated whether object-related action representation can affect the KMI performance, and used eye-movement pattern to explore the difference of cognitive processing between individuals with and without ASD. This finding suggests that individuals with ASD has some trouble in KMI performance with more error and spend more time. Nonetheless, the activation of action representation is typical, the incorrect action can elicit more engagement of KMI in individuals with ASD. What’s more, the eye-movement is different between the two groups, as the ASD groups are object-centered, whereas the TD groups are hand-centered. We believe that object should be used as aids in the motor simulation disorder intervention in individuals with ASD, which would help to improve the intervention efficiency and effects.
Ethical Approval
The studies involving human participants were reviewed and approved by the Human Ethics Committees of Liaoning Normal University (Ethical Number: LL2023058). The university health participants signed the consent form independently; The health participants in high school and all of the ASD participants were signed by their guardians. In addition to the guardians, the ASD adults also signed the consent form by themselves. All methods were carried out in accordance with relevant guidelines and regulations/Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
The health university participants and the ASD adults have consented to the submission of the case report to the journal. In addition, the guardians of the health participants in high school and all of the ASD participants have also consented to the submission of the case report to the journal.
Funding
This study was funded by The State Social Science Fund funds key projects in the later stage (20FSHA001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Association AP. Diagnostic and Statistical Manual of Mental Disorders.
2. Sommerville JA, Decety J. Weaving the fabric of social interaction: articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychon B Rev. 2006;13(2):179–200. doi:10.3758/BF03193831
3. Gallese V, Sinigaglia C. How the body in action shapes the self. J Consciousness Stud. 2011;18(7–8):117–143.
4. Decety J, Grèzes J. The power of simulation: imagining one’s own and other’s behavior. Brain Res. 2006;1079(1):4–14. doi:10.1016/j.brainres.2005.12.115
5. Grangeon M, Guillot A, Collet C. Postural control during visual and kinesthetic motor imagery. Appl Psychophys Biof. 2011;36(1):47–56. doi:10.1007/s10484-011-9145-2
6. Smits-Engelsman BC, Wilson PH. Age-related changes in motor imagery from early childhood to adulthood: probing the internal representation of speed-accuracy trade-offs. Hum Movement Sci. 2013;32(5):1151–1162. doi:10.1016/j.humov.2012.06.006
7. Guillot A, Collet C. Duration of mentally simulated movement: a review. J Motor Behav. 2005;37(1):10–20. doi:10.3200/JMBR.37.1.10-20
8. Ogawa T, Shimobayashi H, Hirayama J-I, Kawanabe M. Asymmetric directed functional connectivity within the frontoparietal motor network during motor imagery and execution. NeuroImage. 2022;247:118794. doi:10.1016/j.neuroimage.2021.118794
9. Macuga KL, Frey SH. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 2012;59(3):2798–2807. doi:10.1016/j.neuroimage.2011.09.083
10. Parsons LM. Imagined spatial transformation of one’s body. J Exp Psychol Gen. 1987;116(2):172. doi:10.1037/0096-3445.116.2.172
11. Sekiyama K, Kinoshita T, Soshi T. Strong biomechanical constraints on young children’s mental imagery of hands. Roy Soc Open Sci. 2014;1(4):140118. doi:10.1098/rsos.140118
12. Conson M, Mazzarella E, Frolli A, et al. Motor imagery in Asperger syndrome: testing action simulation by the hand laterality task. PLoS One. 2013;8(7):e70734. doi:10.1371/journal.pone.0070734
13. Gardner MR, Brazier M, Edmonds CJ, Gronholm PC. Strategy modulates spatial perspective-taking: evidence for dissociable disembodied and embodied routes. Front Hum Neurosci. 2013;7:457. doi:10.3389/fnhum.2013.00457
14. Chen Y-T, Tsou K-S, Chen H-L, Wong -C-C, Fan Y-T, C-t W. Functional but inefficient kinesthetic motor imagery in adolescents with autism spectrum disorder. J Autism Dev Disord. 2018;48(7):784–795. doi:10.1007/s10803-017-3367-y
15. Wild KS, Poliakoff E, Jerrison A, Gowen E. Goal-Directed and Goal-Less Imitation in Autism Spectrum Disorder. J Autism Dev Disord. 2011;42(8):1739–1749. doi:10.1007/s10803-011-1417-4
16. Freud E, Plaut DC, Behrmann M. ‘What’is happening in the dorsal visual pathway. Trends Cognit Sci. 2016;20(10):773–784. doi:10.1016/j.tics.2016.08.003
17. Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cognitive Neurosc. 2003;15(1):30–46. doi:10.1162/089892903321107800
18. Van Elk M, Van Schie H, Bekkering H. Action semantics: a unifying conceptual framework for the selective use of multimodal and modality-specific object knowledge. Phys Life Rev. 2014;11(2):220–250. doi:10.1016/j.plrev.2013.11.005
19. D’Entremont B, Yazbek A. Imitation of Intentional and Accidental Actions by Children with Autism. J Autism Dev Disord. 2007;37(9):1665–1678. doi:10.1007/s10803-006-0291-y
20. Caligiore D, Borghi AM, Parisi D, Baldassarre G. TRoPICALS: a computational embodied neuroscience model of compatibility effects. Psychol Rev. 2010;117(4):1188–1228. doi:10.1037/a0020887
21. Federico G, Brandimonte MA. Tool and object affordances: an ecological eye-tracking study. Brain Cogn. 2019;135:103582. doi:10.1016/j.bandc.2019.103582
