Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Effect of Angiotensin Receptor Blocker and Angiotensin Converting Enzyme Inhibitor on Kidney Function and Blood Potassium Level in Indonesian Type 2 Diabetes Mellitus with Hypertension: A Three-Month Cohort Study
Authors Puspita FM, Yunir E, Agustina PS , Sauriasari R
Received 10 March 2021
Accepted for publication 10 June 2021
Published 7 September 2021 Volume 2021:14 Pages 3841—3849
DOI https://doi.org/10.2147/DMSO.S310091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Febriana M Puspita,1 Em Yunir,2 Putri S Agustina,1 Rani Sauriasari1
1Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia; 2Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Correspondence: Rani Sauriasari
Faculty of Pharmacy, Universitas Indonesia, Depok, 16424, Indonesia
Tel +62-21-7270031
Fax +62-21-7863433
Email [email protected]
Purpose: National formulary restrictions in Indonesia (2019) require estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 to be able to prescribe telmisartan and valsartan and ACE-I intolerance to be able to prescribe irbesartan and candesartan. These restrictions are based on economic considerations and differ from American Diabetes Association (ADA) (2020) guidelines which allow equal use of angiotensin II receptor blockers (ARB) and angiotensin-converting enzyme inhibitors (ACE-I) without restriction. Since there is a need to evaluate the different effects of ACE-I and ARB in the Indonesian hypertensive type 2 diabetes mellitus (T2DM) population, we compare their effects on urine albumin-to-creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), and blood potassium level.
Patients and Methods: A prospective cohort study at RSUPN Dr. Cipto Mangunkusumo Hospital was conducted in 123 T2DM patients. We followed the study subjects prospectively for three months using a validated questionnaire, health record, and laboratory data.
Results: After 3 months of observation, there were no significant changes, except increased BMI values (p = 0.046) in the ACE-I group, and decreased LDL value (p = 0.016) and HDL value (p = 0.004) in the ARB group. Multivariate analysis showed that the consumption of ACE-I or ARB was not associated with a decrease/constant of UACR or increase potassium level, even after adjusting by confounding variables. Interestingly, we found ARB was more likely to increase eGFR, but the significance was lost once the duration of ACE-I/ARB use was entered into the model. In addition, BMI > 25 kg/m2 was a significant factor associated with decreased/constant UACR, maleness was significant for increased eGFR, and declining systolic blood pressure for increase in potassium level.
Conclusion: ACE-I and ARB have a similar effect on UACR and blood potassium level, but ARB slightly increased eGFR compared to ACE-I within three months of consumption.
Keywords: type 2 diabetes mellitus, UACR, eGFR, chronic kidney disease, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers
Introduction
Diabetes mellitus (DM) and hypertension are the main causes of end-stage renal disease (ESRD). According to a survey by the Indonesian Renal Registry, DM accounted for 21% and hypertension 51% of all cases of ESRD.1 It is known that blood-pressure control slows the progression of renal-function decline in DM with hypertension. Research conducted at Dr. Hasan Sadikin Hospital in Indonesia showed that, based on serum creatinine, urea, and eGFR values, the antihypertensive therapy profile used could control the kidney function of T2DM patients with chronic kidney disease (CKD) complications during the three months of the study. In that study, ARB was more frequently prescribed than ACE-I.2 In many other countries, ARBs were more preferable,3 however in middle-income countries ACE-I is more preferable. In Indonesia, this statement was supported by National Formulary restrictions in prescribing ARB.
According to ADA (2020), both angiotensin-converting enzyme inhibitors (ACE-I) and ARBs are first-line therapy for T2DM patients with albuminuria (UACR ≥ 30 mg/g) in reducing the risk of progressive kidney disease.4 However, national formulary restrictions in Indonesia (2019) require estimated glomerular filtration rate (eGFR) results of less than 60 mL/min/1.73 m2 for prescription of telmisartan and valsartan and ACE-I intolerance present after at least one month’s prescription for switching to irbesartan or candesartan. These restrictions are solely based on economic considerations.5
Evidence about which antihypertensive group is more effective is still unclear. Kishore reported that ARB had a better effect on UACR outcome than ACE-I.6 However, several studies have showed no difference in outcomes using ACE-I or ARB.7–9 In contrast, a systematic review and meta-analysis of 110 randomized control trials by Xie et al found that ACE-I was slightly better than ARB in reducing the odds of kidney failure from 39% to 30%.10 Moreover, ACE-I and ARB could cause hyperkalemia, and this can become worse with renal insufficiency and diabetes. Bandak et al reported that ACE-I has a higher risk than ARB in increasing blood potassium levels.11 In our previous study, a cross-sectional study by Agustina et al found that ACE-I and ARB had an equal effect on albuminuria and hyperkalemia in Indonesian hypertensive T2DM patients, even after correction by confounding variables.12 A cross-sectional design does not represent the longitudinal effects of the examined treatments, and so cohort studies are needed to deliver data relating to these issues.
