Back to Journals » Drug Design, Development and Therapy » Volume 14
Edaravone Ameliorates Renal Warm Ischemia-Reperfusion Injury by Downregulating Endoplasmic Reticulum Stress in a Rat Resuscitation Model
Authors Fu ZY , Wu ZJ, Zheng JH , Li N , Lu JY, Chen MH
Received 17 April 2019
Accepted for publication 5 December 2019
Published 15 January 2020 Volume 2020:14 Pages 175—183
DOI https://doi.org/10.2147/DDDT.S211906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Zhao-Yin Fu, Zhi-Jiang Wu, Jun-Hui Zheng, Nuo Li, Jun-Yu Lu, Meng-Hua Chen
Department of Critical Care Medicine, Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530007, People’s Republic of China
Correspondence: Meng-Hua Chen
Department of Critical Care Medicine, Second Affiliated Hospital of Guangxi Medical University, 166 Daxuedong Road, Nanning, Guangxi 530007, People’s Republic of China
Tel +86 771 327 7186
Email [email protected]
Background: This study was conducted to explore whether the effect of edaravone (5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol3-one, EDR) can ameliorate renal warm ischemia-reperfusion injury (IRI) by modulating endoplasmic reticulum stress (ERS) and its downstream effector after cardiac arrest (CA) and cardiopulmonary resuscitation (CPR) in a rat model.
Methods: The rats (n=10) experienced anaesthesia and intubation followed by no CA inducement were defined as the Sham group. Transoesophageal alternating current stimulation was employed to establish 8 min of CA followed by conventional CPR for a resuscitation model. The rats with successful restoration of spontaneous circulation (ROSC) randomly received EDR (3 mg/kg, EDR group, n=10) or equal volume normal saline solution (the NS group, n=10). At 24 hr after ROSC, serum creatinine (SCR), blood urea nitrogen (BUN) levels, and cystatin-C (Cys-C) levels were determined and the protein level of glucose-regulated protein (GRP78), C/EBP homologous protein (CHOP), extracellular signal-regulated kinase (ERK), phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), Bax/Bcl-2, and caspase-3 were detected by Western blot method.
Results: At 24 hrs after ROSC, SCR, BUN and Cys-C were obviously increased and the proteins expression, including GRP78, CHOP and p-ERK1/2, cleaved-caspase 3 Bax/Bcl-2 ratio, were significantly upregulated in the NS group compared with the Sham group (p< 0.05). The remarkable improvement of these adverse outcomes was observed in the EDR group (p< 0.05).
Conclusion: In conclusion, we found that EDR ameliorates renal warm IRI by downregulating ERS and its downstream effectors in a rat AKI model evoked by CA/CPR. These data may provide evidence for future therapeutic benefits of EDR against AKI induced by CA/CPR.
Keywords: renal warm ischemia-reperfusion injury, edaravone, endoplasmic reticulum stress, cardiac arrest, cardiopulmonary resuscitation
Introduction
During ischemia, multiple signalling pathways, which are closely relative with inflammatory and metabolic, play pro-apoptosis roles in cells.1 However, the subsequent blood perfusion restoration may course more severe injury known as ischemia-reperfusion injury (IRI). Renal ischaemia-reperfusion injury (IRI) is known as one of the most common causes of acute kidney injury (AKI) and secondary to various clinical conditions, such as kidney grafting and resuscitation.2,3 Reactive oxygen species (ROS) play an important role in the development of IRI. It has been reported that ROS burst induces endoplasmic reticulum stress (ERS), mitogen-activated protein kinases (MAPK) and cell death.4,5
ER is an intracellular organelle that plays a pivotal role in protein synthesis and folding, Ca2+ storage and signalling.6 As ER is stimulated by Ca2+ overload, ischaemia or hypoxia, its homeostasis changes followed by ERS.7 Growing evidences showed that when the stimuli is excessive or persistent, ERS of renal tubule epithelial cells is an initial response and plays a major pathogenic role in renal IRI.8–10 Thus, ERS inhibition may be a novel treatment for renal IRI.
