Back to Journals » Drug Design, Development and Therapy » Volume 18
Dynasore Alleviates LPS-Induced Acute Lung Injury by Inhibiting NLRP3 Inflammasome-Mediated Pyroptosis
Authors Shan M, Wan H, Ran L, Ye J, Xie W, Lu J, Hu X, Deng S, Zhang W, Chen M, Wang F, Guo Z
Received 12 October 2023
Accepted for publication 12 April 2024
Published 23 April 2024 Volume 2024:18 Pages 1369—1384
DOI https://doi.org/10.2147/DDDT.S444408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Mengtian Shan,1 Huimin Wan,1 Linyu Ran,1 Jihui Ye,1 Wang Xie,2 Jingjing Lu,1 Xueping Hu,1 Shengjie Deng,1 Wenyu Zhang,1 Miao Chen,3 Feilong Wang,1 Zhongliang Guo1,4
1Department of Pulmonary and Critical Care Medicine, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China; 2Department of Respiratory Medicine, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China; 3Department of Emergency, The First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China; 4Department of Respiratory Medicine, Ji’an Hospital, Shanghai East Hospital, Shanghai, Jiangxi, People’s Republic of China
Correspondence: Zhongliang Guo; Feilong Wang, Shanghai East Hospital, School of Medicine, Tongji University, 150st Jimo Road, Pudong, Shanghai, 200092, People’s Republic of China, Tel +86-21-38804518, Fax +86-21-20334513, Email [email protected]; [email protected]
Background: Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are clinically severe respiratory disorders without available pharmacological therapies. Dynasore is a cell-permeable molecule that inhibits GTPase activity and exerts protective effects in several disease models. However, whether dynasore can alleviate lipopolysaccharide (LPS)-induced ALI is unknown. This study investigated the effect of dynasore on macrophage activation and explored its potential mechanisms in LPS-induced ALI in vitro and in vivo.
Methods: Bone marrow-derived macrophages (BMDMs) were activated classically with LPS or subjected to NLRP3 inflammasome activation with LPS+ATP. A mouse ALI model was established by the intratracheal instillation (i.t.) of LPS. The expression of PYD domains-containing protein 3 (NLRP3), caspase-1, and gasdermin D (GSDMD) protein was detected by Western blots. Inflammatory mediators were analyzed in the cell supernatant, in serum and bronchoalveolar lavage fluid (BALF) by enzyme-linked immunosorbent assays. Morphological changes in lung tissues were evaluated by hematoxylin and eosin staining. F4/80, Caspase-1 and GSDMD distribution in lung tissue was detected by immunofluorescence.
Results: Dynasore downregulated nuclear factor (NF)-κB signaling and reduced proinflammatory cytokine production in vitro and inhibited the production and release of interleukin (IL)-1β, NLRP3 inflammasome activation, and macrophage pyroptosis through the Drp1/ROS/NLRP3 axis. Dynasore significantly reduced lung injury scores and proinflammatory cytokine levels in both BALF and serum in vivo, including IL-1β and IL-6. Dynasore also downregulated the co-expression of F4/80, caspase-1 and GSDMD in lung tissue.
Conclusion: Collectively, these findings demonstrated that dynasore could alleviate LPS-induced ALI by regulating macrophage pyroptosis, which might provide a new therapeutic strategy for ALI/ARDS.
Keywords: acute lung injury, acute respiratory distress syndrome, NLRP3 inflammasome, pyroptosis, dynasore, inflammation
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are clinically severe respiratory disorders that lead to high morbidity and mortality worldwide.1,2 No available pharmacological therapy is specific and effective in the treating ALI/ARDS. Although the underlying mechanism of the development of ALI/ARDS remains to be elucidated, the excessive influx of inflammatory cells and an imbalance in the inflammatory response contribute to the pathogenesis of this life-threatening disease.3,4 Among the infiltrating inflammatory cells, macrophages play a key role in promoting inflammation and lung tissue damage by producing proinflammatory cytokines during ALI/ARDS development. Exposure to endotoxin induces macrophages to release tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12 through transcriptional up-regulation and IL-1β through the activation of the PYD domains-containing protein 3 (NLRP3) inflammasome. Thus, exploring new potential drugs to target these transcriptional factors, as well as the NLRP3 inflammasome, is essential for treating ALI/ARDS in clinical practices.