Since the effect of these two groups of drugs is influenced by variability between individuals, an in-depth investigation is needed to assess their effectiveness in the Indonesian population. Therefore, using a prospective cohort study, we aimed to develop more detailed comparisons of the efficacies of ACE-I and ARB specific to Indonesian T2DM patients, measured by UACR and estimated glomerular filtration rate (eGFR), and also by their side effects on increasing blood potassium level (hyperkalemia).
Patients and Methods
A prospective cohort study was conducted at Cipto Mangunkusumo Hospital (RSCM), Jakarta, a national referral hospital in Indonesia, from November 2018 to April 2019. All study subjects were followed for three months.
Patients
This research involved T2DM outpatients with hypertension attending Cipto Mangunkusumo Hospital. Patient enrollment criteria (Figure 1) were patients with ACE-I or ARB prescriptions for at least two months and aged over 18 years. Pregnant or breastfeeding patients, patients taking corticosteroids or birth-control pills, patients with severe liver disease, patients with severe anemia, and patients on dialysis or with ESRD were excluded from this study. During the study, we kept in contact with the study subjects or their caregivers by mobile phone to prevent them from dropping out altogether or missing follow-up sessions. The doctors or nurses involved also reminding them about their monthly visits.
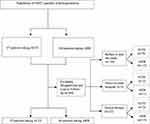 |
Figure 1 Flowchart of patient enrollment. |
Study Approval and Informed Consent
This study was approved by the Ethical Committee of the Medical Faculty, Cipto Mangunkusumo Hospital (FKUI-RSCM), Universitas Indonesia, Jakarta, Indonesia (No. 222/UN2.F1/ETIK/2018) and was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients enrolled had given informed consent.
Clinical Data Collection
The variables analyzed were sociodemographics, BMI, adherence, and clinical data. The clinical data were blood pressure, blood lipids, blood glucose, UACR, and eGFR. Weight was measured with a digital scale and height with a Microtoise. Patient adherence was scored using pill counts and a Morisky, Green, and Levine Medication Adherence Questionnaire (MAQ).13 Blood pressure was measured with a digital blood-pressure monitor and fasting blood glucose with a glucometer. UACR was measured with NycoCard™ U-Albumin (Abbott, USA) and eGFR was calculated using the CKD-EPI equation. Fasting lipid profiles (triglycerides, HDL cholesterol, total cholesterol, LDL cholesterol) and creatinine were measured using ARCHITECT c8000 Clinical Chemistry Analyzer (Abbott, USA).
Statistical Analysis
Data analysis was performed using IBM SPSS statistical software, Version 22 for Windows (IBM, New York, USA). The confidence interval used was 95% with α = 0.05. To handle missing data, we conducted available case analysis. This technique enables the removal of cases from analysis where they have missing values but includes those cases where all values are present. As a result, sample sizes vary between the variables used. Data processing included univariate, bivariate, and multivariate analysis. First, data distribution was examined with the Kolmogorov–Smirnov test. Comparison between the groups (ACE-I, ARB) of baseline data and three-month-data differences were performed using the paired t-test (for parametric data) and the Wilcoxon test (for non-parametric data). Chi-square testing was used to compare the categorical variables. We conducted binomial logistic regression for multivariate analysis. Prior to carrying out multivariate analysis, we performed a variable selection using the bivariate test, in which the selected variables with p value < 0.25 were included in the model. Our study objective is to compare the effects of ACE-I and ARB on urine albumin-to-creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), and blood potassium level. Therefore, we considered using the backward likelihood ratio method for adjusting the confounders in the multivariate analysis. We also conducted goodness-of-fit testing and multicollinearity diagnostics. A p < 0.05 was required for statistically significant consideration.