Extracellular signal-regulated kinase 1/2 (ERK1/2) is a MAPK that is phosphorylated rapidly following renal injury.11 As a downstream effector mechanism of ERS, activation of ERK1/2 has been suggested to be a regulator of renal IRI.12–15 For example, ERS may course cell death at least by a classical BAX/BAK-dependent apoptotic response that can be inhibited by the ERK1/2 signalling pathway.16 However, it is unclear whether the ERS could modulate the ERK signalling pathways to ameliorate renal warm IRI evoked by the process of cardiac arrest/cardiopulmonary resuscitation (CA/CPR).
Edaravone (5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol3-one, EDR) is a novel free-radical scavenger that has been shown to prevent ERS induced by hypoxia and ischaemia.17,18 Although EDR is identified most recently as a protective factor for the development of renal IRI caused by renal arterial or hilar clamping,19–21 poor evidence can answer that whether EDR has the same protective effect in renal warm IRI evoked by CA/CPR. Experimental evidence suggests that GRP78 and CHOP activation is correlated with apoptosis as an ERS downstream event in renal IRI, and ERK activation is an important downstream mechanism of ERS. Therefore, we aimed to verify the hypothesis that EDR play a protective role on renal IRI by downregulated GRP78/CHOP/ERK pathway in a rat CA/CPR model.
Materials and Methods
Preparation of Experimental Rats
This animal study was approved by the Animal Ethics Committee of Guangxi Medical University (Animal Experimental Ethical Inspection no. 201811030). All animals received treatment in strict adherence to the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals. Anaesthetics were titrated in all surgical procedures to avoid unnecessary pain. Male Sprague-Dawley rats weighing 200–230 g were purchased from the Experimental Animal Center of Guangxi Medical University (China, Nanning). Animals were maintained at constant temperature (23 ± 2°C) with a 12 h light-dark cycle and free access to water and food.
Experimental Cardiac Arrest Rat Model
Animal Preparation
All rats fasted for 12 h but had free access to water before the operation. Experimental rats were intraperitoneally injected with sodium pentobarbital (45 µg/g) for anaesthesia, and an additional dose of 10 µg/g was supplemented at hourly intervals. Standard Lead II Electrocardiograph was used to monitor heart rhythm. A twenty-gauge catheter containing 5 IU/mL of sodium heparin saline was inserted into the right femoral vein for drug delivery, and another identical catheter was inserted into the right femoral artery for haemodynamic monitoring. Pressure transducers were connected to a four-channel physiological recorder (BL-420 E Biosystems, Chengdu Technology & Market Co. Ltd., China). After the 5 mins baseline electrocardiograph and physiologic measurements, temperature probes were placed into the rectum. During the experiment, the rectal temperature was adjusted to approximately 37°C using a heat lamp or ice pack.
Renal Warm Ischemia-Reperfusion Injury Induced by the Cardiac Arrest/Cardiopulmonary Resuscitation Model
The rat cardiac arrest (CA) model was established according to our previously reported method.22 Briefly, CA was induced by alternating current (12 V) from a stimulator through a pacing electrode placed in the oesophagus, as confirmed by a decrease in mean arterial pulse pressure (<10 mmHg) and by the appearance of asystole on the electrocardiograph (ECG). Cardiopulmonary resuscitation was initiated 8 min after the induction of CA with mechanical chest compressions (180 per minute) and effective ventilation (TV 8 mL/kg, respiration rate 40/min, and positive end-expiratory pressure 0 cm H2O, oxygen concentration 100%) using a small animal ventilator with capacity control mode. After 1 min of CPR, one dose of epinephrine (0.4 µg/g) was given through the left femoral vein catheter. When ROSC was clarified by ECG activity with visible systole23 and mean arterial pressure (MAP) ≥ 50 mmHg for ≥1 min, chest compressions were stopped. If ROSC is not achieved within 3 min of the onset of cardiopulmonary resuscitation, it is defined as a failure, and the animal is excluded from the study. After achieving ROSC, rats randomly received edaravone (3 mg/kg, n=10, EDR group) or equal volume normal saline solution (n=10, NS group). The sham-operated rats only received the same experimental preparation without CA induction (n=10). The rats were individually fed in cages with dry litter and placed in a quiet room with air conditioning-adjusted temperature (room temperature 26°C).