The NLRP3 inflammasome is a cytoplasmic protein complex of the innate immune system composed of the sensor molecule NOD-like receptor (NLR) family protein NLRP3, the apoptosis-associated speak-like protein (ASC), and the effector caspase-1.5–7 The activation of the NLRP3 inflammasome requires two signals. The first signal is primed by pathogen- or danger-associated molecular patterns (DAMPs) through Toll-like receptors, which upregulate the expression of NLRP3, and pro-IL-1β or pro-IL-18 by activating the nuclear factor (NF)-κB pathway. The second signal is mediated by diverse extracellular stimuli, which are often referred to as NLRP3 agonists, leading to the assembly of the inflammasome complex and eventual activation.4,8 Once activated, the NLRP3 inflammasome processes pro-caspase-1 into mature caspase-1, which, in turn, cleaves cytokine precursors to mature forms and causes gasdermin D (GSDMD) to form holes in the cell membrane, resulting in the maturation and release of IL-1β and IL-18.9 These NLRP3 inflammasome-mediated pyroptotic cell death and cytokine production processes are critical for host defenses during infection. However, the excessive activation of the NLRP3 inflammasome is associated with the progression of inflammation. Recent studies showed that the NLRP3 inflammasome played a critical role in the development of ALI.10–12 Inflammasome activation in infected macrophages was shown to be the key driver of COVID-19 pathology, and inhibiting the NLRP3 inflammasome pathway reversed chronic lung pathology.13 While the activation of the NLRP3 inflammasome helps in host protection against invading pathogens, excessive NLRP3 inflammasome-mediated inflammation can lead to lung tissue injury and organ dysfunction.14 Therefore, the NLRP3 inflammasome has been recognized as an essential target in the treatment of ALI/ARDS.12
Dynasore is a cell-permeable small molecule that non-competitively inhibits the GTPase activity of dynamin1, dynamin2, and dynamin-related protein 1 (Drp1).15 Since GTPase controls a wide range of cellular responses, regulating its activities has been recognized as a promising approach to treating various diseases. The inhibition of GTPase by dynasore has demonstrated protective effects in several disease models, such as mitigating oxidative stress on the ocular surface, reducing heart ischemia/reperfusion injury, and ameliorating spinal cord injury.16–18 However, whether dynasore can alleviate macrophage activation and LPS-induced ALI is unknown. This study found that dynasore significantly reduced key proinflammatory cytokine production from macrophages by inhibiting the NF-κB pathway and activating NLRP3 inflammasome. Importantly, pretreatment with dynasore attenuated lung injury in an LPS-induced mouse model of ALI. Thus, the study findings suggest a new potential target for treating of ALI/ARDS.
Materials and Methods
Materials
Dynasore (minimum purity > 99%) (Cat# 2897) and mouse DuoSet kits for IL-1β/IL-1F2, TNF-α, IL-6, and IL-12 were purchased from R&D Systems (Minneapolis, MN, USA). The molecular structure of dynasore is illustrated in Supplementary Figure S1. LPS from Escherichia coli O111:B4 (Cat# L4391) was purchased from Sigma-Aldrich (St Louis, MO, USA). The XTT Cell Viability Kit (Cat# 9095) was purchased from Cell Signaling Technology (Danvers, MA, USA). MitoSOXTM Red mitochondrial superoxide indicator (Cat# M36008) was obtained from Thermo Scientific (Waltham, MA, USA). Dulbecco’s modified Eagle medium (DMEM) and heat-inactivated fetal bovine serum (HIFBS) were purchased from Gibco (Waltham, MA, USA). Recombinant murine colony-stimulating factor (M-CSF; Cat# 315–02) was purchased from PeproTech (Waltham, MA, USA).
Mouse Strains
Male C57BL/6J mice (6 to 8 weeks old) weighing 20–25 g were purchased from Shanghai Model Organisms Center. Mice were maintained in a 12 h light/dark cycle under specific pathogen-free conditions. All procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals established by the US National Institutes of Health and were approved by the Ethics Committee of Animal Experiments of Tongji University (No. TJBB03722101).
LPS-Induced Model of Acute Lung Injury
The sample size calculation for the animal experiments was determined using the Resource Equation Approach.19 Based on the computational formula, the required sample size for animal experiments ranged from 4 to 6 animals per group. In our experiments, each group comprised n=6, consistent with the calculated algorithm. The mice were randomly divided into 8 groups of 6 mice each: control group, dynasore 10 mg group, dynasore 30 mg group, dynasore 50 mg group, LPS group, dynasore 10 mg + LPS group, dynasore 30 mg + LPS group, and dynasore 50 mg + LPS group. In the dynasore groups, mice were pretreated with dynasore at 10 mg/kg, 30 mg/kg, or 50 mg/kg by intraperitoneal injection for 3 h before the intratracheal instillation (i.t.) of phosphate-buffered saline (PBS)/LPS. In the LPS group and Dynasore + LPS groups, the ALI model was established by the intratracheal instillation (i.t.) of LPS (dissolved in 50 μL of sterile PBS) at a dose of 10 mg/kg as described.20 Mice in the control group and Dynasore groups were given an intratracheal instillation of isovolumetric sterile PBS. The mice were euthanized by an overdose of inhaled carbon dioxide, followed by irrigation or lung tissue collection.
