Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Effect of Fluid and Caffeine Management on Quality of Life in Older Women with Overactive Bladder in Rural Korea: A Pilot Study
Authors Park J , Lee K , Lee K
Received 12 October 2023
Accepted for publication 21 March 2024
Published 10 April 2024 Volume 2024:17 Pages 1549—1559
DOI https://doi.org/10.2147/JMDH.S441256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jeongok Park,1 Kyoungjin Lee,2 Kayoung Lee3
1College of Nursing, Mo-Im Kim Nursing Research Institute, Yonsei University, Seoul, South Korea; 2College of Nursing, Kyungbok University, Gyeonggi-do, South Korea; 3College of Nursing, Gachon University, Incheon, South Korea
Correspondence: Kayoung Lee, Gachon University College of Nursing, 191 Hambakmoero, Yeonsu-Gu, Incheon, 21936, South Korea, Tel +82-32-820-4227, Email [email protected]
Purpose: This study aimed to investigate the effectiveness of the simplified intervention, consisting of fluid and caffeine management alone in older women with overactive bladder symptoms.
Patients and Methods: A quasi-experimental pretest-posttest design was used. Rural, community-dwelling older women were recruited at four senior centers in South Korea. Of the 63 participants initially enrolled, 34 met the inclusion criteria. One group (n = 15) used fluid and caffeine management alone (FM), and the other group (n = 12) used a combination of fluid and caffeine management and pelvic floor muscle training (FM+PFMT). Urinary symptom-specific health-related quality of life was measured using the Korean version of KHQ. Sleep quality was measured using the Pittsburgh Sleep Quality Index. After the intervention, participants were assessed 4 and 8 weeks. A linear mixed model was used for the analysis.
Results: The mean age of the participants was 74.44 ± 5.67 years. Among the nine domains of KHQ, impact on life and physical limitations decreased significantly in both groups, without significant between-group differences. Sleep/energy increased in both groups, and the scores in the FM+PFMT group were significantly improved. The number of micturition episodes per day and the quality of sleep did not differ significantly between the two groups.
Conclusion: A simplified intervention, consisting of fluid and caffeine management alone can be considered as the first-line intervention to improve health-related quality of life in rural, community-dwelling, older women with overactive bladder symptoms. Healthcare providers should consider providing a relatively simple, but equally effective intervention to maximize the adherence and effectiveness.
Keywords: overactive urinary bladder, behavior therapy, quality of life, treatment adherence and compliance, nursing
Introduction
Overactive bladder (OAB) is defined as the presence of
urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection or other obvious pathology.1
According to the population-based studies, the prevalence of OAB syndrome is 11.8–16.5% in adults aged 18 years and older in Europe, Canada, and the US, and 20% in adults aged 40 years and older in Asia.2–4 The prevalence is 30–40% in adults aged 75 years and older.3 OAB is not life-threatening but, it may have a negative effect on quality of life.5
A combination of behavioral and pharmacologic interventions is effective for managing OAB in older adults.6 Behavioral interventions can be categorized into lifestyle modification and training techniques.7 Lifestyle modification includes fluid management and weight control, avoiding bladder irritants in the diet, and timed voiding.7 Training techniques include bladder training with pelvic floor muscle (PFM) contraction to control urinary urgency, and regular pelvic floor muscle training (PFMT).7
Fluid management with caffeine reduction is simple and easy to implement in community-dwelling older adults with OAB.8 Previous studies have shown that reducing caffeinated coffee consumption and appropriate fluid intake is associated with alleviation of OAB symptoms.9,10 OAB symptoms and urinary incontinence (UI) can be made worse by excessive fluid intake and incontinence, whereas restriction of fluids may promote urinary urgency and frequency due to an increase in urine concentration, which may irritate the bladder mucosa.11 The daily volume of fluid intake should be approximately 1500 mL or 30 mL/kg per 24 hours.12 Hashim and Abrams13 found that OAB symptoms significantly decreased in patients who decreased their fluid intake by 25%. In their study,13 participants’ average fluid intake and output volumes were greater than 1500 mL and 1700 mL per day, respectively. That is, fluid reduction may only be effective on OAB symptoms in adults who have appropriate fluid intake.13 In contrast, Park et al8 reported that the average volume of fluid intake in older women living in a rural area in Korea was approximately 934 mL per day. Thus, health care providers should confirm patients’ daily fluid intake status before advising them to increase or decrease fluid intake.