Renal Function Analysis
The serum of the experimental rats was taken from the carotid artery at 24 h after ROSC. The values of serum creatinine (SCR), blood urea nitrogen (BUN), and cystatin-C (Cys-C) were monitored in the Department of Laboratory, the Second Affiliated Hospital of Guangxi Medical University.
Western Blot Analysis
Rats from each experimental group were anaesthetized and then sacrificed to take kidneys for Western blot detection at 24 hrs after ROSC. The expression levels of GRP 78, p-PERK, and CHOP in renal tissues were assessed by Western blot analysis. The prepared kidney tissues were weighed and homogenized in a glass homogenizer containing 1:10 (w/v) ice-cold whole cell lysis buffer (Beyotime Biotechnology, China, P0013B). The lysed protein was collected and centrifuged at 14,000 × g for 15 mins at 4°C. The BCA Protein Assay Kit (Beyotime Biotechnology, China, P0010) was used to determine total tissue protein concentration. The tissue total protein (10–20 µL) of protein lysates was separated by 10–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membranes (Millipore, USA, 0.22-μm pore diameter). The membranes were blocked with PBST containing 5% bovine serum albumin for 1 h and then incubated with primary antibody overnight at 4°C. β-Actin was used for normalization. The primary antibodies were as follows: primary antibodies ERK 1/2 (ab184699) and p-ERK1/2 (ab76299) were purchased from Abcam Plc, Cambridge, UK, and MFN2(11925). β-Actin (CST, 4970S), GRP 78 (CST, 3183S), CHOP (CST, 2895S), Bax (CST, 14796S), Bcl-2 (Abcam, 182858), and GAPDH (Abcam, 181602). The membrane was washed three times with PBST and then incubated with secondary antibody (Cell Signalling Technology, USA, #5151, 1:15,000). Membranes were quantified by using a Western blot detection system with a Li-cor Odyssey Scanner imaging densitometer, and the results of the bands detected were quantified using ImageJ software (v1.33, NIH, Bethesda, MD, USA).
Statistical Analysis
All data are expressed as the mean ± standard deviations. Statistical analysis software is SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables between groups were compared using the Student’s t-test. Groups were compared using one-way ANOVA followed by the Student–Newman–Keul test for post hoc comparisons. p < 0.05 was considered statistically significant.
Results
Edaravone Improves Renal Function After CA/CRP
To examine the kidney function, we compared Cys-C, SCR, BUN and Cys-C in all groups. The values of Cys-C, SCR and BUN were significantly increased in NS group compared with the Sham group (p < 0.05), suggesting an adverse outcome of renal function. Treatment of edaravone obviously decreased the level of Cys-C, SCR and BUN (p < 0.05), suggesting a protective effect on renal function (Figure 1).
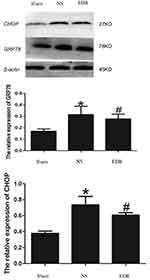
EDR Reduced Endoplasmic Reticulum Stress Induced by CA/CPR
We perform the Western blot detection to determine the effect of EDR on the endoplasmic reticulum stress (ERS) induced by CA/CPR. The result showed that the expression of glucose-regulated protein (GRP78) and C/EBP homologous protein (CHOP) presented remarkable upregulation in the NS group (p < 0.05), while treatment of EDR significantly reduced the level of GRP78 and CHOP (p < 0.05). (Figure 2).
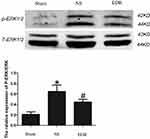
EDR Inhibits Phosphorylation of Extracellular Signal-Regulated Kinase 1/2
As shown in Figure 3, compared with the sham group, p-ERK1/2 was significantly elevated in the NS group (p < 0.05), while the expression of p-ERK1/2 was reduced in EDR group (p < 0.05).