Bone Marrow-Derived Macrophage Isolation and Culture
Bone marrow-derived macrophages (BMDMs) were isolated from C57BL/6J mice as previously described.21,22 Briefly, the tibia and femora were isolated and pulverized to obtain bone marrow cells. The cells were suspended and grown in DMEM (10% HIFBS and 1% penicillin-streptomycin) containing 20 ng/mL of M-CSF at 37 °C and 5% CO2 in a humidified incubator. On days 4 and 6, half the volume of fresh growth medium was added. After 7 days in culture, nonadherent cells were removed by vigorous washing and the adherent macrophages were harvested and plated. Cell purity of isolated BMDMs was analyzed by flow cytometry and confirmed to be more than 99%, using CD45, F4/80, and CD11b as the main markers (Flow cytometry gating strategy as shown in Supplementary Figure S2). The cells were plated in DMEM (10% HIFBS and 1% penicillin-streptomycin) at 5×105 cells/well in 24-well culture plates or 1×106 cells/well in 12-well culture plates for further experimentation.
Macrophage Activation
BMDMs were seeded at 1×106 /mL for the experiments. The cells were untreated or treated with different concentrations of dynasore (5 μM, 10 μM, 25 μM, and 50 μM) for 1 h and stimulated with LPS (100 ng/mL) for 24 h to induce classical activation.23 The cells were primed with LPS (500 ng/mL) for NLRP3 inflammasome activation for 3 h before adding 5 mM adenosine triphosphate (ATP) for 30 min.24,25
Lactate Dehydrogenase Activity
The CyQUANTTM LDH Cytotoxicity Assay Kit (Invitrogen, Waltham, MA, USA) was used to quantify lactate dehydrogenase (LDH) release from the BMDMs as a measure of cell death following inflammasome stimulation. Freshly harvested supernatants were used in this assay. Supernatant (50 μL) was added to 50 μL of the reaction mixture and incubated in the dark at room temperature for 30 min. Stop solution (50 μL) was added to terminate the reaction, and absorbance was measured at 490 nm and 680 nm using a microplate reader. Lysis buffer was added to untreated cells 45 min before harvesting and served as maximum LDH release controls.
Mitochondrial Reactive Oxygen Species (ROS) Measurement
BMDMs were seeded in a 24-well plate overnight and left untreated or treated with different concentrations of dynasore for 1 h. The cells were then stimulated with LPS for 3 h and loaded with 5 μM MitoSOX at 37 °C 10 min later. Cells were acquired on a FACS Aria II flow cytometer, and the data were analyzed using FlowJo v.9.5.2 software.
Cell Viability
Cell viability was analyzed using the XTT assay. BMDMs were seeded in 96-well plates overnight, incubated with different concentrations of dynasore (5 μM, 10 μM, 25 μM, and 50 μM) for 1 h, and then stimulated with LPS (100 ng/mL) for 3 h or 24h. Absorbance was read at 450 nm after 1 to 3 h of incubation with 50 μL of XTT detection solution at 37 °C.
Quantification of Cytokines
Cytokines were quantified using IL-1β/IL-1F2, TNF-α, IL-6, and IL-12 DuoSet kits. The assays were conducted according to the manufacturer’s instructions, with appropriately diluted cell supernatants added to each plate. Absorbance was measured at 450 nm and 540 nm on a microplate reader. Absorbance values were corrected by subtracting background absorbance, and cytokine concentrations were subsequently obtained by extrapolation from a standard curve plot.
Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from BMDMs using TRIzol reagent (Invitrogen, Waltham, MA, USA), and cDNA was synthesized using a Transcriptor First Strand cDNA Synthesis Kit (Thermo Scientific, Rockford, IL, USA). Real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed using PowerUpTM SYBRTM Green Master Mix (Thermo Scientific, Rockford, IL, USA). Gene expression was normalized to that of with β-actin as the housekeeping gene and measured by the 2−ΔCt method. The relative gene expression was assayed by 2−ΔΔCt. IL-1β, IL-6, IL-12, NLRP3, Caspase-1, and GSDMD primers used in this study were synthesized by Sangon Biotech (Shanghai, China), and the sequences are shown in Table 1.
 |
Table 1 Sequences of the Primers Used to Quantitate Gene Expression |
Western Blotting
After different treatments and stimulation, the cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors. The cell lysate was boiled in sodium dodecyl sulfate (SDS) sample buffer for 5 min at 95–100°C, separated by SDS- polyacrylamide gel electrophoresis, and transferred to polyvinylidene membranes. The membranes were blocked for 1 h in Tris-buffered saline (TBS) plus 5% nonfat dry milk and then incubated with primary antibodies overnight at 4 °C. The primary antibodies were: mouse anti-IL-1β antibody (Ab; Cat# AF-401; R&D Systems, Minneapolis, MN, USA); rabbit anti-NLRP3 Ab (Cat# 15101), anti-cleaved GSDMD Ab (Cat# 10137), anti-NF-κB p65 Ab (Cat# 8242), anti-phospho-NF-κB p65 (Ser616) Ab (Cat# 3033), anti-DRP1 (D6C7) Ab (Cat #8570), and anti-phosphorylated DRP1 (Ser616) Ab (Cat# 3455); Cell Signaling Technology (Danvers, MA, USA); and rabbit anti-pro-caspase-1+p10+p12 (Cat# ab179515), and rabbit anti-β actin (Cat# ab8227; Abcam, Cambridge, MA, USA). After washing three times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1h at room temperature in TBST plus 5% nonfat dry milk. Western blotting substrates were added for 5 minutes after washing three times.
