Back to Journals » Biologics: Targets and Therapy » Volume 18
Effect of N-Acetylcysteine on Cisplatin Toxicity: A Review of the Literature
Authors Zavala-Valencia AC, Velasco-Hidalgo L, Martínez-Avalos A, Castillejos-López M , Torres-Espíndola LM
Received 11 October 2023
Accepted for publication 8 December 2023
Published 16 January 2024 Volume 2024:18 Pages 7—19
DOI https://doi.org/10.2147/BTT.S438150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Angeles Citlali Zavala-Valencia,1,2 Liliana Velasco-Hidalgo,3 Armando Martínez-Avalos,3 Manuel Castillejos-López,4 Luz-María Torres-Espíndola1
1Laboratory of Pharmacology, National Institute of Pediatrics, Mexico City, Mexico; 2Iztacala Faculty of Higher Studies, Tlalnepantla, México; 3Oncology Service, National Institute of Pediatrics, Mexico City, México; 4Hospital Epidemiology and Infectology Unit, National Institute of Respiratory Diseases Ismael Cosío Villegas, Mexico City, Mexico
Correspondence: Luz-María Torres-Espíndola; Manuel Castillejos-López, Email [email protected]; [email protected]
Abstract: N-acetylcysteine (NAC) is a membrane-permeable cysteine precursor capable of enhancing the intracellular cysteine pool, enhancing cellular glutathione (GSH) synthesis, and thus potentiating the endogenous antioxidant mechanism. Late administration of NAC after cisplatin has been shown in different in vivo studies to reduce the side effects caused by various toxicities at different levels without affecting the antitumor efficacy of platinum, improving total and enzymatic antioxidant capacity and decreasing oxidative stress markers. These characteristics provide NAC with a rationale as a potentially effective chemo protectant in cisplatin-based therapeutic cycles. NAC represents a potential candidate as a chemoprotective agent to decrease toxicities secondary to cisplatin treatment. It suggests that it could be used in clinical trials, whereby the effective dose, timing, and route should be adjusted to optimize chemoprotection. This review provides an overview of the effect of NAC on cisplatin toxicity, a drug widely used in the clinic in adults and children.
Keywords: cisplatin, toxicity, chemo protectant, oxidative stress, antioxidant
Introduction
Childhood cancer is a major contributor to the mortality rate in the world.1 While cancer treatments have improved survival rates, the toxic response to treatment, and the formation of free radicals have caused more deaths than the disease.2 Cancer treatment is currently surgery, chemotherapy, and radiotherapy; chemotherapy is multi-pharmacological; numerous medications can impact the antioxidant status during therapy.3 Cisplatin is a widely used chemotherapy drug due to its effectiveness and broad range of applications in the clinic. The main mechanism behind the antineoplastic activity of cisplatin is its ability to crosslink with DNA, which inhibits transcription and replication. However, the clinical use of cisplatin is limited due to its severe toxicity (nephrotoxicity, ototoxicity, neurotoxicity) and dose-dependent side effects.4 Antioxidants, such as N-acetyl cysteine, are said to prevent this toxicity. N-acetylcysteine (NAC) is a precursor of L-cysteine acetylated and reduced glutathione. NAC is an over-The-counter, safe, and well-tolerated dietary supplement.5–7 This review summarized evidence for NAC as a protective agent against cisplatin toxicity.
It’s important to consider the role of antioxidants in the impact of excessive oxygen consumption on the body. Reactive oxygen species (ROS) can cause damage to proteins, cell membranes, DNA, and RNA, potentially leading to the development and advancement of cancer.8–12 Antioxidants are crucial in neutralizing free radicals and preventing oxidative damage. They work by donating electrons to free radicals, interrupting chain radical reactions, and deactivating metals. The body’s natural antioxidant system includes enzymatic antioxidants like superoxide dismutase SOD, catalase (CAT), glutathione peroxidase (GPx), and Thioredoxin (Trx), as well as hydrophilic and lipophilic antioxidants. By decreasing cellular damage parameters such as oxidative stress and DNA mutations, antioxidants help protect against the harmful effects of ROS13,14
Oxidative stress arises from the imbalance of prooxidants and antioxidants, leading to biomolecule changes and disease development. Cancer cells escalate ROS production due to mitochondrial dysfunction, metabolic alterations, and genetic mutations, activating protumorigenic signaling pathways. While moderate ROS levels promote cell functions, high levels induce cell death. Cancer cells enhance antioxidant capacity but are insufficient against high ROS levels and supports protumor signaling.15–19
Cisplatin
Cisplatin, chemically referred to as cis-diamminedichloroplatinum (CDDP), was first synthesized by M. Peyrone in 1844. The scientific community largely ignored this compound until 1965, when Rosenberg found that the electrolyte product of platinum electrodes could impede the cell division of Escherichia coli. This was later identified as CDDP.4,20,21
It was the first platinum compound approved by the FDA for the treatment of cancer in 1978, under the name cisplatin; since then, it has been used as a chemotherapeutic agent in different types of cancers, including bladder, breast, cervix esophagus, head and neck, ovarian, prostate, small cell and non-small cell lung, stomach, testicular cancers, Hodgkin’s and non-Hodgkin’s lymphomas, melanoma, mesothelioma, multiple myeloma, neuroblastoma, sarcomas, medulloblastoma and osteosarcomas.20–22 Cisplatin is a compound consisting of a central platinum (Pt) atom coordinated with two ammonia (NH3) ligands and two chloride (Cl) ion ligands, forming a planar quadrilateral.22