Caffeine promotes detrusor muscle instability and has a diuretic effect. A high caffeine intake is associated with OAB.14 Specifically, older women in rural areas of South Korea (hereafter, Korea) have a high prevalence of lack of a formal education, overweight or obesity, and high caffeine consumption compared with the general population.6,15,16 Thus, lifestyle change behavioral interventions should be considered as the first option for older women in rural areas, and interventions should be simple to initiate and maintain.
PFMT was also effective in alleviating various lower urinary symptoms, such as urgency, stress or mixed UI.17 Because PFM contraction decreases detrusor pressure, increases urethral pressure, and suppresses the micturition reflex, patients with OAB are taught to perform PFM contractions when they feel an urge to urinate, instead of rushing to the toilet. Consequently, patients with OAB who perform PFM contractions may feel comfortable going to the toilet and avoid urinary leakage. In addition, PFMT may cause pelvic floor changes, and can normalize urethral pressure and the micturition reflex.18 Thus, correct contractions of the PFM and continued adherence to PFMT are crucial for the effectiveness of this approach.
However, correctly contracting the PFM and continued adherence to PFMT can be challenging for community-dwelling and rural-living older women. Talasz et al19 found that about 66% of older women were unable to perform PFM contractions, while 22% of them performed insufficient PFM contractions. Approximately one-third of women performed correct PFM contractions after receiving brief instructions,20 but PFMT adherence tended to decrease over time.21 Considering the balance of efficacy, simplicity, and long-term adherence, it is not clear which behavioral interventions should first be recommended for community-dwelling older adults. Thus, in this study, we compared the effectiveness of using fluid and caffeine management alone versus using a combination of fluid and caffeine management with PFMT among community-dwelling older women with OAB in Korea.
Materials and Methods
Design
The current study had a quasi-experimental pretest-posttest design.
Participants
Community-dwelling older women who had OAB symptoms were recruited through four senior centers. These senior centers, which are places where older adults build friendships and participate in social activities, were selected from Gapyeong, Gyeonggi Province, South Korea. The four senior centers were randomized to two FM and two FM+PFMT sites. The enrolled participants were assigned to the FM or FM+PFMT group based on their place of residence, geographically close to a particular senior center, to minimize intervention-control contamination.
Participants were considered to have OAB if they answered “yes” on the question, “In the past month, have you had any experience of the sudden urge to urinate?” and answered “about once a daily”, “2–4 times a day”, or “5 times a day or more” on the question, “how often do you have a sudden desire to urinate that is difficult to defer?” The first question was originally developed by Irwin et al2 and used in the Korean study.22 The second question was one from Overactive Bladder Symptom Score.23 To check for a urinary tract infection, a urine dipstick test was used. Using an ultrasound bladder scanner (BioCon-700, Mcube Technology, Seoul, Korea), potential participants’ post-void residual urine volume was measured within 5 min after they voided. To avoid confounding effects, we excluded older women who had a urinary tract infection, had more than 100 mL of post-void residual urine, or had taken medicine for lower urinary tract symptoms.