Bronchoalveolar Lavage Fluid (BALF) Collection
BALF was collected by instilling 1 mL of ice-cold PBS (pH 7.4) intratracheally using a 20-gauge blunt needle, aspirated back after 30s.26 The recovered fluid was centrifuged at 1500 rpm for 5 min at 4 °C, and the supernatant was collected and stored at −80 °C for subsequent experiments (eg, quantification of IL-1β, IL-6, and IL-12 cytokines).
Hematoxylin & Eosin (H&E) Staining
Lung tissue was fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin. Subsequently, 5-μm sections were sliced for H&E staining or immunofluorescence analysis. Histopathological changes were observed by microscopy. Lung injury scores were calculated according to the recommendations of the American Thoracic Society.27
Immunofluorescent Double Labeling
After permeabilizing the tissues and blocking non-specific binding sites, the tissue sections were incubated with the following primary antibodies diluted in serum: rabbit anti-GSDMD pro+ NT Ab (Cat# AF4012; Affinity Biosciences, Changzhou, China) and mouse anti-caspase-1 Ab (Cat# sc56036; Santa Cruz Biotechnology, Beijing, China) in a humidified chamber overnight at 4 °C. Tissue sections were incubated with anti-rabbit and anti-mouse IgG (H+L) secondary antibodies for 1 h at room temperature in the dark. After immunostaining, the sections were incubated with 1 μg/mL of DAPI for 5 min in the dark to label the nuclei. The sections were visualized under a Nikon fluorescent microscope connected to a digital camera. The number of positive cells (red or green) and the total number of cells (blue) were counted using the counting function of ImageJ software.
Statistical Analysis
The data are presented as the mean ± standard deviation (SD). For analyses with sample sizes of n=6, Welch-ANOVA with Games-Howell post-hoc test was employed to assess differences among three or more groups, utilizing the R programming language (R version 4.2.3). For datasets with sample sizes n=3 or 4, permutation tests conducted in the R programming language were used to evaluate differences among three or more groups. QQ plots were utilized to assess data distribution prior to conducting the analyses in the R language. A p-value of < 0.05 was considered significant.
Results
Dynasore Inhibits the Classical Activation of Macrophages by LPS
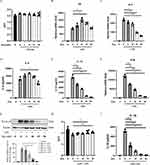
BMDMs were pretreated with different concentrations of dynasore followed by classical activation by LPS stimulation to explore the effects of dynasore on macrophage-mediated inflammation. As shown in Figure 1A, pretreatment with dynasore did not impact BMDM cell viability after 24 hours of LPS stimulation. However, dynasore significantly reduced both mRNA and protein levels of key proinflammatory cytokines including IL-6 and IL-12 (Figure 1B–E).
The effects of dynasore on the production of IL-1β, which is another classic cytokine produced from classically activated macrophages, were examined. To this end, a well-established LPS priming (signal 1) plus ATP stimulation (signal 2) model was used to study IL-1β processing. As shown in Figure 1F and G, dynasore treatment significantly reduced both mRNA and protein levels of pro-IL-1β in BMDMs primed by LPS. Dynasore also inhibited the release of mature IL-1β after ATP stimulation in a dose-dependent manner and slightly increased cell activity (Figure 1H and I). These data demonstrate that dynasore could effectively inhibit proinflammatory cytokine production from classically activated macrophages.
Dynasore Inhibits NLRP3 Inflammasome Activation and Pyroptosis in Macrophages
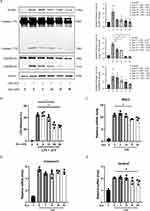
This study is based on the successful induction of NLRP3 inflammasome activation in BMDMs by the first signal LPS primer followed by the second signal ATP, as shown in Supplementary Figure S3. As IL-1β secretion was significantly decreased by dynasore after LPS plus ATP stimulation (Figure 1I), whether dynasore could affect NLRP3 inflammasome activation and mediated its pyroptosis in macrophages was investigated. Dynasore effectively inhibited the release of caspase-1 p10, an active form of caspase-1 and an indicator of NLRP3 activation, after LPS plus ATP stimulation (Figure 2A). GSDMD N-terminal and LDH release were also reduced by dynasore treatment (Figure 2A and B), indicating that NLRP3-mediated pyroptotic cell death was blocked. The mRNA expression of NLRP3, caspase-1, and GSDMD was similarly downregulated by dynasore treatment after LPS plus ATP stimulation (Figure 2C–E). These data suggest that dynasore inhibited NLRP3 inflammasome activation, downstream IL-1β cleavage, and pyroptosis in macrophages.