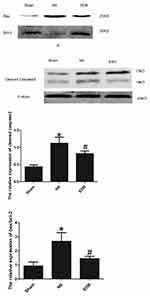
EDR Decreases Caspase-3 and the Bax/Bcl-2 Ratio
Compared with the Sham group, cleaved caspase-3 and the Bax/Bcl-2 ratio were significantly upregulated in the NS group (p < 0.05); by the contrast, the expression of cleaved caspase-3 and the Bax/Bcl-2 ratio were significantly decreased in the EDR group. (p < 0.05). (Figure 4).
Discussion
In this study, we found that renal warm ischaemia/reperfusion injury (IRI) induced by cardiac arrest/cardio-pulmonary resuscitation (CA/CPR) substantially upregulated the expression of Glucose Regulated Protein 78 (GRP78) and C/EBP-homologous protein (CHOP) at 24 hrs post-ROSC. EDR treatment ameliorated renal dysfunction and protected against renal damage, including a significant reduction in SCR, BUN, and Cys-C. Compared to the NS group, GRP78, CHOP, p-ERK1/2, caspase-3 expression and Bax/Bcl-2 ratio were significantly decreased in the EDR group, suggesting protective effect of EDR against endoplasmic reticulum stress (ERS) and apoptosis.
In our previous study, rats subjected to CA/CPR presented with excessive ROS production in the brain tissue.5 Overdose of ROS-induced ERS, which plays an important role in the development of several organ IRI in rats.24–26 while EDR can be used to reduce or block ROS-induced ERS to reduce organ IRI.27 Hence, the purpose of our current study is to investigate the renal protective potential of EDR, a potent free-radical scavenger against renal warm IRI induced by CA/CPR.
The current data are consistent with previous results demonstrating that EDR pre-treatment protects against warm IRI in a variety of tissues and organs, including the heart and brain.28,29 In addition, a recent clinical study suggests that EDR may be a useful medication to protect kidney function in patients with acute ischaemic stroke.21 Overall, our findings are consistent with recently published data demonstrating that EDR exerts beneficial effects against organ ischaemic damage, including renal IRI.30 As mentioned in the previous study, the animal model of the protective effect of EDR on renal IRI is mostly due to clamping of the renal artery or renal pedicle. However, there may be high incidence of vascular injury, infection, hilar injury, venous congestion, cold renal ischaemia during animal operation, and the observation period is only at the early phase of reperfusion.31–33 Importantly, these models did not fully mimic the most common clinical features of AKI after ROSC. Hence, we used the methods of transoesophageal alternating current stimulation to establish 8 min of CA in a rat model followed by conventional CPR to avoid the adverse effects and create a model of renal warm IRI that is close to clinical characteristics.
As an ERS downstream event, activation of GRP78/CHOP pathway is the key cellular response of ERS-induced apoptosis.9,34,35 Downregulation of GRP78/CHOP by intermedin significantly decreased apoptosis.10 In addition, a previous study revealed that EDR ameliorated early renal dysfunction and injury evoked by ischaemia/reperfusion in mice subjected to 45 min of bilateral renal IRI.33 However, it is unclear whether EDR exerts protection against ERS in renal warm IRI evoked by CA/CPR. Our current research showed that EDR can ameliorate renal warm IRI by down-regulating GRP78 and CHOP expression, which was similar with previous studies regarding with EDR protection on dilated cardiomyopathy, spatial memory,28,29 cerebral ischaemia36 and autoimmune myocarditis.37
Studies have suggested that EDR can alleviate oxidative damage by inhibiting ERK1/2 activation.38,39 ERK1/2 is a member of the MAPK family, which plays an important role in cell survival and death.40 Inhibition of ERK by EDR can alleviate oxidative damage. What is more, the ERK1/2 signalling pathway has also been shown to be involved in ERS-mediated apoptosis.41,42 Studies have demonstrated that IR dramatically increases phosphorylated ERK1/2 levels in the kidney.43,44 In addition, multiple studies proved that ERK activation was commonly protective in renal IRI.44,45 By the contrast, IRI dramatically increased phosphorylated ERK1/2 expression and promoted apoptosis have also been reported.46 In our present work, we found that the expression of phosphorylated ERK1/2 was significantly elevated which accompanied with renal function decrease at 24 h after ROSC in NS group. This result was consistent with our previous research, in which we found that inhibiting the expression of phosphorylated ERK1/2 can protect the brain from IRI in rat experienced CA/CPR.47