Sample Size
For sample size calculation, we applied repeated-measures analysis of variance, with an effect size of 0.8 and a confidence level of 0.05, using the G*Power program (version 3.1.2). The required sample size was 27 per group. Considering a possible dropout rate of 30%, the recruitment target was set to 35 participants per group. Sixty-three participants eventually enrolled through the recruitment announcement. Of the 63 participants who were initially enrolled, 34 met the inclusion criteria. For the pilot study, a minimum sample size of 12 per group was recommended.24
A CONSORT flow diagram, illustrating the flow of participants from recruitment to analysis, is presented in Figure 1. Of the 34 participants, 18 were assigned to the FM group, and 16 were assigned to the FM+PFMT group. Twenty-seven participants (79.4%) completed data collection at baseline and received the intervention.
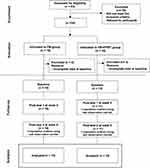 |
Figure 1 Flow chart of the research process. |
Intervention
The FM+PFMT group received education regarding fluid and caffeine management, combined with PFMT, and the FM group received education regarding fluid and caffeine management only. The principal investigator provided one-time group education at the senior center. The detailed content and timing of the education are presented in Table 1. None of the participants had any health problems requiring restriction of fluid intake. To ensure the appropriate volume of fluid intake a day, which is approximately 1500mL, participants were instructed to use a 300 mL water bottle to measure their daily fluid intake. They were advised increase or decrease their fluid intake based on their daily amount. In addition, for caffeine management, participants received information on caffeinated coffee and energy drinks, and were encouraged to avoid them.
 |
Table 1 Overview of the Content of the Intervention |
The participants in the FM+PFMT group received verbal instructions on PFMT, based on the literature.25 The standard established recommendation for PFMT was provided (ie, three sets of 10 exercises, performed three to four times a week). It was also recommended that participants conduct fast twitching of the PFM several times whenever they felt urgency, before going to the toilet. To monitor adherence to fluid and caffeine management and PFMT, participants were asked to write a daily log recording whether they performed the intervention.
Measurement
Health-Related Quality of Life
Urinary symptom-specific health-related quality of life (HRQOL) was the primary outcome and measured using the Korean version of the King’s Health Questionnaire (KHQ).26,27 The instrument is a 21-item questionnaire based on nine domains including “general health perceptions”, “impact on life”, “role limitation”, “physical limitation”, “personal relationship”, “social limitation”, “emotion”, “sleep/energy”, and “incontinency severity”. The score for each domain ranges from 0 to 100. A higher score indicates a lower HRQOL. Cronbach’s α for the Korean version was 0.9228 and that obtained in this study was 0.84.
Sleep Quality
Sleep quality was the secondary outcome and was assessed using the Korean version of the Pittsburgh Sleep Quality Index (PSQI-K).29,30 The instrument is a 19-item, self-rated questionnaire and the scores range from 0 to 21. A higher score indicates lower sleep quality. Cronbach’s α of the Korean version was 0.8429 and that in this study was 0.67.
Participants’ Characteristics
Information was collected on participants’ age, level of education, living status, self-reported health status, number of micturition episodes per day, and self-reported comorbidities.
Data Collection
In this study, data were collected from February to April 2018. At the four senior centers, face-to-face, structured interviews were conducted by trained research assistants. After collecting the baseline data, the interventions were provided by the principal investigator on the same day at the rural senior center. After the intervention was provided, the research assistants who participated in the data collection called all participants once a week to monitor their adherence to the intervention and encourage them to maintain adherence. Data were collected at 4 and 8 weeks after the intervention for posttest 1 and posttest 2, respectively.
Ethical Considerations
This study complies with the ethical principles of the Declaration of Helsinki. This study was approved by the institutional review board of Yonsei University (No. Y-2017-0106). Written informed consent was obtained. All participants were informed of the aims, procedures, and possible benefits and risks of the study, and their right to withdraw at any time during the research process. They were informed that all data would be used for research purposes only.