Dynasore Downregulates NF-κB Signaling and Drp1/ROS/NLRP3 Inflammatory Pathways
NF-κB signaling pathway plays a crucial role in the regulation of innate immunity and inflammation. NF-κB has been recognized as a key transcription factor of proinflammatory cytokines in classically activated macrophages. The effects on NF-κB p65 and its phosphorylated form were examined to explore the potential mechanism of how dynasore inhibited proinflammatory cytokine production in LPS-stimulated macrophages. NF-κB p65 and p-p65 were downregulated in macrophages by dynasore treatment (Figure 3A). Compared to the control group, the nuclear translocation of NF-κB p65 was induced in macrophages by LPS stimulation. However, the immunofluorescence intensity of cellular (or nuclear) NF-κB p65 was reduced by dynasore treatment in a dose-dependent manner (Figure 3B and C). These data indicate that dynasore could directly inhibit NF-κB signaling in classically activated macrophages and, thus, reduce proinflammatory cytokine production.
Next, how dynasore inhibited NLRP3 inflammasome activation and mediated its pyroptosis in macrophages was analyzed. Dynasore is a chemical inhibitor of Drp1, which is a GTPase that regulates mitochondrial fission.28 During cellular stress, Drp1 localizes to the mitochondrial outer membrane and, in turn, is phosphorylated at Ser616. This process induces mitochondrial fission to promote the production of reactive oxygen species (ROS), which is an important driver of NLRP3 inflammasome activation.29–31 We found that Drp1 phosphorylation at the Ser616 site was inhibited by dynasore in LPS-stimulated macrophages (Figure 3D). Mitochondrial ROS production was also decreased after dynasore treatment (Figure 3E and F). Thus, dynasore might inhibit NLRP3 inflammasome activation through the Drp1/ROS/NLRP3 axis.
Dynasore Alleviates Lipopolysaccharide-Induced Acute Lung Injury in vivo
Mice were treated with dynasore at doses of 10 mg/kg, 30 mg/kg, or 50mg/kg before the intratracheal instillation (i.t.) of LPS (10 mg/kg) to determine whether dynasore could alleviate LPS-induced ALI and explore the optimal protective concentration of dynasore in vivo. The levels of proinflammatory cytokines in both BALF and serum were significantly reduced by dynasore pretreatment (Figure 4A–D). The inflammatory cytokines IL-1β in BALF and IL-6 in serum were significantly decreased in the dynasore 30mg group compared to the dynasore 10 mg group (p < 0.05). Doses of 30 mg/kg and 50 mg/kg dynasore similarly prevented increases in proinflammatory cytokines (IL-1β and IL-6) in BALF and serum (Figure 4A–D). Therefore, dynasore at a dose of 30 mg/kg was used as the optimal protective concentration in the ALI model in subsequent experiments.
Compared to the control group, LPS administration successfully induced acute lung tissue injury, as indicated by lung histology (Figure 5A). However, dynasore (30 mg/kg) pretreatment significantly reduced lung injury scores, shown by alleviated tissue damage and less alveolar hemorrhage, thickening of the alveolar wall, and the infiltration of inflammatory cells (Figure 5A and B). The levels of proinflammatory cytokines in both BALF and serum, including IL-1β and IL-6, were also significantly reduced by dynasore (30 mg/kg) treatment (Figure 5C–G). As dynasore reduces NLRP3 inflammasome activation in macrophages, pyroptosis-related protein expression was analyzed in lung tissue to evaluate the inhibition of NLRP3 inflammasome activation. As shown in Figure 6, the co-expression of F4/80 (main marker of macrophages), caspase-1 (localized in the cytoplasm) and GSDMD (localized in the cell membrane) was upregulated by LPS administration. Compared to the LPS group, the co-expression of F4/80, caspase-1 and GSDMD was significantly reduced in the dynasore 30 mg/kg + LPS group (Figure 6). Thus, these data show that dynasore pretreatment alleviated LPS-induced acute lung injury by decreasing proinflammatory cytokines production and macrophages pyroptosis in vivo.
Discussion
The findings of this study demonstrated that dynasore treatment alleviated macrophage activation in vitro and LPS-induced ALI in vivo. Dynasore reduced key proinflammatory cytokine production in macrophages by inhibiting NF-κB pathways and NLRP3 inflammasome, processes likely linked to the Drp1/ROS pathway (Figure 7). Our findings suggest a new potential target for ALI/ARDS therapeutic intervention.