22. Jaskula B, Pancerz K, Szkola J. Toward synchronization of EEG and eye-tracking data using an expert system extended.
23. Wright DJ, Wood G, Franklin ZC, Marshall B, Riach M, Holmes PS. Directing visual attention during action observation modulates corticospinal excitability. PLoS One. 2018;13(1):e0190165. doi:10.1371/journal.pone.0190165
24. Duchowski AT. Eye Tracking Methodology: Theory and Practice. London: Springer; 2003.
25. Gog TV, Kester L, Nievelstein F, Giesbers B, Paas F. Uncovering cognitive processes: different techniques that can contribute to cognitive load research and instruction. Comput Hum Behav. 2009;25(2):325–331. doi:10.1016/j.chb.2008.12.021
26. Poiroux E, Cavaro-Ménard C, Leruez S, Lemée JM, Richard I, Dinomais M. What do eye gaze metrics tell us about motor imagery? PLoS One. 2015;10(11):e0143831. doi:10.1371/journal.pone.0143831
27. Bruton AM, Holmes PS, Eaves DL, Franklin ZC, Wright DJ. Neurophysiological markers discriminate different forms of motor imagery during action observation. Cortex. 2020;124:119–136. doi:10.1016/j.cortex.2019.10.016
28. Baroncohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females. J Autism Dev Disord. 2001;31(1):5–17. doi:10.1023/A:1005653411471
29. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997.
30. Finke EH, Wilkinson KM, Hickerson BD. Social referencing gaze behavior during a videogame task: eye tracking evidence from children with and without ASD. J Autism Dev Disord. 2017;47(2):415–423. doi:10.1007/s10803-016-2968-1
31. Unema PJA, Pannasch S, Joos M, Velichkovsky BM. Time course of information processing during scene perception: the relationship between saccade amplitude and fixation duration. Vis Cogn. 2005;12(3):473–494. doi:10.1080/13506280444000409
32. Pearson A, Marsh L, Hamilton A, Ropar D. Spatial transformations of bodies and objects in adults with autism spectrum disorder. J Autism Dev Disord. 2014;44(9):2277–2289. doi:10.1007/s10803-014-2098-6
33. Markman AB, Brendl CM. Constraining theories of embodied cognition. Psychol Sci. 2005;16(1):6–10. doi:10.1111/j.0956-7976.2005.00772.x
34. Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front Psychol. 2011;2:51. doi:10.3389/fpsyg.2011.00051
35. Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav R. 2012;36(4):1292–1313. doi:10.1016/j.neubiorev.2012.02.007
36. Vanmarcke S, Van de Cruys S, Moors P, Wagemans J. Intact animacy perception during chase detection in ASD. Sci Rep-UK. 2017;7(1):11851. doi:10.1038/s41598-017-12204-x
37. Burnett HG, Jellema T. (Re-) conceptualisation in Asperger’s syndrome and typical individuals with varying degrees of autistic-like traits. J Autism Dev Disord. 2013;43(1):211–223. doi:10.1007/s10803-012-1567-z
38. Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. J Cognitive Neurosci. 2008;20(1):1–19. doi:10.1162/jocn.2008.20013
39. Mazzarella E, Ramsey R, Conson M, Hamilton A. Brain systems for visual perspective taking and action perception. Soc Neurosci. 2013;8(3):248–267. doi:10.1080/17470919.2012.761160
40. Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi:10.1037/a0015640
41. van Elk M, van Schie HT, Bekkering H. Action semantic knowledge about objects is supported by functional motor activation. J Exp Psychol Human. 2009;35(4):1118. doi:10.1037/a0015024
42. Tipper SP, Paul MA, Hayes AE. Vision-for-action: the effects of object property discrimination and action state on affordance compatibility effects. Psychon B Rev. 2006;13(3):493–498. doi:10.3758/BF03193875
43. Rumiati RI, Bekkering H. To imitate or not to imitate? How the brain can do it, that is the question! Brain Cogn. 2003;53(3):479–482. doi:10.1016/S0278-2626(03)00208-2