Data Analysis
Descriptive statistics were used to describe participants’ demographics and disease-related characteristics. The homogeneity between the FM+PFMT and FM groups was evaluated using the independent t-test, the chi-square test, and Fisher’s exact test. Between-group differences were analyzed using a linear mixed model. All statistical analyses were based on the intention-to-treat population. For the missing data, last observation carried forward (LOCF) method was used. The data were analyzed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
The baseline characteristics of the 27 participants are presented in Table 2. There were no significant differences in the general characteristics of the FM and FM+PFMT groups. The mean age of the participants was 74.44 ± 5.67 years. Thirty-three percent of the participants did not have any formal education, and 51.9% of them had completed elementary school education (6 years of education). The mean body mass index was 25.59 ± 3.69 kg/m2, and 70.4% of the participants were overweight or obese. Fifty-two percent of participants lived with their spouses. The most prevalent health problems were hypertension (55.6%) and musculoskeletal disease (40.7%).
 |
Table 2 Comparison of General Characteristics and Main Variables Between the FM and FM+PFMT Groups at Baseline (N=27) |
The mean number of micturition episodes per day was 7.93 ± 3.31. The mean sleep quality score measured by the PSQI-K was 10.19 ± 3.52, meaning that participants’ sleep quality was poor. On the KHQ, participants reported slightly poor general health perceptions (56.48 ± 24.61) and a minor impact on life related to their bladder problems (41.97 ± 32.80). Participants reported that the other domains of KHQ were not strongly affected by their bladder problems, as follows: role limitations (17.28 ± 22.40), physical limitations (25.31 ± 28.26), social limitations (13.58 ± 23.13), personal relationships (4.17 ± 11.79; n = 8), and emotions (11.11 ± 21.35). Regarding the personal relationships with their partner, only eight participants responded to the question because 13 participants (48.2%) were widowed. Most participants with spouses responded as “not applicable” to the questions, “Does your bladder problem affect your relationship with your partner?” and “Does your bladder problem affect your sex life?”, and the data were treated as missing according to KHQ scoring system. Therefore, the variables regarding personal relationships were not included in the linear mixed model analysis.
The mean values, standard deviations, and results of the linear mixed model analysis for outcome variables are presented in Table 3. Regarding the primary outcome of urinary symptom–specific HRQOL, the scores on the KHQ domains for “the impact on life” and “physical limitations” related to bladder problems decreased significantly over time in both groups (p = 0.010, p = 0.027, respectively). However, in both groups, “incontinence severity” fluctuated over time (p = 0.036). “Sleep/energy” increased over time in both groups (p = 0.006), and a significant group-by-time interaction effect was noted (p = 0.049; Figure 2). While the scores of “sleep/energy” in the FM group improved minimally, these scores improved significantly in the FM+PFMT group. No significant differences between two groups by time were found in the other domains of the KHQ. Regarding the secondary outcome—the quality of sleep—measured by the PSQI-K or the number of micturition episodes per day, there were no differences between the two groups over time.
 |
Table 3 Linear Mixed Model Analysis of the Outcome Variables |
 |
Figure 2 Score changes of sleep/energy by group over time. |
Discussion
Current guidelines have suggested the use of behavior therapy that combines training techniques with habit changes as a first-line treatment for OAB.1,30 However, unassisted interventions, including Kegel exercises, are often difficult for older women to follow because they struggle to identify the correct muscles to confirm whether they are performing the exercise properly. In this study, we showed that, over time, behavioral interventions—both a simplified fluid and caffeine management and a combination of fluid and caffeine management with PFMT—were significantly effective in improving the impact of OAB symptoms on life and the physical limitations related to OAB symptoms, with no significant differences between the two groups. These findings support our hypothesis that a simplified intervention, ie, fluid and caffeine management alone, can be considered as a first-line behavioral intervention, as it is equally effective in alleviating the effects of OAB symptoms on life and physical limitations as is PFMT combined with fluid and caffeine management.