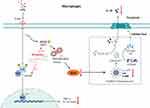 |
Figure 7 The signaling mechanism diagram of dynasore alleviates inflammation in macrophages and acute lung injury. |
Macrophages are the most abundant immune cells in lung tissue and play a critical role in maintaining tissue homeostasis by initiating host defenses upon pathogen stimulation.32 However, the excessive inflammatory response mediated by macrophages promotes ALI/ARDS.33,34 Therefore, the timely regulation of the inflammatory signaling pathway in macrophages is critical for re-establishing tissue homeostasis. Inflammatory disorders caused by the excessive production of proinflammatory cytokines are key factors in the development of ALI/ARDS. Exposure to endotoxin induces macrophages to release TNF-α, IL-6, and IL-12 through the transcriptional up-regulation of the NF-κB pathway and IL-1β by NLRP3 inflammasome activation. While targeting NF-κB pathways has long been recognized as a potential strategy to suppress excessive inflammation, recent studies showed that NLRP3 inflammasome-mediated IL-1β cleavage and pyroptotic cell death contribute significantly to ALI/ARDS development.35–38 The NLRP3 inflammasome processes pro-caspase-1 into mature caspase-1, which, in turn, cleaves cytokine precursors to mature forms and causes GSDMD to form holes in the cell membrane, resulting in the release of IL-1β and other inflammatory cytokines.39 Subsequently, the excessive influx of inflammatory cells and the release of many proinflammatory factors increase the permeability of the alveolar epithelium and induce the destruction of the alveolar-capillary barrier, leading to pulmonary edema and ALI.2 Importantly, inhibiting NLRP3 inflammasome activation led to the alleviation of ALI.11,12 Therefore, the NLRP3 inflammasome has been recognized as an essential target for the treatment of ALI/ARDS.
Dynasore is a cell-permeable small molecule that inhibits the GTPase activity of dynamin1, dynamin2, and Drp1.15 Since GTPase controls a wide range of cellular responses, regulating its activities has been recognized as a promising approach to treating different diseases, including inflammatory diseases. Recent studies have revealed the critical roles of Rho GTPase and RAB26 GTPase in regulating pulmonary endothelial cell permeability and preventing vascular leakage, which are critical in the development of acute lung injury.40,41 The application of dynasore to inhibit GTPase activities has demonstrated protective effects in several disease models.17,18 Dynasore protected cardiac lusitropy and limited cell damage by maintaining mitochondrial morphology and intracellular ATP in stressed cells.17 Moreover, dynasore significantly enhanced motor function by inhibiting the activation of the neuronal mitochondrial apoptotic pathway after spinal cord injury.18 Consequently, dynasore’s inhibition of GTPase activity may play a crucial role in the pathogenesis and progression of acute lung injury. In the present study, dynasore was shown to be effective in alleviating macrophage activation in vitro and LPS-induced acute lung injury in vivo.
Macrophages were pretreated with different concentrations of dynasore followed by classical activation by LPS stimulation to explore the effects of dynasore on macrophage-mediated inflammation. Dynasore pretreatment significantly reduced proinflammatory cytokines, including IL-6 and IL-12. Dynasore treatment also significantly decreased the protein levels of pro-IL-1β primed by LPS and inhibited the release of IL-1β after LPS priming plus ATP stimulation in a dose-dependent manner. These data suggest that dynasore effectively inhibited the classical activation of macrophages. Then, whether dynasore affected NLRP3 inflammasome activation and mediated its pyroptosis in macrophages was investigated. Dynasore effectively reduced caspase-1 p10, GSDMD N-terminal, and LDH release after LPS plus ATP stimulation. Thus, the data indicate that dynasore treatment inhibited both NLRP3 inflammasome activation and pyroptosis in macrophages.
The NF-κB signaling pathway plays a crucial role in regulating innate immunity and inflammation. NF-κB has been recognized as an early transcription factor that manipulates immune responses, is rapidly activated, and increases proinflammatory cytokines in macrophages during ALI.42,43 Activation of the NF-κB pathway can upregulate the expression of NLRP3, pro-IL-1β, or pro-IL-18 in the first signal of the NLRP3 inflammasome. Thus, the effects on NF-κB p65 and its phosphorylated form by dynasore were analyzed to explore the potential mechanism of how dynasore inhibited proinflammatory cytokines production in LPS-stimulated macrophages. The results suggest that NF-κB p65, p-p65, and NF-κB p65 nuclear translocation were downregulated by dynasore treatment. These data indicate that dynasore could directly inhibit NF-κB signaling during the classical activation of macrophages, reducing proinflammatory cytokine production.
We next tested how dynasore inhibited NLRP3 inflammasome activation and mediated its pyroptosis in macrophages. Dynasore is a chemical inhibitor of Drp1, which is a GTPase that regulates mitochondrial fission.29,30 Zhang et al showed that Drp1 hyperactivation suppressed glycolysis by inhibiting hexokinase 1 and further activated the NLRP3 inflammasome.30 Rayamajhi et al observed that the hyperactivation of Drp1 led to mitochondrial damage, aberrant mitochondrial fission, the perinuclear aggregation of damaged mitochondria, and increased ROS production following infection.29 The NLRP3 inflammasome can be activated by components of cellular metabolites, ROS, or DAMPs.44,45 During cellular stress, Drp1 localizes to the mitochondrial outer membrane and is phosphorylated. This process induces mitochondrial fission to promote the production of mitochondrial ROS, which is associated with NLRP3 inflammasome activation.46,47 The results of this study indicated that Drp1 phosphorylation at the Ser616 site was inhibited in LPS-stimulated macrophages by dynasore treatment. ROS production was also decreased after dynasore treatment. Therefore, dynasore might inhibit NLRP3 inflammasome activation through the Drp1/ROS/NLRP3 axis.