Apoptosis is widely considered to be the main mechanism that induces cell death in renal IRI.48,49 Bax and caspase-3 are the most important downstream effectors for the ERK pathway50,51 that have also been demonstrated to involve in ERS-induced apoptosis.52–54 In renal cells, ischaemia activates Bax55 and inhibits Bcl-2,56 resulting in an increased Bax/Bcl-2 ratio.57 On the other hand, caspases are well-known drivers of apoptotic cell death, cleaving cellular proteins that provide critical links in cell regulatory networks controlling dying cells.58 Active caspase-3 leads to DNA fragmentation and formation of apoptotic bodies.59 Caspase-3 and Bax have been demonstrated to mediate ERS-induced apoptosis.52–54 Our previous study demonstrated that inhibition of ischaemia-induced ERK1/2 kinase activity can reduce Bax and caspase-3 expression and improve organ function.47 In the present study, we showed that EDR treatment decreased phosphorylation of ERK1/2 and apoptotic parameters including caspase-3 and the Bax/Bcl-2 ratio. These results suggested a reno-protective effect of EDR on a CA/CPR model. As mentioned previously, ERK activation is an important downstream mechanism of ERS.12 We hold the opinion that EDR acts as an anti-apoptotic agent by downregulating GRP78/CHOP/ERK signal pathway.
Graphene was discovered in 2004 and its application in nanomaterials has been developing rapidly for drug delivery.60 Graphene nanomaterials can enhance the efficacy in chemotherapy applications,61 increase drug loading capacity,62 and presents no toxicity to cells.63 In addition, some improved graphene nanomaterials are able to cross the cell membrane and then accumulated more in the cell cytoplasm compared with the traditional ones. The capacity of transmembrane makes it possible to delivery drug to the targeted organ or cells more effectively.64 Furthermore, a series of triggered drug delivery systems consist of graphene have been reported. The systems realize controlling drug delivery remotely and adjusting dosing regimens on demand.65,66 Therefore, it is worth to determine whether delivery of EDR targeting renal with graphene nanomaterials could obtain a better outcome in renal warm ischemia-reperfusion injury post CA/CPR.
In conclusion, we found that EDR ameliorates renal warm IRI by downregulating ERS and its downstream effectors in a rat AKI model evoked by CA/CPR. These data may provide evidence for future therapeutic benefits of EDR against AKI induced by CA/CPR.
Ethics Approval and Consent to Participate
The present study was approved by the Committee on the Ethics of Animal Experiments and Human Subject Research of the Guangxi Medical University.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The research was supported by the National Natural Science Foundation of China (grant no.81860333) and Scientific Research and Technological Development Project of Qinzhou City (grant no.201616814).
Disclosure
Zhao-yin Fu reports grants from National Natural Science Foundation of China and Scientific Research and Technological Development Project of Qinzhou City, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Antonia L, Pasquale D, Antonio C, et al. Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: differential modulation by rapamycin. J Am Soc Nephrol. 2004;15(10):2675. doi:10.1097/01.ASN.0000139932.00971.E4
2. Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to risk/injury/failure/loss/end-stage, acute kidney injury network, and kidney disease: improving global outcomes classifications. J Crit Care. 2013;28(4):389–396. doi:10.1016/j.jcrc.2012.12.008
3. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364(9447):1814–1827. doi:10.1016/S0140-6736(04)17406-0
4. Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol. 2009;112(1):e1–e9. doi:10.1159/000210573
5. Nguyen Thi PA, Chen MH, Li N, Zhuo XJ, Xie L. PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev. 2016;2016(10):1–13. doi:10.1155/2016/3723762