Results
During the research, 167 participants were enrolled, but 44 were dropped because they refused to participate in the follow-up study, moved to another hospital, or underwent a switch in therapy. A total of 123 outpatients was analyzed (Figure 1). Table 1 shows the basic characteristics of the study subjects. The majority of participants in this study (56.9%) were female, and the average age of the subjects in both groups was below 60. Most of the participants in both the ACE-I and ARB groups were non-smokers. Participants in both groups were mostly unemployed (78.2% of the ACE-I group and 75% of the ARB group). The percentage of academy/university graduates was slightly higher in the ARB group (41.2%) than in the ACE-I group (34.5%). The average duration of DM in subjects who took ACE-I was eight years, shorter than the ARB group (ten years). The average duration of hypertension in the ACE-I group was six years, shorter than the ARB group (ten years). In the ACE-I group, we found that the most-used therapy was oral antidiabetics (41.8%), whereas in the ARB group it was a combination of oral and injection therapy (39.7%). In both groups, patients mostly had a history of using ACE-I/ARB for more than six months before this study. After three months of observation, there were no significant differences in clinical parameters, including UACR, eGFR, and potassium level, except for BMI in the ACE-I group, and LDL and HDL levels in the ARB group (Table 2). Bivariate analysis was used to choose the variables included in multivariate analysis, with p < 0.25 as the significance level for criterion selection. Interaction tests were not performed since bivariate analysis did not identify statistically significant subgroup differences.
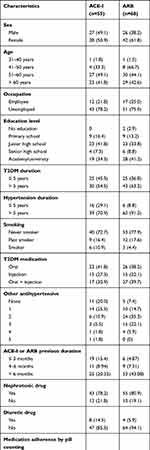 |
Table 1 Baseline Characteristics of Study Subjects |
 |
Table 2 Clinical Characteristics Before and After Observation |
Bivariate analysis showed that there was an insignificant correlation between BMI and eGFR in both groups. However, as BMI and UACR showed a significant correlation the author included BMI into the multivariate analysis. Logistic regression analysis showed that the consumption of ACE-I or ARB was not associated with a decreased/constant in UACR, even after being controlled with confounding variables (Table 3). However, we found that BMI > 25 was a significant factor associated with decreased or maintained UACR. In terms of increased eGFR, we found that ARB was more likely to increase eGFR compared to ACE-I (Table 4). Men were also more likely to have an increase in eGFR (Table 4). However, the significance of this result was lost after the duration of use of ACE-I/ARB was entered in the model. As can be seen in Table 5, neither drug (ACE-I or ARB) was associated with increase in potassium level, even after control by confounding variables. Declining systolic blood pressure was the only significant factor associated with increase in potassium levels. According to goodness-of-fit testing, all models showed p > 0.05, indicating that all models fit the data. Multicollinearity test results also showed that the obtained VIF values were between 1 and 1.5, indicating that there were no multicollinearity symptoms.
 |
Table 3 Multivariate Analysis for Decreased/Constant UACR |
 |
Table 4 Multivariate Analysis for Increased/Constant eGFR |
 |
Table 5 Multivariate Analysis for Increased Potassium Level |
Discussion
The average BMI of study subjects at baseline in both groups was in the overweight range (≥25 kg/m2). The significant BMI increase in the ACE-I group supports a prior study by Parikh et al that found women who consumed ACE-I to have higher waist circumferences.14 The increased BMI over time associated with ACE-I use may be related to improved glucose uptake via translocation of glucose transporters to the cell surface, as previously reported.14,15 The ARB group experienced a decrease in LDL and HDL cholesterol after three months of observation, but still within the normal range. These findings were supported by a previous study which found that ARB has positive effects on lipid profiles.16 It is possible that the lipid-lowering property of ARB is due to a number of mechanisms, including its activation of PPAR-γ, which regulates lipid metabolism.16 Decreased LDL can reduce the risk of coronary artery disease complications, but low HDL cholesterol can increase the risk of worsening the condition in DM patients.17
Most of the patients in this study were middle-aged or elderly and had T2DM and hypertension with a duration of more than five years. Proteinuria is reported as having been present for less than ten years.18 Compliance in taking medication will affect drug levels in the blood and the ability to maintain a constant blood-sugar level, blood pressure, and lipid profile.19 Based on medication-adherence measurement using the pill-counting method and the MAQ questionnaire, we found that our study subjects showed relatively high compliance, thus providing a fairly robust sample in which to analyze therapeutic effectiveness.
Most of the patients were taking metformin in combination with sulfonylurea or insulin, and there was no significant difference in the proportion of metformin users between the two groups. However, given that metformin has an effect on renal function, we put metformin in the “nephrotoxic drug” variable category as a potential confounder. Our study results showed that nephrotoxic drugs did not have a significant effect on eGFR, UACR, and hyperkalemia.