44. Xie R, Sun X, Yang L, Guo Y. Characteristic executive dysfunction for high‐functioning autism sustained to adulthood. Autism Res. 2020;13(12):2102–2121. doi:10.1002/aur.2304
45. Chen X, Wan L, Qin W, et al. Functional preservation and reorganization of brain during motor imagery in patients with incomplete spinal cord injury: a pilot fMRI study. Front Hum Neurosci. 2016;10(46):1–10. doi:10.3389/fnhum.2016.00046
46. Randerath J, Martin KR, Frey SH. Are tool properties always processed automatically? The role of tool use context and task complexity. Cortex. 2013;49(6):1679–1693. doi:10.1016/j.cortex.2012.08.016
47. Koelewijn T, van Schie HT, Bekkering H, Oostenveld R, Jensen O. Motor-cortical beta oscillations are modulated by correctness of observed action. Neuroimage. 2008;40(2):767–775. doi:10.1016/j.neuroimage.2007.12.018
48. Ridderinkhof KR, W PM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi:10.1016/j.bandc.2004.09.016
49. DiCriscio AS, Miller SJ, Hanna EK, et al. Brief report: cognitive control of social and nonsocial visual attention in autism. J Autism Dev Disord. 2016;46(8):2797–2805. doi:10.1007/s10803-016-2804-7
50. Klin A, Jones W, Schultz R, Volkmar F, Frith U, Hill EL. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc London Ser B. 2003;358(1430):345–360. doi:10.1098/rstb.2002.1202
51. Sabatos-Devito M, Schipul SE, Bulluck JC, Belger A, Baranek GT. Eye tracking reveals impaired attentional disengagement associated with sensory response patterns in children with autism. J Autism Dev Disord. 2016;46(4):1319–1333. doi:10.1007/s10803-015-2681-5
52. Kim T, Frank C, Schack T. A systematic investigation of the effect of action observation training and motor imagery training on the development of mental representation structure and skill performance. Front Hum Neurosci. 2017;11:499. doi:10.3389/fnhum.2017.00499
53. Cumming J, Williams SE. Introducing the revised applied model of deliberate imagery use for sport, dance, exercise, and rehabilitation. Move Sport Sci. 2013;82:69–81. doi:10.1051/sm/2013098
54. McNeill E, Toth AJ, Harrison AJ, Campbell MJ. Cognitive to physical performance: a conceptual model for the role of motor simulation in performance. Int Rev Sport Exer P. 2020;13(1):205–230. doi:10.1080/1750984X.2019.1689573
55. Romano-Smith S, Wood G, Wright D, Wakefield C. Simultaneous and alternate action observation and motor imagery combinations improve aiming performance. Psychol Sport Exerc. 2018;38:100–106. doi:10.1016/j.psychsport.2018.06.003
56. Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58(1):25–45. doi:10.1146/annurev.psych.57.102904.190143
57. Romano Smith S, Wood G, Coyles G, Roberts JW, Wakefield CJ. The effect of action observation and motor imagery combinations on upper limb kinematics and EMG during dart‐throwing. Scand J Med Sci Spor. 2019;29(12):1917–1929. doi:10.1111/sms.13534
58. Bub DN, Masson ME, Cree GS. Evocation of functional and volumetric gestural knowledge by objects and words. Cognition. 2008;106(1):27–58. doi:10.1016/j.cognition.2006.12.010
59. Fati W, Song L, Shurui G, Luyue L, Weiyi R. Online learning concentration level recognition combining facial cues and eye movement features. China Educ Technol. 2022;11:37–44.
60. Grossmann T, Missana M, Krol KM, Dehaene-Lambertz G. The neurodevelopmental precursors of altruistic behavior in infancy. PLoS Biol. 2018;16(9):e2005281. doi:10.1371/journal.pbio.2005281
61. Banire B, Al-Thani D, Qaraqe M, Khowaja K, Mansoor B. The effects of visual stimuli on attention in children with autism spectrum disorder: an eye-tracking study. IEEE Access. 2020;8:225663–225674. doi:10.1109/ACCESS.2020.3045042
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.