6. Bravo R, Parra V, Gatica D, et al. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301(301):215–290.
7. Asma MB, Mohamed Amine Z, Thierry H, et al. Attenuation of endoplasmic reticulum stress and mitochondrial injury in kidney with ischemic postconditioning application and trimetazidine treatment. J Biomed Sci. 2012;19(1):71. doi:10.1186/1423-0127-19-71
8. Montie HL, Kayali F, Haezebrouck AJ, Rossi NF, Degracia DJ. Renal ischemia and reperfusion activates the eIF 2 alpha kinase PERK. Biochim Biophys Acta. 2005;1741(3):314–324. doi:10.1016/j.bbadis.2005.04.007
9. Mi RN, Kim JI, Sang JH, Lee TJ, Park KM. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim Biophys Acta. 2015;1852(9):1895–1901. doi:10.1016/j.bbadis.2015.06.004
10. Wang Y, Tian J, Qiao X, et al. Intermedin protects against renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress. BMC Nephrol. 2015;16(1):169. doi:10.1186/s12882-015-0157-7
11. Masaki T. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol. 2004;15(7):1835–1843. doi:10.1097/01.ASN.0000130623.66271.67
12. Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278(31):29317–29326. doi:10.1074/jbc.M302368200
13. Alderliesten M, De GM, Oldenampsen J, et al. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol. 2007;171(2):452–462. doi:10.2353/ajpath.2007.060805
14. Choi DE, Jeong JY, Choi H, et al. ERK phosphorylation plays an important role in the protection afforded by hypothermia against renal ischemia-reperfusion injury. Surgery. 2017;161(2):444–452. doi:10.1016/j.surg.2016.07.028
15. Liu WH, Liu HB, Gao DK, et al. ABCG2 protects kidney side population cells from hypoxia/reoxygenation injury through activation of the MEK/ERK pathway. Cell Transplant. 2013;22(10):1859–1868. doi:10.3727/096368912X657206
16. Darling NJ, Balmanno K, Cook SJ. ERK1/2 signalling protects against apoptosis following endoplasmic reticulum stress but cannot provide long-term protection against BAX/BAK-independent cell death. PLoS One. 2017;12(9):e0184907. doi:10.1371/journal.pone.0184907
17. Kikuchi K, Takeshige N, Miura N, et al. Beyond free radical scavenging: beneficial effects of edaravone (Radicut) in various diseases. Exp Ther Med. 2012;3(1):3. doi:10.3892/etm.2011.352
18. Qi X, Okuma Y, Hosoi T, Nomura Y. Edaravone protects against hypoxinlschemia-lnduced endoplasmic reticulum dysfunction. J Pharmacol Exp Ther. 2004;311(1):388–393. doi:10.1124/jpet.104.069088
19. Tahara M, Nakayama M, Jin MB, et al. A radical scavenger, edaravone, protects canine kidneys from ischemia-reperfusion injury after 72 hours of cold preservation and autotransplantation. Transplantation. 2005;80(2):213–221. doi:10.1097/01.TP.0000165092.07375.C9
20. Matsuyama M, Hayama T, Funao K, et al. Treatment with edaravone improves the survival rate in renal warm ischemia-reperfusion injury using rat model. Transplant Proc. 2006;38(7):2199–2200. doi:10.1016/j.transproceed.2006.06.077
21. Kamouchi M, Sakai H, Kiyohara Y, Minematsu K, Hayashi K, Kitazono T. Acute kidney injury and edaravone in acute ischemic stroke: the Fukuoka Stroke Registry. J Stroke Cerebrovasc Dis. 2013;22(8):e470–e476. doi:10.1016/j.jstrokecerebrovasdis.2013.05.018
22. Chen M-H, Liu T-W, Xie L, et al. A simpler cardiac arrest model in rats. Am J Emerg Med. 2007;25(6):623–630. doi:10.1016/j.ajem.2006.11.033
23. Deng G, Yonchek JC, Quillinan N, et al. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J Neurosci Methods. 2014;222:34–41. doi:10.1016/j.jneumeth.2013.10.015
24. Jassem W, Heaton ND. The role of mitochondria in ischemia|[sol]|reperfusion injury in organ transplantation. Kidney Int. 2004;66(2):514–517. doi:10.1111/j.1523-1755.2004.761_9.x
25. Toth A, Nickson P, Mandl A, Bannister ML, Toth K, Erhardt P. Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc Haematol Disord. 2007;7(3):205–218.