The absorption of cisplatin is facilitated through the copper transporter 1 (CTR1) and organic cation transporters (OCTs).20,21 Cisplatin is resistant to hydrolysis outside cells because of the high concentration of chloride ions in the blood (around 100 mM). After entering cells, cisplatin undergoes a slow hydrolysis process, forming cationic mono aqua and di aqua complexes. This occurs due to the relatively low concentration of chloride ions, which ranges from 4 to 22 mM. The complexes [Pt(NH3)2Cl(OH2)]+ and [Pt(NH3)2(OH2)2]2+ are derived from cisplatin and are known for their high reactivity towards cellular targets. These complexes, consisting of mono and di-aqua molecules, are prone to hydrolysis and can react rapidly.4,21,22 (DNA is the main biological target).4,20 This product, which is hydrolyzed, can react with proteins’ sulfhydryl groups and nucleic acids’ purine bases.20,22,23 Cisplatin attaches to the reactive N7 center on purine residues, creating crosslinking complexes. These complexes comprise 90% 1.2 intracatenary d(GpG) adducts and 10% 1.3 intracatenary d(ApG) adducts. These mechanisms are responsible for the cytotoxicity of cisplatin, along with monofunctional adducts and interstrand crosslinks.4,20
The effects of cisplatin-induced adducts and crosslinks include DNA unwinding, bending, replication, and transcription inhibition, which can lead to DNA strand breaks, impaired cell division, and initiation of pro-apoptotic pathways.22,23
Furthermore, CP can raise ROS levels, leading to mitochondrial dysfunction and promoting lipid peroxidation (LPO). CP can also activate the intrinsic apoptosis pathway by triggering mitochondrial permeabilization, releasing cytochrome C (cyt C), and stimulating caspase expression. Additionally, it can activate the extrinsic apoptosis pathway through the induction of Fas receptor signaling. It has been reported that CP can sensitize cancer cells to cell death by altering intracellular calcium levels.21,24
Cisplatin Toxicity
Cisplatin has certain limitations in dosage and clinical application due to its tendency to cause toxic side effects. The most common are nephrotoxicity, ototoxicity, neurotoxicity, gastrointestinal toxicity, and hematological toxicity. While less frequent, other side effects include cardiotoxicity, hepatotoxicity, retinal toxicity, inappropriate antidiuretic hormone syndrome, hypersensitivity reactions, and reproductive toxicity, which may still occur in some cases.4,20,21,24
Nephrotoxicity
Approximately 25–35% of adults experience nephrotoxicity.25 and 70% of pediatric patients during therapeutic cycles.26 Various factors, including elevated levels of ROS, mitochondrial dysfunction, inflammation, DNA damage, and induction of apoptosis, can cause renal toxicity.27 Cisplatin mainly accumulates in the proximal and distal tubules of the kidneys, causing epithelial cell death and impairing tubular reabsorption. This activates the tubuloglomerular feedback mechanism and, together with tubular obstruction, reduces glomerular filtration.28
One proposed mechanism for activating inflammatory responses involves inflammasomes containing the pyrin domain 3 (NLRP3) of the Toll-like receptor 4 (TLR4) and NLR family, which are pattern recognition receptors. The activation of nuclear factor-kB (NF-kB) triggers the expression of NLPR3 and pro-interleukin (IL)-1beta (pro-IL-1β). NF-kB can be activated by different stimuli like lipopolysaccharide binding to TLR4 and ROS. Caspase-1 activation mediated by NLRP3 results in the cleavage of pro-IL-1β and pro-IL-18, which are now known to play a role in Cisplatin-induced nephrotoxicity.29
Ototoxicity
Cisplatin therapy often leads to permanent sensorineural hearing loss, which is a common complication,30 between 40% and 80% of adults and at least 50% of children are left with permanent hearing loss.31,32 Cisplatin-induced hearing loss is essential because it affects the quality of life and later affects childhood access to speech and spoken language development. Language and communication are fundamental to psychosocial development, cognition, learning, and literacy.33
Cisplatin ototoxicity is caused by multiple mechanisms,32,34 such as the production of reactive oxygen species, depletion of the antioxidant glutathione and its regenerative enzymes, increased lipid peroxidation rate, oxidative modifications of proteins, nucleic acid damage through activation of the caspases system, S-nitrosylation of cochlear proteins, and the resulting apoptosis of inner ear cells.32,35
Long-term stepwise retention has been demonstrated with each cycle of platinum treatment in the cochlea after chemotherapy,31,36 damaging hair cells essential for maintaining hearing and spiral neurons, supporting cells, and vascular veins.37 Thus, three cochlear regions are implicated in CP toxicity: the organ of Corti, spiral ganglion neurons, and the vascular stria.31
Neurotoxicity
Platinum-based chemotherapy often causes neurotoxicity, which reduces the effective dose and may lead to treatment discontinuation.38 Cisplatin-induced peripheral neuropathy (Cis-PN) is a type of nerve damage that affects the sensory nerves in the hands and feet. It can cause a symmetrical distribution of symptoms known as “glove and stocking distribution”, which may also extend to the elbows and knees. It is associated with cumulative doses above 300–350 mg/m2.39 The drug is linked to adverse effects in 30–50% of patients who complete the course, causing debilitating symptoms in 10% of them.40–42
Cis-PN causes numbness, tingling (paresthesia), burning, pain, reduced vibrational sensitivity in the fingers and toes, and decreased ankle reflexes.38
In the peripheral nervous system, the sensory neurons in the dorsal root ganglion (DRG) are the primary site where cisplatin accumulates. This vulnerability can be attributed to several factors, including the lack of a blood-brain barrier (BBB), a lower amount of detoxifying glutathione, and specific membrane transporters called organic cation transporters (OCTs).43,44 Neurotoxicity caused by cisplatin has been linked to damage in mitochondrial and nuclear DNA.45