Multivariate analysis by adjusting confounding factors showed no differences in the effect of ACE-I and ARB on UACR (Table 3). We found that BMI > 25 kg/m2 also tended to decrease/maintain a constant UACR compared to BMI ≤ 25 kg/m2. This is not in line with the review of Luther and Brown, who state that the renin-angiotensin-aldosterone system is activated in obesity, thus increase the blood pressure, which in turn increases the risk of microalbuminuria.15 However, in our study, most subjects were overweight, not obese (BMI > 30 kg/m2). We also found that the combination of ARB and being male was significantly more likely to increase eGFR, even after adjusting by confounders (Table 4). However, the duration of ACE-I and ARB treatment eliminated the significance of ARB to the increase in eGFR. Prolonged use of ACE-I/ARB can reduce systemic blood pressure and dilation of efferent arterioles through the renin-angiotensin-aldosterone system inhibitor. Proper control of glomerular pressure can reduce hyperfiltration, improve glomerular filtration rates, and reduce the incidence of microalbuminuria.20 Compared with ACE-I, which plays a role in changing angiotensin-I to angiotensin II, the mechanism of action of ARBs is directly against the angiotensin receptor, enabling angiotensin II to be optimally inhibited.21 Inhibition of angiotensin II by ARB can induce mesangial constriction and increase the glomerular filtration coefficient, thereby more potential in increasing eGFR.22
Men were also shown to be more likely to increase or maintain a constant eGFR than women (Table 4). Among men, higher baseline sex-hormone-binding globulin (SHBG) level was associated with reduced eGFR risk.23 In addition, nitric oxide (NO) in renal arteries in women was higher and oxidative stress was lower compared to male T2DM patients.24 However, men have skeletal muscle mass higher than women25 and lower muscle mass in older men is likely to lead to an overestimation of eGFR calculation.26
In addition, there was no significant difference in the effect of ACE-I and ARB on potassium levels. Only declined systolic blood pressure was more likely to increase the potassium level. This mechanism seems to be related to a side effect of ACE-I and ARB in causing hyperkalemia. Hyperkalemia is an acute electrolyte abnormality that has the potential to cause life-threatening arrhythmias.27 The effect of antihypertensive treatments on decreasing systolic blood pressure is associated with the renin-angiotensin-aldosterone system, which decreases aldosterone secretion and increases potassium levels, and this could be a cautionary point for long-term use.28
This study was an observational study, and we therefore could not adjust the drug types and doses administered. However, we conducted the study only on a single site and only collected data from outpatients who received similar types and doses of drugs within the three months of observation. The given types of drugs and doses were prescribed according to the patients’ conditions and hospital formulary and treatment guidelines. Change in blood pressure as the clinical outcome of the antihypertensive given to the patients was considered as a confounding variable and included in the multivariate analysis. The prospective cohort nature of our study design is one of our study’s advantages. In addressing compliance issues, we collected medication-adherence data using two instruments: the MAQ questionnaire and the pill-counting method and applied multivariate analysis. Our study subjects showed relatively high compliance. Therefore, our study results could minimize the bias effect of patients’ compliance.
In many countries, ARB is more preferable than ACE-I. However, in lower and middle-income countries, ACE-I is still more widely used, due to economic consideration. Therefore, the results of this study can serve as a decision-making tool particularly for the lower and middle-income countries government.
Conclusion
ACE-I and ARB had a similar effect on UACR and blood potassium level, but ARB was slightly more likely to increase eGFR compared to ACE-I within three months of consumption.
Acknowledgments
The authors are grateful to PUTI KI Grant from Directorate of Research and Development Universitas Indonesia (No. NKB-752/UN2.RST/HKP.05.00/2020) for financial support. We also thank patients at Cipto Mangunkusumo Hospital, Jakarta, Indonesia for their great contribution to this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Indonesian Renal Registry. 10th Report of Indonesian Renal Registry 2017; 2017. Available from: https://www.indonesianrenalregistry.org/.
2. Rachmaini F, Amalia L, Rahayu C. Therapeutic Profile of Antihypertensive and Antihyperlipidemia Towards Kidney Function in Type 2 Diabetes Mellitus Patients with Chronic Kidney Disease Complication at Dr. Hasan Sadikin Hospital. Pharm Sci Res. 2020;7(1). doi:10.7454/psr.v7i1.1066
3. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
4. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020 [published correction appears in Diabetes Care. 2020 Aug;43(8):1977–1978]. Diabetes Care. 2020;43(Suppl 1):S111–S134. doi:10.2337/dc20-S010
5. Decree of Ministry of Health Republic of Indonesia No. HK.01.07/MENKES/813/2019 on National Formulary; 2019. Available from: https://jdih.kemkes.go.id.