26. Hayashi T, Saito A, Okuno S, Ferranddrake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25(1):41–53. doi:10.1038/sj.jcbfm.9600005
27. Srinivasan K, Sharma SS. Edaravone offers neuroprotection in a diabetic stroke model via inhibition of endoplasmic reticulum stress. Basic Clin Pharmacol Toxicol. 2012;110(2):133–140. doi:10.1111/j.1742-7843.2011.00763.x
28. Arumugam S, Thandavarayan RA, Veeraveedu PT, et al. Beneficial effects of edaravone, a novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol Med. 2012;16(9):2176–2185. doi:10.1111/jcmm.2012.16.issue-9
29. Tian A, Ma H, Zhang R, Cui Y, Wan C. Edaravone improves spatial memory and modulates endoplasmic reticulum stress-mediated apoptosis after abdominal surgery in mice. Exp Ther Med. 2017;14(1):355–360. doi:10.3892/etm.2017.4489
30. Karaa M, Kuyucu Y, Tuli A, Tap O. The effect of edaravone on ischemia–reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):197–202. doi:10.1016/j.ejogrb.2012.02.026
31. Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012;83(6):721–727. doi:10.1016/j.resuscitation.2011.11.030
32. Di PR, Impellizzeri D, Mondello P, et al. Palmitoylethanolamide reduces early renal dysfunction and injury caused by experimental ischemia and reperfusion in mice. Shock. 2012;38(4):356–366. doi:10.1097/SHK.0b013e318267bbb9
33. Chiazza F, Chegaev K, Rogazzo M, et al. A nitric oxide-donor furoxan moiety improves the efficacy of edaravone against early renal dysfunction and injury evoked by ischemia/reperfusion. Oxid Med Cell Longev. 2015;2015:804659. doi:10.1155/2015/804659
34. Wang CL, Liu C, Niu LL, Wang LR, Hou LH, Cao XH. Surfactin-induced apoptosis through ROS-ERS-Ca2+-ERK pathways in HepG2 cells. Cell Biochem Biophys. 2013;67(3):1433–1439. doi:10.1007/s12013-013-9676-7
35. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi:10.1038/nature07203
36. Qi X, Okuma Y, Hosoi T, Nomura Y. Edaravone protects against hypoxia/ischemia-induced endoplasmic reticulum dysfunction. J Pharmacol Exp Ther. 2004;311(1):388–393. doi:10.1124/jpet.104.069088
37. Shimazaki H, Watanabe K, Veeraveedu PT, et al. The antioxidant edaravone attenuates ER-stress-mediated cardiac apoptosis and dysfunction in rats with autoimmune myocarditis. Free Radic Res. 2010;44(9):1082–1090. doi:10.3109/10715762.2010.499904
38. Kawasaki T, Kitao T, Nakagawa K, et al. Nitric oxide‐induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2010;55(13):1325–1333. doi:10.1002/glia.20541
39. Zhao ZY, Luan P, Huang SX, et al. Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci Ther. 2013;19(3):163–169. doi:10.1111/cns.12044
40. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi:10.1016/j.bbadis.2009.12.009
41. Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279(47):49420–49429. doi:10.1074/jbc.M407700200
42. Cheng-Chieh H, Takaharu I, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278(31):29317–29326. doi:10.1074/jbc.M302368200
43. Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277(3):2040–2049. doi:10.1074/jbc.M107525200
44. Zou YR, Zhang J, Wang J, Peng L, Li GS, Wang L. Erythropoietin receptor activation protects the kidney from ischemia/reperfusion-induced apoptosis by activating ERK/p53 signal pathway. Transplant Proc. 2016;48(1):217–221. doi:10.1016/j.transproceed.2016.01.009
45. Kyriazis I, Kagadis GC, Kallidonis P, et al. PDE5 inhibition against acute renal ischemia reperfusion injury in rats: does vardenafil offer protection? World J Urol. 2013;31(3):597–602. doi:10.1007/s00345-012-0980-4
46. Mr VDB, Kapeller R, Pratt JC, Chang JH, Burakoff SJ. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J Biol Chem. 1999;274(16):11178–11185. doi:10.1074/jbc.274.16.11178
47. Nguyen Thi PA, Chen MH, Li N, Zhuo XJ, Xie L. PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev. 2016;2016:3723762. doi:10.1155/2016/3723762
48. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Chapter Six – cell Biology of Ischemia/Reperfusion Injury. Int Rev Cell Mol Biol. 2012;298:229–317.