Glutathione Synthesis and Functions
Glutathione (GSH) is a detoxifying agent in cells and one of the central mechanisms by which ROS are removed.5 The tripeptide, γ-L-glutamyl-L-cysteinyl glycine, is found in all mammalian tissues at 1 to 10 mM levels. It serves several vital functions, including antioxidant defense, xenobiotics detoxification, cell cycle progression, apoptosis regulation, cysteine storage, redox potential maintenance, immune function modulation, and fibrogenesis.46 GSH, found inside cells, is produced from three amino acids: cysteine, glutamine, and glycine. The formation of γ-glutamyl cysteine and glycine, crucial components of GSH, is made possible by the enzyme glutamate-cysteine ligase (GCL) and the catalyst glutathione synthetase (GSS). GSS facilitates the reaction between γ-glutamyl cysteine and glycine, which ultimately results in the formation of GSH.47
Cysteine has the lowest concentration of the mentioned precursors, which limits the rate of GSH synthesis during oxidative stress.5
N-Acetyl Cysteine
NAC is a man-made version of the natural amino acid L-cysteine.5 It is a thiol, membrane-permeable cysteine precursor capable of enhancing the intracellular cysteine pool, releasing thiols from proteins through disulfide cleavage, thereby increasing GSH levels and enhancing GSH-dependent detoxification activity of H2 O2 by its antioxidant enzymes (its glutathione peroxidase it and thioredoxin).47
NAC is a commonly prescribed medication for treating acetaminophen (paracetamol) overdose. It replenishes the depleted GSH stores in hepatocytes during detoxification. Additionally, NAC has been found to have mucolytic properties, which means it can break down disulfide bonds in mucus glycoproteins, reducing viscosity.5,48–50
The chemical structure consists of a sulfhydryl functional group (-SH) plus an acetyl group (-COCH 3) attached to the amino group (NH 2);51–53 thiols (RSH) can be oxidized by radicals and act as electron-pair donors.53 NAC has direct antioxidant properties because its free thiol group can interact with reactive oxygen and nitrogen species like hydroxyl radical (-OH), nitrogen dioxide (-NO2), carbon trioxide ion (CO3--), and thiyl radical (RS-), as well as nitroxyl (HNO) which is the reduced and protonated form of nitric oxide (-NO).54
When reacting with species such as superoxide radical anion (O 2--), hydrogen peroxide (H 2 O 2), and peroxynitrite (ONOO-), relatively slower reactions take place.51,53 However, O 2 – generated within cells is rapidly converted to H 2 O 2 by local SOD, the substance can transform into water through a reaction catalyzed by either CAT or GPX. In the case of GPX, the process involves the oxidation of GSH to glutathione disulfide, which is then converted back to GSH by glutathione reductase, thioredoxin, and glutaredoxin.5
Likewise, NAC is an effective reducing agent of protein disulfides through the classical thiol-disulfide exchange mechanism. An S N 2 reaction mechanism involves the attacking NAC thiolate binding to the central sulfur of the disulfide in a single step, releasing the exiting thiol (R″SH)., a capacity to restore systemic reserves of small thiols and reduce SH groups of proteins, regulating redox conditions as an antioxidant mechanism.50
The SH group in NAC is a mild Lewis base, which makes it highly reactive with mild Lewis acids such as various toxic metals. The high effectiveness of NAC against toxic metals with mild Lewis acid properties is related to its stable binding to the SH group, producing inactive complexes. The formation of these complexes inside cells prevents the toxic binding of metals to the Cys-SH protein, while their extracellular production blocks the cellular uptake of metals.55,56
Chemoprotection by N-acetylcysteine frequently results from the inactivation of primary toxicants or reactive electrophiles that arise as metabolites or lipid peroxidation products. The most toxic lipid oxidation products are α, β-unsaturated aldehydes such as acrolein, crotonaldehyde, and 4-hydroxy-2-nonenal (4HNE). The toxicity of these carbonyl compounds is caused by their very high reactivity with protein-SH groups through Michael’s addition at the β-carbon of the double bond (R-CH=CH-CHO). The SH group of NAC rapidly forms Michael adducts with α, β -unsaturated aldehydes, which prevents their conjugation to proteins and the resulting toxic effects.55,57
In addition to its antioxidant effect, NAC has an anti-inflammatory effect.58,59 N-acetylcysteine has been shown to decrease IL-6 levels in hemodialysis patients. TNF-α and IL-1β have also decreased in mouse models treated with n-acetylcysteine. N-acetylcysteine inhibits the activation of redox-sensitive nuclear factor kappa B, which stimulates the expression of proinflammatory genes at times of oxidative stress, releasing many inflammatory cytokines.60
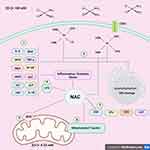
In the case of cisplatin, as mentioned earlier, a lower chlorine concentration is found at the intracellular level; the chloride ions in cisplatin are replaced by water molecules, forming the cationic complexes monoaqua and diaqua,20 species with a much higher avidity for nucleophilic sites on macromolecules, including DNA and proteins. The reactivity of aqueous species is modulated by the level of available molecules with free thiol groups, as in the case of NAC, thiol-containing molecules have high avidity for aqueous cisplatin species, preventing their binding to other cellular targets, mechanism underlying the highly effective cytoprotective properties of NAC, over those of other antioxidants28 (Figure 1).