Mice were treated with dynasore before the intratracheal instillation of LPS to determine whether dynasore could prevent LPS-induced ALI in vivo. Compared to the control group, LPS administration successfully induced acute lung tissue injury. However, dynasore pretreatment significantly reduced lung injury scores, reflected by less tissue damage and alveolar hemorrhage, thickening of the alveolar wall, and infiltration of inflammatory cells. In addition, the levels of proinflammatory cytokines in both BAL and serum, including IL-1β and IL-6, were significantly reduced by dynasore treatment. As dynasore reduces NLRP3 inflammasome activation in macrophages, whether dynasore affected macrophages pyroptosis-related pathways in vivo was investigated. The co-expression of F4/80, caspase-1 and GSDMD was also upregulated by LPS administration. Compared to LPS-only administration, the co-expression of F4/80, caspase-1 and GSDMD was significantly reduced after dynasore treatment, indicating that dynasore pretreatment alleviated LPS-induced ALI by decreasing proinflammatory cytokine production and macrophages pyroptosis in vivo.
The limitations of this study are as follows: The unavailability of commercially viable agonists for enhancing NLRP3 inflammasome activity presented a significant challenge. Furthermore, the inherent low transfection efficiency of the lentiviral vector, particularly in non-proliferating primary BMDMs overexpressing NLRP3, prevented the execution of rescue experiments using dynasore to inhibit the NLRP3 inflammasome.
In summary, the study findings revealed that dynasore, a cell-permeable small molecule, alleviated macrophage activation in vitro and LPS-induced ALI in vivo. Dynasore reduced key proinflammatory cytokine production by macrophages by inhibiting NF-κB pathways and NLRP3 inflammasome, processes likely linked to the Drp1/ROS pathway. Our study might reveal a new target for the treatment of ALI/ARDS.
Conclusion
This study revealed that dynasore treatment effectively mitigated macrophage activation in vitro and alleviated LPS-induced ALI in vivo. Dynasore exerted its effects by reducing the production of crucial proinflammatory cytokines in macrophages through the inhibition of NF-κB pathways and the NLRP3 inflammasome. These processes are likely associated with the Drp1/ROS pathway. These findings offer a promising new target for therapeutic intervention in ALI/ARDS.
Abbreviations
ALI, Acute Lung Injury; ARDS, Acute Respiratory Distress Syndrome; ATP, Adenosine Triphosphate; BALF, Bronchoalveolar Lavage Fluid; BMDMs, Bone Marrow-Derived Macrophages; DAMP, Danger-Associated Molecular Patterns; Drp1, Dynamin-related protein 1; GSDMD, Gasdermin D; H&E, Hematoxylin & Eosin; LPS, Lipopolysaccharide; NLR, NOD-like receptor; ROS, Reactive Oxygen Species.
Funding
This work was supported by the Top-level Clinical Discipline Project of Shanghai Pudong (PWYgf2021-05), the National Natural Science Foundation of China (82070076 and 82000082), the National Natural Science Foundation of JiangXi Province (20192ACBL20050), and Provincial Natural Science Foundation of Anhui (2008085QH353).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi:10.1001/jama.2016.0291
2. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi:10.1001/jama.2017.21907
3. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi:10.1038/s41572-019-0069-0
4. Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167(1):183–191. doi:10.1016/j.trsl.2015.04.015
5. He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi:10.1016/j.tibs.2016.09.002
6. Meyers AK, Zhu X. The NLRP3 inflammasome: metabolic regulation and contribution to inflammaging. Cells. 2020;9(8). doi:10.3390/cells9081808
7. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi:10.1146/annurev.immunol.021908.132715
8. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
9. Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature18590
10. Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–1234. doi:10.1164/rccm.201201-0003OC
11. Grailer JJ, Canning BA, Kalbitz M, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192(12):5974–5983. doi:10.4049/jimmunol.1400368
12. Yang HH, Duan JX, Liu SK, et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics. 2020;10(11):4749–4761. doi:10.7150/thno.43108
13. Sefik E, Qu R, Junqueira C, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606(7914):585–593. doi:10.1038/s41586-022-04802-1
14. Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation-The negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–869. doi:10.1038/ni.3772
15. Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–850. doi:10.1016/j.devcel.2006.04.002