6. Kishore P. Comparison of efficacy of ARB’S and ACEI’S in treatment of microalbuminuria with type II diabetes. World J Pharm Pharm Sci. 2017;1850–1859. doi:10.20959/wjpps20178-9840
7. Xu R, Sun S, Huo Y, et al. Effects of ACEIs versus ARBs on proteinuria or albuminuria in primary hypertension: a meta-analysis of randomized trials. Medicine (Baltimore). 2015;94(39):e1560. doi:10.1097/MD.0000000000001560
8. Tahir M, Hassan WU, Muhamamd H, Alam A. Angiotensin II receptor blockers (ARBs) in management of micro albuminuria among normotensive type 2 diabetic patients. PAFMJ. 2016;66(2):258–261.
9. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–1444. doi:10.1136/bmj.321.7274.1440
10. Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian Network Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis. 2016;67(5):728–741. doi:10.1053/j.ajkd.2015.10.011
11. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6(7):e005428. doi:10.1161/JAHA.116.005428
12. Agustina PS, Yunir E, Prawiroharjo P, Damanik J, Sauriasari R. Comparison of effects of ACEIs and ARBs on albuminuria and hyperkalemia in Indonesian hypertensive type 2 diabetes mellitus patients. Int J Hypertens. 2020;2020:5342161. doi:10.1155/2020/5342161
13. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
14. Parikh JS, Randhawa AK, Wharton S, Edgell H, Kuk JL. The association between antihypertensive medication use and blood pressure is influenced by obesity. J Obes. 2018;2018:4573258. doi:10.1155/2018/4573258
15. Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32(12):734–739. doi:10.1016/j.tips.2011.07.006
16. Kyvelou SM, Vyssoulis GP, Karpanou EA, et al. Effects of antihypertensive treatment with angiotensin II receptor blockers on lipid profile: an open multi-drug comparison trial. Hellenic J Cardiol. 2006;47(1):21–28.
17. Tarigan T, Yunir E, Subekti I, Pramono L, Martina D. Profile and analysis of diabetes chronic complications in outpatient diabetes clinic of Cipto Mangunkusumo Hospital, Jakarta. Med J Indones. 2015;24(3):156–162. doi:10.13181/mji.v24i3.1249
18. Abdulqawi Ali A, Abdullatif Daiffallah A, Hussein AJ. Prevalence of proteinuria among type 2 diabetic patients in Dhamar Governorate, Yemen. Int J Diabetes Clin Res. 2019;6(2). doi:10.23937/2377-3634/1410106
19. Hening WN, Sartika RAD, Sauriasari R. Effect of hospital pharmacist counseling on clinical outcomes of type 2 diabetes mellitus outpatients. J Res Pharm Pract. 2019;8(3):155–161. doi:10.4103/jrpp.JRPP_19_67
20. Choe EY, Wang HJ, Kwon O, et al. Variants of the adiponectin gene and diabetic microvascular complications in patients with type 2 diabetes [published correction appears in Metabolism. 2017 Feb;67:115]. Metabolism. 2013;62(5):677–685. doi:10.1016/j.metabol.2012.11.005
21. Dézsi CA. Differences in the clinical effects of angiotensin-converting enzyme inhibitors and Angiotensin receptor blockers: a critical review of the evidence. Am J Cardiovasc Drugs. 2014;14(3):167–173. doi:10.1007/s40256-013-0058-8
22. Kobori H, Mori H, Masaki T, Nishiyama A. Angiotensin II blockade and renal protection. Curr Pharm Des. 2013;19(17):3033–3042. doi:10.2174/1381612811319170009
23. Kim C, Ricardo AC, Boyko EJ, et al. Sex hormones and measures of kidney function in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2019;104(4):1171–1180. doi:10.1210/jc.2018-01495
24. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi:10.1161/01.HYP.0000107251.49515.c2
25. Nankivell BJ, Nankivell LFJ, Elder GJ, Gruenewald SM. How unmeasured muscle mass affects estimated GFR and diagnostic inaccuracy. EClinicalMedicine. 2020;29–30:100662. doi:10.1016/j.eclinm.2020.100662
26. Tap L, Boyé NDA, Hartholt KA, van der Cammen TJM, Mattace-Raso FUS. Association of estimated glomerular filtration rate with muscle function in older persons who have fallen. Age Ageing. 2018;47(2):269–274. doi:10.1093/ageing/afx180
27. Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):e156–e166. doi:10.1111/j.1755-5922.2010.00258.x
28. Sousa AG, Cabral JV, El-Feghaly WB, De sousa LS, Nunes AB. Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J Diabetes. 2016;7(5):101–111. doi:10.4239/wjd.v7.i5.101
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