The antioxidant properties of NAC may be attributed to the following mechanisms:
- It has a direct antioxidant effect on specific oxidative species.50
- The ability to act as a precursor of cysteine enhances cellular GSH synthesis and potentiates the endogenous antioxidant mechanism as an indirect antioxidant effect of NAC.54
- The compound can break disulfide bonds and restore thiol stores, which help regulate the redox state.50
- The ability of the free thiol of NAC to bind to mono aqua and di aqua cation complexes, preventing their binding to other cellular targets.28
- The conversion of thiols derived from NAC into hyperactivated thiols (hydropersulfides) serves as direct oxidant scavengers and protective coatings for critical protein thiols in metabolism.5 (Table S1)
Mitochondrial Transfer
Mitochondrial transfer involves introducing mitochondrial genes or the mitochondria into a host cell. This can result in major changes to the host’s bioenergetic state and impact cell differentiation, inflammatory processes, cell survival, and drug resistance. Communication between the donor and recipient cells is essential for mitochondrial transfer, and it can be regulated by various structures, including extracellular vesicles (EV), tunneling nanotubes (TNT), and communicating junctions (GJ), among others.61
Mitochondria in neighboring cells can transfer to tumor cells, improving mitochondrial function and promoting tumor growth and metastasis.47 In addition, considering several studies,61–65 correlate mitochondrial transfer with chemoresistance and with the recovery of cancer cells after treatment.
N-acetylcysteine has been found to inhibit mitochondrial transfer from stromal cells to cancer cells.47,64,66,67 however, the precise mechanism by which NAC carries out this action has not yet been determined. As a result, the attenuation of mitochondrial transfer has only been attributed to scavenging ROS.47
NAC in Cancer
There is controversy surrounding the involvement of antioxidants and ROS in cancer.68 Epidemiological studies on synthetic antioxidant supplementation are inconclusive and contradictory due to the antioxidant versus prooxidative properties of antioxidants and the involvement of antioxidants in intracellular signaling and redox regulation, which modulate proliferation, apoptosis, and gene expression.69
NAC is a widely used antioxidant with therapeutic potential. NAC has been reported to have therapeutic benefits in various types of cancer: Kwon Y. J. reports that triple-negative breast cancer (TNBC) generates high levels of ROS and depends on ROS signaling to survive and progress malignantly by affecting the interaction between cancer cells and the tissue microenvironment (TME). However, NAC treatment can effectively decrease ROS production and ROS-mediated signaling, hindering cell survival, metastasis, and drug resistance in TNBC cells.48
Monti et al suggested that NAC alone may inhibit breast cancer cell growth.70
Amini et al propose enhancing the chemotherapeutic index for peritoneal surface malignancies of gastrointestinal origin with bromelain, N-acetylcysteine, and chemotherapeutic agents.71
According to Fen et al, low concentrations of NAC suppress pancreatic stellate cell (PSCs) activation and reduce oxidative stress, resulting in a quiescent state, inhibiting PSC viability, invasiveness, and migration, attenuating cancer-stromal interactions.72
Jurkowska et al analyzed the effect of NAC on the proliferation of human neuroblastoma SH-SY5Y cells; the addition of NAC to the medium at non-cytotoxic concentrations resulted in the inhibition of SH-SY5Y cell proliferation after 48 h of culture (P <0.05), increased in 3-mercapto pyruvate sulfurtransferase (MPST) activity, and, intracellular level of sulfur sulfane in these cells.73
In an endogenous mouse model of malignant melanoma, human malignant melanoma cells’ migration and invasive properties increased with the combination of NAC and the soluble vitamin E analog Trolox.74 Similarly, N-acetylcysteine and vitamin E accelerated lung cancer progression in mice by reducing survival and increasing tumor progression by disrupting the ROS-p53 axis.75
NAC in Protection Against Adverse Events During Cisplatin Chemotherapy in Experimental Animal Models and Humans
Nephrotoxicity
As shown in Table 1, NAC has been shown to have a nephroprotective effect in different in vivo studies when used as a concomitant treatment after receiving cisplatin therapy, acting as a chemoprotective, without affecting the antitumor efficacy of platinum, decreasing significantly (p= 0.001) the levels of BUN, and creatinine, achieving significant improvement (p= 0.001) in results of histological studies of hemorrhage, necrosis and damage to tubule cells, significantly improving (p ≤ 0.05) stress markers nephritic oxidative and inflammation, reducing LPO levels, and significantly (p < 0.05) increasing total antioxidant capacity (TAC) and enzymatic antioxidant activity28,54,76–79
 |
Table 1 Evidence of NAC in Protection Against Adverse Events During Cisplatin Chemotherapy in Experimental Animal Models |
Ototoxicity