49. Mori S, Sawada T, Okada T, Kubota K. Erythropoietin and its derivative protect the intestine from severe ischemia/reperfusion injury in the rat. Surgery. 2008;143(4):556–565. doi:10.1016/j.surg.2007.12.013
50. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2010;58(11):621–631. doi:10.1080/15216540600957438
51. Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for αMβ2 integrin clustering or activation in the control of apoptosis via regulation of Akt and ERK survival mechanisms. J Cell Biol. 2000;151(6):1305–1320. doi:10.1083/jcb.151.6.1305
52. Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403(6765):98–103. doi:10.1038/47513
53. Jimbo A, Fujita E, Kouroku Y, et al. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp Cell Res. 2003;283(2):156–166. doi:10.1016/S0014-4827(02)00033-2
54. Zong WX, Chi L, Georgia H, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162(1):59–69. doi:10.1083/jcb.200302084
55. Havasi A, Li Z, Wang Z, et al. Hsp27 inhibits bax activation and apoptosis via a phosphatidylinositol 3-Kinase-dependent mechanism. J Biol Chem. 2008;283(18):12305–12313. doi:10.1074/jbc.M801291200
56. Chien CT, Chang TC, Tsai CY, Shyue SK, Lai MK. Adenovirus-mediated bcl-2 gene transfer inhibits renal ischemia/reperfusion induced tubular oxidative stress and apoptosis. Am J Transplant. 2015;5(6):1194–1203.
57. Dae Eun C, Young JJ, Beom Jin L, et al. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297(2):362–370. doi:10.1152/ajprenal.90609.2008
58. Wigle JC, Estlack LE, Schuster KJ. Retinal illumination to protect against laser damage. Spienewsroom. 2013.
59. Cao G, Pei W, Lan J, et al. Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci. 2001;21(13):4678. doi:10.1523/JNEUROSCI.21-13-04678.2001
60. Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Controlled Release. 2014;173(1):75–88. doi:10.1016/j.jconrel.2013.10.017
61. Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6(4):537–544. doi:10.1002/smll.v6:4
62. Yang X, Zhang X, Ma Y, Yi H, Wang Y, Chen Y. Superparamagnetic graphene oxide–fe3O4nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem. 2009;19(18):2710–2714. doi:10.1039/b821416f
63. Pan Y, Bao H, Sahoo NG, Wu T, Li L. Water‐soluble Poly(N‐isopropylacrylamide)–graphene sheets synthesized via click chemistry for drug delivery. Adv Funct Mater. 2011;21(14):2754–2763. doi:10.1002/adfm.201100078
64. Oliveira E, Macedo C, Collares C, Sakamoto-Hojo E, Donadi E, Passos G. Quantum-dot-conjugated graphene as a probe for simultaneous cancer-targeted fluorescent imaging, tracking, and monitoring drug delivery. Bioconjug Chem. 2013;24(3):387–397. doi:10.1021/bc3004809
65. Dinggeng H, Xiaoxiao H, Kemin W, Zhen Z, Xue Y, Xuecai L. Remote-controlled drug release from graphene oxide-capped mesoporous silica to cancer cells by photoinduced pH-jump activation. Langmuir. 2014;30(24):7182–7189. doi:10.1021/la501075c
66. Yin PT, Shah S, Chhowalla M, Lee KB. Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem Rev. 2015;115(7):2483–2531. doi:10.1021/cr500537t
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.