In this study, there were no significant differences between the intervention and FM+PFMT groups in any of the outcomes, except for the sleep and energy variables in the KHQ. Considering that the effect of PFMT on OAB depends on several factors, such as the frequency and extent of training, adherence to the training procedure, functional performance of PFM, and satisfaction with the intervention,31,32 one possible explanation of the negative findings in the current study is that the FM+PFMT group participants might not have performed PFMT correctly based on unsupervised verbal instructions, without vaginal palpation or ultrasound monitoring. According to previous systematic reviews, supervised PFMT programs were more effective in reducing UI than non-supervised programs.33–35
In reality, however, it is not possible to provide supervised PFMT programs to women living in rural areas, because of limited facilities and human resources. In rural areas of Korea, there are few specialized healthcare providers that can care for patients with OAB and that can supervise PFMT interventions, as these are mainly provided by secondary or tertiary hospitals. In addition, for women who live in rural areas, far from specialized clinics, there are limited resources for learning how to perform PFMT in home-based exercises or for attending supervised PFMT programs. Therefore, to change behaviors in Korean women living in rural areas, it is important to consider the tolerability, simplicity, and ease of adherence to behavioral interventions, in addition to the efficacy of the interventions.
This study had several limitations. First, the evaluation of intervention adherence and compliance may not have been accurate because the study data were based on self-reporting via daily log sheets and a telephone follow-up interview. Specifically, all participants were encouraged to consume 1500 mL of fluids. For older women living in rural areas, with low education levels, we designed a simple logging method using simple marking rather than writing the amount of fluid intake. However, we found that it was not easy for these women to mark a daily log consistently, while following the intervention instructions. For future studies, we suggest that a pre-survey should be conducted to identify which intervention logging methods are appropriate for older women living in rural areas (eg, using daily accomplishment stickers, rather than writing in a diary).
Second, during the intervention process, it was difficult to ensure accurate evaluation of whether participants performed PFMT properly, because we did not employ an objective measure of effective contractions of the PFM, such as a biofeedback devices or digital muscle testing. Instead, based on the daily log sheet and a telephonic follow-up interview, we considered them to be compliant with the verbal instructions of PFMT if they had completed 60% of the full instructions (three sets of 10 muscle training exercises per day). Thus, future studies should include objective measurements that test PFM contraction, to improve the internal validity of such an experimental study.
Third, in this study, data were collected at 4 and 8 weeks after the intervention. Depending on the severity of their OAB, some participants experienced the effect of the intervention within 2 weeks and up to 3 months or longer.36,37 Therefore, future studies need to consider a longer follow-up period to better measure the effects of PFMT and the fluid moderation intervention. Moreover, to avoid confounding effects, the current study excluded older women who had a urinary tract infection, had more than 100 mL of post-void residual urine, or were taking medication for lower urinary tract symptoms. In addition, for increasing the accuracy of the amount of participants’ intake and output and urinary symptoms of OAB, future studies should consider including a voiding diary or pad use.
Additionally, in the current study, 27 participants of the 34 enrolled participants remained, which implies a 20.6% attrition rate. This attrition rate may be attributed to seasonal effects, because this study was conducted from February to April 2018, which is a busy farming season. Therefore, we suggest that considering farming events is important when conducting research in rural areas. As another strategy to decrease the attrition rate during the intervention period, regular monitoring, such as encouraging intervention adherence by a telephone call once a week or using an online-video individual/group meeting, can be considered. It is also important to include gatekeepers, such as community nurses and leaders, in an advisory board to identify user-friendly interventions and to develop alternative retention plans, thereby maximizing the benefit–burden ratio.38 Future intervention studies including older adults living in rural areas should consider the benefits of the potentially helpful intervention and social interactions, as well as the burdens of discomfort, time, and accessibility, associated with study participation.