Table 2, shows in vivo studies and clinical trials set out in NAC show a potential otoprotective effect against ototoxicity caused by platinum, administered four h after CP without altering otoacoustic emissions (OAE) or auditory brainstem responses (ABR) and avoiding negative histopathological findings (p <0.05). Since no significant changes in auditory thresholds were recorded in the NAC-treated groups (T9,10) a higher reduced/oxidized glutathione ratio GSH/GSSG was observed compared to the other groups p ≤ 0.001.80–82,90–92
 |
Table 2 Evidence of NAC in Protection Against Adverse Events During Cisplatin Chemotherapy in Humans |
In animal models, NAC is usually administered through intratympanic administration. This route is ideal as it does not interfere with the antineoplastic ability of cisplatin, and it effectively delivers the drug to the inner ear in concentrated amounts.81,91,93
Neurotoxicity
For neurotoxicity assessment, the studies in Table 1 evaluated the effect of NAC concomitant to PC therapy, showing improved oxidant/antioxidant status; fewer structural alterations were found in myelin and axoplasm. GSH, SOD levels, and GPx, GST, SOD, and CAT activities were increased. Reversing proinflammatory cytokines, prooxidant, and proapoptotic effects of cisplatin. Decreasing levels of BAX, Inducible nitric oxide synthase (iNOS), tumor necrosis factor-alpha (TNF-a), malondialdehyde (MDA), and nuclear factor κB (NF-kB) (p<0.05).83–85
Other Toxicities
In the evaluation of other toxicities, such as cardiotoxicity, as shown in Table 1, late or concomitant administration of NAC to cisplatin therapy has shown a reduction in oxidative stress and cardio dynamic stress parameters, decreasing levels of MB isoenzyme of creatine kinase (CK-MB), lactate dehydrogenase (LDH), Cardiac Myosin Light Chains 1(CMLC-1), cardiac troponin T (cTnl), total oxidizing capacity (TOC), lipid hydroperoxide (ROOH), increasing levels of TAC, and showing a milder degree of interstitial oedema, vacuolization and improvement of hemorrhage.86,87
In the case of hepatotoxicity, Table 1. We found decreased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzyme activities. NAC promoted B-cell lymphoma 2 (Bcl-2) signaling and decreased p53 signaling. NAC+CP treatment attenuated the effects of CP on MDA in liver tissue, CAT, SOD, and GSH.88,89
Administering NAC after cisplatin treatment can provide delayed chemoprotection. This is because it helps maintain cisplatin’s early DNA alkylation mechanism in rapidly dividing cancer cells while also providing antioxidant protection against ROS, likely the main cause of toxicity in differentiated cells.76
Conclusion
The evidence in the literature displayed in the current review indicates that the NAC administration can be a promising approach to address some current issues related to cisplatin and other conditions. NAC can improve the intracellular cysteine pool, enhance GSH synthesis, and boost endogenous antioxidant capacity through different enzymes. These findings indicate that NAC administration is a potential candidate for chemoprotection due to its ability to react with ROS, which can decrease side effects and improve both patient treatment and long-term outcomes.
Further human clinical studies are urgently needed as most studies have only been reported in animal models. Hence, it is necessary to define the effective dose and the optimal route of administration for better chemoprotection. This could aid in developing personalized therapies, reducing toxicity and complications associated with treatments. As a result, it would lower the costs of medical care and improve the quality of life of patients receiving chemotherapy.
Acknowledgments
Citlaltepetl Salinas Lara, Ph.D., director of the MEDICI program of the Iztacala Faculty of Higher Studies, is acknowledged for helping medical surgeon graduates reach the different health institutes.
Funding
Program E022 of the National Institute of Pediatrics has supported this paper.
Disclosure
The authors declare no conflict of interest.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int, J, Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
2. Al-Tonbary Y, Al-Haggar M, El-Ashry R, El-Dakroory S, Azzam H, Fouda A. Vitamin e and N-acetylcysteine as antioxidant adjuvant therapy in children with acute lymphoblastic leukemia. Adv Hematol. 2009;2009:689639. doi:10.1155/2009/689639
3. Singh K, Bhori M, Kasu YA, Bhat G, Marar T. Antioxidants as precision weapons in war against cancer chemotherapy-induced toxicity - Exploring the armoury of obscurity. Saudi Pharm J. 2018;26(2):177–190. doi:10.1016/j.jsps.2017.12.013
4. Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32(8):1469–1486. doi:10.1021/acs.chemrestox.9b00204
5. Raghu G, Berk M, Campochiaro PA, et al. The multifaceted therapeutic role of N-Acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr Neuropharmacol. 2021;19(8):1202–1224. doi:10.2174/1570159X19666201230144109
6. Rogliani P, Matera MG, Page C, Puxeddu E, Cazzola M, Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir Res. 2019;20(1):104. doi:10.1186/s12931-019-1078-y
7. Calverley P, Rogliani P, Papi A. Safety of N-acetylcysteine at high doses in chronic respiratory diseases: a review. Drug Saf. 2021;44(3):273–290. doi:10.1007/s40264-020-01026-y
8. Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4(2):108–122. doi:10.1002/brb3.208
9. Wahabi K, Perwez A, Rizvi MA. Antioxidant in Cancer. Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Chakraborti S, eds. Singapore: Springer; 2022.
10. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi:10.1016/j.cub.2014.03.034
11. Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–1228. doi:10.1089/ars.2012.4529
12. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi:10.1016/j.ejmech.2015.04.040
13. Colovic MB, Vasic VM, Djuric DM, Krstic DZ. Sulphur-containing amino acids: protective role against free radicals and heavy metals. Curr Med Chem. 2018;25(3):324–335. doi:10.2174/0929867324666170609075434
14. He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–553. doi:10.1159/000485089
15. Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants - An overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209:112891. doi:10.1016/j.ejmech.2020.112891
16. Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–2298. doi:10.1083/jcb.201804161
17. Kong H, Chandel NS. Regulation of redox balance in cancer and T cells. J Biol Chem. 2018;293(20):7499–7507. doi:10.1074/jbc.TM117.000257
18. Khan SU, Fatima K, Aisha S, Hamza B, Malik F. Redox balance and autophagy regulation in cancer progression and their therapeutic perspective. Med Oncol. 2022;40(1):12. doi:10.1007/s12032-022-01871-0
19. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi:10.1016/j.semcdb.2017.05.023
20. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
21. Ghosh SC. The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi:10.1016/j.bioorg.2019.102925
22. Zhang J, Ye ZW, Tew KD, Townsend DM. Cisplatin chemotherapy and renal function. Adv Cancer Res. 2021;152:305–327. doi:10.1016/bs.acr.2021.03.008
23. Domingo IK, Latif A, Bhavsar AP. Pro-inflammatory signalling prropels cisplatin-induced toxicity. Int J Mol Sci. 2022;23(13):7227. doi:10.3390/ijms23137227
24. Abadi AJ, Mirzaei S, Mahabady MK, et al. Curcumin and its derivatives in cancer therapy: potentiating antitumor activity of cisplatin and reducing side effects. Phytother Res. 2022;36(1):189–213. doi:10.1002/ptr.7305
25. Sato K, Watanabe S, Ohtsubo A, et al. Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer. 2016;16(1):222. doi:10.1186/s12885-016-2271-8
26. Finkel M, Goldstein A, Steinberg Y, Granowetter L, Trachtman H. Cisplatinum nephrotoxicity in oncology therapeutics: retrospective review of patients treated between 2005 and 2012. Pediatr Nephrol. 2014;29(12):2421–2424. doi:10.1007/s00467-014-2935-z
27. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20(12):3011. doi:10.3390/ijms20123011
28. Sancho-Martínez SM, Prieto-García L, Prieto M, et al. N-acetylcysteine transforms necrosis into apoptosis and affords tailored protection from cisplatin cytotoxicity. Toxicol Appl Pharmacol. 2018;349:83–93. doi:10.1016/j.taap.2018.04.010
29. Badr AM, Al-Kharashi LA, Attia H, et al. TLR4/inflammasomes cross-talk and pyroptosis contribute to N-Acetyl cysteine and chlorogenic acid protection against cisplatin-induced nephrotoxicity. Pharmaceuticals. 2023;16(3):337. doi:10.3390/ph16030337
30. Ramkumar V, Mukherjea D, Dhukhwa A, Rybak LP. Oxidative stress and inflammation caused by cisplatin ototoxicity. Antioxidants. 2021;10(12):1919. doi:10.3390/antiox10121919
31. Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8(1):1654. doi:10.1038/s41467-017-01837-1
32. Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. 2016;2016:1809394. doi:10.1155/2016/1809394
33. Freyer DR, Brock P, Knight K, et al. Interventions for cisplatin-induced hearing loss in children and adolescents with cancer. Lancet Child Adolesc Health. 2019;3(8):578–584. doi:10.1016/S2352-4642(19)30115-4
34. Gonçalves MS, Silveira AF, Teixeira AR, Hyppolito MA. Mechanisms of cisplatin ototoxicity: theoretical review. J Laryngol Otol. 2013;127(6):536–541. doi:10.1017/S0022215113000947
35. Chirtes F, Albu S. Prevention and restoration of hearing loss associated with the use of cisplatin. Biomed Res Int. 2014;2014:925485. doi:10.1155/2014/925485
36. Meijer AJM, Li KH, Brooks B, et al. The cumulative incidence of cisplatin-induced hearing loss in young children is higher and develops at an early stage during therapy compared with older children based on 2052 audiological assessments. Cancer. 2022;128(1):169–179. doi:10.1002/cncr.33848
37. Tang Q, Wang X, Jin H, et al. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021;163:60–71. doi:10.1016/j.ejpb.2021.03.008
38. Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi:10.1155/2011/843019
39. Groen CM, Podratz JL, Pathoulas J, Staff N, Windebank AJ. Genetic reduction of mitochondria complex I subunits is protective against cisplatin-induced neurotoxicity in Drosophila. J Neurosci. 2022;42(5):922–937. doi:10.1523/JNEUROSCI.1479-20.2021
40. Johnson C, Pankratz VS, Velazquez AI, et al. Candidate pathway-based genetic association study of platinum and platinum-taxane related toxicity in a cohort of primary lung cancer patients. J Neurol Sci. 2015;349(1–2):124–128. doi:10.1016/j.jns.2014.12.041
41. Shirmanova MV, Druzhkova IN, Lukina MM, et al. Chemotherapy with cisplatin: insights into intracellular pH and metabolic landscape of cancer cells in vitro and in vivo. Sci Rep. 2017;7(1):8911. doi:10.1038/s41598-017-09426-4
42. Santos NAGD, Ferreira RS, Santos ACD. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem Toxicol. 2020;136:111079. doi:10.1016/j.fct.2019.111079
43. Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? [published correction appears in Neurosci Lett. 2015 Jun 2;596():108]. Neurosci Lett. 2015;596:90–107. doi:10.1016/j.neulet.2014.10.014
44. Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014;34(1):547–550.
45. Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9(9):e106485. doi:10.1371/journal.pone.0106485
46. Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–3153. doi:10.1016/j.bbagen.2012.09.008
47. Kalyanaraman B. NAC, NAC, Knockin’ on Heaven’s door: interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells [published online ahead of print, 2022 Oct 9]. Redox Biol. 2022;57:102497. doi:10.1016/j.redox.2022.102497
48. Kwon Y. Possible beneficial effects of N-acetylcysteine for treatment of triple-negative breast cancer. Antioxidants. 2021;10(2):169. doi:10.3390/antiox10020169
49. Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141(2):150–159. doi:10.1016/j.pharmthera.2013.09.006
50. Aldini G, Altomare A, Baron G, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. doi:10.1080/10715762.2018.1468564
51. Tenório MCDS, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): impacts on human health. Antioxidants. 2021;10(6):967. doi:10.3390/antiox10060967
52. Radomska-Lesnniewska DM, Skopinski P. N-acetylcysteine as an anti-oxidant and anti-inflammatory drug and its some clinical applications. Centr Eur J Immunol. 2012;37:57–66.
53. Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–4129. doi:10.1016/j.bbagen.2013.04.016
54. Abdel-Wahab WM, Moussa FI, Saad NA. Synergistic protective effect of N-acetylcysteine and taurine against cisplatin-induced nephrotoxicity in rats. Drug Des Devel Ther. 2017;11:901–908. doi:10.2147/DDDT.S131316
55. Zhitkovich A. N-Acetylcysteine: antioxidant, aldehyde scavenger, and more. Chem Res Toxicol. 2019;32(7):1318–1319. doi:10.1021/acs.chemrestox.9b00152
56. Luczak MW, Zhitkovich A. Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium (VI), cadmium (II), and cobalt (II). Free Radic Biol Med. 2013;65:262–269. doi:10.1016/j.freeradbiomed.2013.06.028
57. Higashi T, Elmeligy E, Mai Y, et al. Glutathione and cysteines suppress cytotoxicity of gas phase of cigarette smoke by direct reacting with unsaturated carbonyl compounds in the gas phase. Biochem Biophys Res Commun. 2019;509(4):988–993. doi:10.1016/j.bbrc.2019.01.040
58. Okamoto A, Tanaka M, Sumi C, et al. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol. 2016;16(1):104. doi:10.1186/s12871-016-0273-3
59. Gao X, Lampraki EM, Al-Khalidi S, Qureshi MA, Desai R, Wilson JB. N-acetylcysteine (NAC) ameliorates Epstein-Barr virus latent membrane protein 1 induced chronic inflammation. PLoS One. 2017;12(12):e0189167. doi:10.1371/journal.pone.0189167
60. Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84(6):652–659. doi:10.4103/ijdvl.IJDVL_33_18
61. Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P. Mitochondrial Transfer in Cancer: a Comprehensive Review. Int J Mol Sci. 2021;22(6):3245. doi:10.3390/ijms22063245
62. Pasquier J, Guerrouahen BS, Al Thawadi H, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11(1):94. doi:10.1186/1479-5876-11-94
63. Chang JC, Chang HS, Wu YC, et al. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res. 2019;38(1):30. doi:10.1186/s13046-019-1028-z
64. Burt R, Dey A, Aref S, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415–1429. doi:10.1182/blood.2019001398
65. Wang J, Liu X, Qiu Y, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11. doi:10.1186/s13045-018-0554-z
66. Liu D, Gao Y, Liu J, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65. doi:10.1038/s41392-020-00440-z
67. Mistry JJ, Marlein CR, Moore JA, et al. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc Natl Acad Sci U S A. 2019;116(49):24610–24619. doi:10.1073/pnas.1913278116
68. Poljsak B, Milisav I. The role of antioxidants in cancer, friends or foes? Curr Pharm Des. 2018;24(44):5234–5244. doi:10.2174/1381612825666190123112647
69. Šalamon Š, Kramar B, Marolt TP, Poljšak B, Milisav I. Medical and dietary uses of N-Acetylcysteine. Antioxidants. 2019;8(5):111. doi:10.3390/antiox8050111
70. Monti D, Sotgia F, Whitaker-Menezes D, et al. Pilot study demonstrating metabolic and anti-proliferative effects of in vivo anti-oxidant supplementation with N-Acetylcysteine in breast cancer. Semin Oncol. 2017;44(3):226–232. doi:10.1053/j.seminoncol.2017.10.001
71. Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6(2):350–369.