16. Webster A, Chintala SK, Kim J, et al. Dynasore protects the ocular surface against damaging oxidative stress. PLoS One. 2018;13(10):e0204288. doi:10.1371/journal.pone.0204288
17. Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One. 2013;8(4):e60967. doi:10.1371/journal.pone.0060967
18. Li G, Shen F, Fan Z, et al. Dynasore improves motor function recovery via inhibition of neuronal apoptosis and astrocytic proliferation after spinal cord injury in rats. Mol Neurobiol. 2017;54(9):7471–7482. doi:10.1007/s12035-016-0252-1
19. Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101–105. doi:10.21315/mjms2017.24.5.11
20. Huang XT, Liu W, Zhou Y, et al. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radic Biol Med. 2020;146:222–233. doi:10.1016/j.freeradbiomed.2019.11.011
21. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83(1):1–14. doi:10.1002/0471142735.im1401s83
22. Wang F, Zhang S, Vuckovic I, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28(3):463–475. doi:10.1016/j.cmet.2018.08.012
23. Chen J, Fu CY, Shen G, et al. Macrophages induce cardiomyocyte ferroptosis via mitochondrial transfer. Free Radic Biol Med. 2022;190:1–14. doi:10.1016/j.freeradbiomed.2022.07.015
24. Bai D, Du J, Bu X, et al. ALDOA maintains NLRP3 inflammasome activation by controlling AMPK activation. Autophagy. 2022;18(7):1673–1693. doi:10.1080/15548627.2021.1997051
25. Yang F, Ye XJ, Chen MY, et al. Inhibition of NLRP3 inflammasome activation and pyroptosis in macrophages by taraxasterol is associated with its regulation on mTOR signaling. Front Immunol. 2021;12:632606. doi:10.3389/fimmu.2021.632606
26. Zhong WJ, Yang HH, Guan XX, et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J Cell Physiol. 2019;234(4):4641–4654. doi:10.1002/jcp.27261
27. Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi:10.1165/rcmb.2009-0210ST
28. Wang X, Jiang W, Yan Y, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15(12):1126–1133. doi:10.1038/ni.3015
29. Rayamajhi M, Miao EA. The RIP1-RIP3 complex initiates mitochondrial fission to fuel NLRP3. Nat Immunol. 2014;15(12):1100–1112. doi:10.1038/ni.3030
30. Zhang X, Wang R, Hu D, et al. Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci Adv. 2020;6(49). doi:10.1126/sciadv.abb8680
31. Zhang T, Ding S, Wang R. Research Progress of mitochondrial mechanism in NLRP3 inflammasome activation and exercise regulation of NLRP3 inflammasome. Int J Mol Sci. 2021;22(19). doi:10.3390/ijms221910866
32. Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi:10.1038/ni.2419
33. Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi:10.1155/2018/1264913
34. Killien EY, Huijsmans RLN, Ticknor IL, et al. Acute respiratory distress syndrome following pediatric trauma: application of pediatric acute lung injury consensus conference criteria. Crit Care Med. 2020;48(1):e26–e33. doi:10.1097/CCM.0000000000004075
35. Ying Y, Mao Y, Yao M. NLRP3 inflammasome activation by MicroRNA-495 promoter methylation may contribute to the progression of acute lung injury. Mol Ther Nucleic Acids. 2019;18:801–814. doi:10.1016/j.omtn.2019.08.028
36. Kurundkar D, Kurundkar AR, Bone NB, et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight. 2019;4(1). doi:10.1172/jci.insight.120722
37. Ning L, Wei W, Wenyang J, Rui X, Qing G. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. 2020;10(7):e228. doi:10.1002/ctm2.228
38. Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60(4):405–414. doi:10.1111/jpi.12322
39. Hughes MM, O’Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281(1):88–98. doi:10.1111/imr.12608
40. Abedi F, Hayes AW, Reiter R, Karimi G. Acute lung injury: the therapeutic role of Rho kinase inhibitors. Pharmacol Res. 2020;155:104736. doi:10.1016/j.phrs.2020.104736
41. Dong W, He B, Qian H, et al. RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy. 2018;14(10):1677–1692. doi:10.1080/15548627.2018.1476811
42. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi:10.1101/cshperspect.a001651
43. Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology. 2021;29(1):91–100. doi:10.1007/s10787-020-00773-9
44. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi:10.1038/nature09663
45. Puri G, Naura AS. Implication of mitochondrial ROS-NLRP3 inflammasome axis during two-hit mediated acute lung injury in mice. Free Radic Res. 2022;56(1):1–16. doi:10.1080/10715762.2021.2023740
46. Jiang C, Zhang J, Xie H, et al. Baicalein suppresses lipopolysaccharide-induced acute lung injury by regulating Drp1-dependent mitochondrial fission of macrophages. Biomed Pharmacother. 2022;145:112408. doi:10.1016/j.biopha.2021.112408
47. Li L, Mu Z, Liu P, Wang Y, Yang F, Han X. Mdivi-1 alleviates atopic dermatitis through the inhibition of NLRP3 inflammasome. Exp Dermatol. 2021;30(12):1734–1744. doi:10.1111/exd.14412
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.