Finally, this pilot study had a small sample size and was conducted in a single county in Korea. Due to the small size of the senior centers in the rural area, finding participants with OAB was difficult. Nurses at the health center confirmed this difficulty, even with ample time set aside for recruitment. Therefore, generalizing our findings to other older adults with OAB symptoms should be undertaken with caution. Based on the results of this pilot study, a replication study that expands the geographic area and number of participants should be undertaken in the future. Moreover, to improve the generalizability of the study findings, environmental factors and social interactions should be considered for a multilevel assessment of participant characteristics that could affect the results.
Conclusion
This study found that fluid and caffeine management can be considered as the first-line intervention among various behavioral interventions for rural, community-dwelling older women with OAB. For those who have difficulty in performing and adhering to proper PFMT, healthcare providers should consider education on a relatively simple, but equally effective intervention, ie, fluid management including caffeinated drink reduction to maximize adherence and to optimize the effectiveness of behavioral interventions.
Acknowledgments
We would like to express our gratitude to the women from Gapyeong County who participated in this study and to the nurses of the community health centers.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (grant number 2015R1C1A1A01054434); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number 2019R1F1A1062769); and National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number 2021R1C1C2011587).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol. 2019;202(3):558–563. doi:10.1097/JU.0000000000000309
2. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–14; discussion 14–5. doi:10.1016/j.eururo.2006.09.019
3. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi:10.1007/s00345-002-0301-4
4. Chuang YC, Liu SP, Lee KS, et al. Prevalence of overactive bladder in China, Taiwan and South Korea: results from a cross-sectional, population-based study. Low Urin Tract Symptoms. 2019;11(1):48–55. doi:10.1111/luts.12193
5. Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92(7):731–735. doi:10.1046/j.1464-410x.2003.04463.x
6. Macdiarmid SA. Maximizing the treatment of overactive bladder in the elderly. Rev Urol. 2008;10(1):6–13.
7. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572–1580. doi:10.1016/j.juro.2015.01.087
8. Park J, Lee YJ, Lee K, Park S. Coffee intake, health-related quality of life, and associated factors of overactive bladder in older Korean women living in rural South Korea. J Women Aging. 2019;31(5):367–380. doi:10.1080/08952841.2018.1444950
9. Robinson D, Hanna-Mitchell A, Rantell A, Thiagamoorthy G, Cardozo L. Are we justified in suggesting change to caffeine, alcohol, and carbonated drink intake in lower urinary tract disease? Report from the ICI-RS 2015. Neurourol Urodyn. 2017;36(4):876–881. doi:10.1002/nau.23149
10. Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. Br J Nurs. 2002;11(8):560–565. doi:10.12968/bjon.2002.11.8.10165
11. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52–8. doi:10.1038/sj.ejcn.1601902
12. Institute of Medicine. Panel on Dietary Reference Intakes for Electrolytes and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, D.C: National Academies Press; 2005.
13. Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102(1):62–66. doi:10.1111/j.1464-410X.2008.07463.x
14. Kosilov KV, Loparev SA, Ivanovskaya MA, Kosilova LV. Caffeine as a probable factor for increased risk of OAB development in elderly people. Curr Urol. 2016;9(3):124–131. doi:10.1159/000442866
15. Baek JM, Song JY, Lee SJ, et al. Caffeine intake is associated with urinary incontinence in Korean postmenopausal women: results from the Korean national health and nutrition examination survey. PLoS One. 2016;11(2):e0149311. doi:10.1371/journal.pone.0149311