72. Feng H, Moriyama T, Ohuchida K, et al. N-acetyl cysteine induces quiescent-like pancreatic stellate cells from an active state and attenuates cancer-stroma interactions. J Exp Clin Cancer Res. 2021;40(1):133. doi:10.1186/s13046-021-01939-1
73. Jurkowska H, Wróbel M. Inhibition of human neuroblastoma cell proliferation by N-acetyl-L-cysteine as a result of increased sulfane sulfur level. Anticancer Res. 2018;38(9):5109–5113. doi:10.21873/anticanres.12831
74. Le Gal K, Ibrahim MX, Wiel C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7(308):308re8. doi:10.1126/scitranslmed.aad3740
75. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6(221):221ra15. doi:10.1126/scitranslmed.3007653
76. Muldoon LL, Wu YJ, Pagel MA, Neuwelt EA. N-acetylcysteine chemoprotection without decreased cisplatin antitumor efficacy in pediatric tumor models. J Neurooncol. 2015;121(3):433–440. doi:10.1007/s11060-014-1657-1
77. Güntürk I, Yazici C, Köse SK, Dağli F, Yücel B, Yay AH. The effect of N-acetylcysteine on inflammation and oxidative stress in cisplatin-induced nephrotoxicity: a rat model. Turk J Med Sci. 2019;49(6):1789–1799. doi:10.3906/sag-1903-225
78. Shalby AB, Assaf N, Ahmed HH. Possible mechanisms for N-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicol Mech Methods. 2011;21(7):538–546. doi:10.3109/15376516.2011.568985
79. Huang S, You J, Wang K, et al. N-Acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Biomed Res Int. 2019;2019:4805853. doi:10.1155/2019/4805853
80. Somdaş MA, Güntürk İ, Balcıoğlu E, Avcı D, Yazıcı C, Özdamar S. Protective effect of N-acetylcysteine against cisplatin ototoxicity in rats: a study with hearing tests and scanning electron microscopy. Braz J Otorhinolaryngol. 2020;86(1):30–37. doi:10.1016/j.bjorl.2018.08.002
81. Chen BC, Lin LJ, Lin YC, Lee CF, Hsu WC. Optimal N-acetylcysteine concentration for intratympanic injection to prevent cisplatin-induced ototoxicity in Guinea pigs. Acta Otolaryngol. 2022;142(2):127–131. doi:10.1080/00016489.2022.2038796
82. Mohan S, Smyth BJ, Namin A, Phillips G, Gratton MA. Targeted amelioration of cisplatin-induced ototoxicity in Guinea pigs. Otolaryngol Head Neck Surg. 2014;151(5):836–839. doi:10.1177/0194599814544877
83. Zaki SM, Mohamed EA, Motawie AG, Abdel Fattah S. N-acetylcysteine versus progesterone on the cisplatin-induced peripheral neurotoxicity. Folia Morphol. 2018;77(2):234–245. doi:10.5603/FM.a2017.0090
84. Abdel-Wahab WM, Moussa FI. Neuroprotective effect of N-acetylcysteine against cisplatin-induced toxicity in rat brain by modulation of oxidative stress and inflammation. Drug Des Devel Ther. 2019;13:1155–1162. doi:10.2147/DDDT.S191240
85. Vukovic R, Kumburovic I, Joksimovic Jovic J, et al. N-Acetylcysteine protects against the anxiogenic response to cisplatin in rats. Biomolecules. 2019;9(12):892. doi:10.3390/biom9120892
86. Gunturk EE, Yucel B, Gunturk I, Yazici C, Yay A, Kose K. The effects of N-acetylcysteine on cisplatin-induced cardiotoxicity. Bratisl Lek Listy. 2019;120(6):423–428. doi:10.4149/BLL_2019_068
87. Rosic G, Selakovic D, Joksimovic J, et al. The effects of N-acetylcysteine on cisplatin-induced changes of cardiodynamic parameters within coronary autoregulation range in isolated rat hearts. Toxicol Lett. 2016;242:34–46. doi:10.1016/j.toxlet.2015.11.028
88. Coşkun Ö, Öztopuz Ö, Büyük B. Possible protective activity of n-acetyl cysteine against cisplatin-induced hepatotoxicity in rats. Mol Biol Rep. 2021;48(1):637–644. doi:10.1007/s11033-020-06111-0
89. Elsayed A, Elkomy A, Elkammar R, et al. Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats. Sci Rep. 2021;11(1):13979. doi:10.1038/s41598-021-93196-7
90. Sarafraz Z, Ahmadi A, Daneshi A. Transtympanic injections of N-acetylcysteine and dexamethasone for prevention of cisplatin-induced ototoxicity: double blind randomized clinical trial. Int Tinnitus J. 2018;22(1):40–45. doi:10.5935/0946-5448.20180007
91. Riga MG, Chelis L, Kakolyris S, et al. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: a feasible method with promising efficacy. Am J Clin Oncol. 2013;36(1):1–6. doi:10.1097/COC.0b013e31822e006d
92. Orgel E, Knight KR, Chi YY, et al. Intravenous N-Acetylcysteine to prevent cisplatin-induced hearing loss in children: a nonrandomized controlled Phase I trial. Clin Cancer Res. 2023;29(13):2410–2418. doi:10.1158/1078-0432.CCR-23-0252
93. Yoo J, Hamilton SJ, Angel D, et al. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: a pilot randomized study in head and neck cancer patients. Laryngoscope. 2014;124(3):E87–E94. doi:10.1002/lary.24360
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.