16. Lim HS, Hwang JY, Choi JC, Kim M. Assessment of caffeine intake in the Korean population. Food Addit Contam Part a Chem Anal Control Expo Risk Assess. 2015;32(11):1786–1798. doi:10.1080/19440049.2015.1077396
17. Dumoulin C, Hay-Smith J, Frawley H, et al. 2014 consensus statement on improving pelvic floor muscle training adherence International Continence Society 2011 State-of-the-Science Seminar. Neurourol Urodyn. 2015;34(7):600–605. doi:10.1002/nau.22796
18. Bo K, Fernandes ACNL, Duarte TB, Brito LGO, Ferreira CHJ. Is pelvic floor muscle training effective for symptoms of overactive bladder in women? A systematic review. Physiotherapy. 2020;106:65–76. doi:10.1016/j.physio.2019.08.011
19. Talasz H, Gosch M, Enzelsberger H, Rhomberg HP. Female geriatric patients with urinary incontinence symptoms and their control over pelvic floor muscles. Z Gerontol Geriatr. 2005;38(6):424–430. doi:10.1007/s00391-005-0301-2
20. Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165(2):322–7; discussion 7–9. doi:10.1016/0002-9378(91)90085-6
21. Bø K, Hilde G. Does it work in the long term?—A systematic review on pelvic floor muscle training for female stress urinary incontinence. Neurourol Urodyn. 2013;32(3):215–223. doi:10.1002/nau.22292
22. Lee YS, Lee KS, Jung JH, et al. Prevalence of overactive bladder, urinary incontinence, and lower urinary tract symptoms: results of Korean EPIC study. World J Urol. 2011;29(2):185–190. doi:10.1007/s00345-009-0490-1
23. Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome--overactive bladder symptom score. Urology. 2006;68(2):318–323. doi:10.1016/j.urology.2006.02.042
24. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmac Statis. 2005;4(4):287–291.
25. Ben Ami N, Dar G. What is the most effective verbal instruction for correctly contracting the pelvic floor muscles? Neurourol Urodyn. 2018;37(8):2904–2910. doi:10.1002/nau.23810
26. Oh SJ, Park HG, Paick SH, Park WH, Choo MS. Translation and linguistic validation of Korean version of the king’s health questionnaire instrument. Korean J Urol. 2005;46:438–450.
27. Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104(12):1374–1379. doi:10.1111/j.1471-0528.1997.tb11006.x
28. S-J O, Choo M-S, Kim HS, et al. Psychometric properties of the Korean version of the King’s health questionnaire in women with stress urinary incontinence. J Korean Continence Soc. 2005;9(2):115–123. doi:10.5213/jkcs.2005.9.2.115
29. Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16(3):803–812. doi:10.1007/s11325-011-0579-9
30. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
31. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
32. Barton A, Serrao C, Thompson J, Briffa K. Transabdominal ultrasound to assess pelvic floor muscle performance during abdominal curl in exercising women. Int Urogynecol J. 2015;26(12):1789–1795. doi:10.1007/s00192-015-2791-9
33. Bø K, Herbert RD. There is not yet strong evidence that exercise regimens other than pelvic floor muscle training can reduce stress urinary incontinence in women: a systematic review. J Physiother. 2013;59(3):159–168. doi:10.1016/S1836-9553(13)70180-2
34. Berghmans LC, Hendriks HJ, De Bie RA, van Waalwijk van Doorn ES, Bø K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU Int. 2000;85(3):254–263. doi:10.1046/j.1464-410x.2000.00434.x
35. Price N, Dawood R, Jackson SR. Pelvic floor exercise for urinary incontinence: a systematic literature review. Maturitas. 2010;67(4):309–315. doi:10.1016/j.maturitas.2010.08.004
36. Li X, Liu L, He J, Yan J, Wang Y. Analysis of the effectiveness of the application of pelvic floor rehabilitation exercise and the factors influencing its self-efficacy in postoperative patients with cervical cancer. Front Oncol. 2023;13:1118794. doi:10.3389/fonc.2023.1118794
37. Papanikolaou DT, Lampropoulou S, Giannitsas K, Skoura A, Fousekis K, Billis E. Pelvic floor muscle training: novel versus traditional remote rehabilitation methods. A systematic review and meta-analysis on their effectiveness for women with urinary incontinence. Neurourol Urodyn. 2023;42(4):856–874. doi:10.1002/nau.25150
38. Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56(12):2340–2348. doi:10.1111/j.1532-5415.2008.02015.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
