Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Does Nipple-Ward Positive Margin Contribute to a Higher Rate of Re-Excision Procedures After a Lumpectomy with Pathology-Confirmed Positive Margins? A Retrospective Study
Authors Bhimani F , Lin S, McEvoy M, Cavalli A, Obaid L, Chen Y, Gupta A, Pastoriza J, Shihabi A, Feldman S
Received 14 June 2023
Accepted for publication 10 November 2023
Published 21 February 2024 Volume 2024:16 Pages 41—50
DOI https://doi.org/10.2147/BCTT.S425863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Fardeen Bhimani,1,* Sophie Lin,1,* Maureen McEvoy,1,2 Arianna Cavalli,2 Liane Obaid,2 Yu Chen,1 Anjuli Gupta,1,2 Jessica Pastoriza,1,2 Areej Shihabi,1 Sheldon Feldman1,2
1Breast Surgery Division, Department of Surgery, Montefiore Medical Center, Montefiore Einstein Center for Cancer Care, Bronx, New York, USA; 2Albert Einstein College of Medicine, Bronx, New York, USA
*These authors contributed equally to this work
Correspondence: Sheldon Feldman, Department of Surgery, Division of Breast Surgery, Montefiore Medical Center, 1250 Waters Place, 7th Floor, Hutch Tower 1, Bronx, NY, USA, Email [email protected]
Background: Positive margins on lumpectomy specimens are associated with a twofold increased risk of local breast tumor recurrence. Prior literature has demonstrated various techniques and modalities for assessing margin status to reduce re-excision rates. However, there is paucity of literature analyzing which margin contributes to the highest re-excision rates. Therefore, the primary aim of the study was to investigate whether the nipple-ward margins resulted in a higher rate of re-excision in our patient population.
Methods: A retrospective chart review was performed on patients who had re-excision surgery. Nipple-ward margin was identified by correlating radiological and pathological reports. A cut-off of more than 25% was used to demonstrate correlation between nipple-ward margin and re-excision rate.
Results: A total of 98 patients’ data were analyzed, with 41 (41.8%), 14 (14.3%), 5 (5.1%), and 38 (38.8%) diagnosed with DCIS, IDC, ILC, and mixed pathology on their margins, respectively. Overall, 48% (n=47) of the positive margins were nipple-ward, with 44.7% (n=21) reporting DCIS. Upon stratification, 45 (45.9%) cases were single-margin positive, with 26 (57.8%) being nipple-ward. Furthermore, the remaining 53 (54.1%) patients had multiple positive margins, with 21 (39.6.7%) nipple-ward cases.
Conclusion: Positive nipple-ward margins significantly contribute to a higher re-excision rate p < 0.001; 48% of re-excision surgeries had positive nipple-ward margins, and 57.8% of positive single-margin cases were nipple-ward. Taking an additional shave during initial lumpectomy decreases re-excision rates. However, planning a lumpectomy procedure with a more elliptical rather than a spherical resection with additional cavity shave (ie, larger volume) in the nipple-ward direction and minimizing the remaining cavity shaves so the total volume resected remains unchanged. Nevertheless, future studies with larger sample sizes are required to bolster our findings.
Keywords: nipple-ward, breast cancer, re-excision, nipple-ward positive breast cancer, Shave trial, positive margins
Background
Breast cancer is the most common newly diagnosed malignancy among women across the United States.1 For many years, mastectomy was perceived as the only treatment option, even for early-stage breast cancer. However, many studies have demonstrated that a more conservative surgical approach for small breast cancers can lead to similar long-term outcomes in terms of locoregional recurrence and survival. This has led to a transition from surgical management with radical mastectomy to simple mastectomy, to breast-conserving surgery (BCS) with quadrantectomy and then lumpectomy for early breast cancer. BCS, followed by adjuvant radiotherapy, has been shown to be as effective as mastectomy in terms of oncological outcomes.2–4 An important aspect of BCS is the complete excision of the tumor with negative resection margins to ensure lower recurrence rates.5–7 Prior studies have shown that positive microscopic margins on lumpectomy specimens are associated with a two-fold increased risk of ipsilateral breast local tumor recurrence, and as a result, consensus guidelines recommend re-excision to negative margins.8,9 It has been suggested that 20–40% of patients have positive margins requiring re-excision after an initial lumpectomy.10 In fact, a 2015 New England Journal of Medicine editorial describes re-excision as the “other breast cancer epidemic”, underscoring the need for interventions to decrease these avoidable repeat operations.11
Re-excision procedures are a drain on healthcare resources and are associated with decreased patient satisfaction.12 Furthermore, re-excision procedures have the potential to increase patient anxiety, adversely affect cosmesis, and delay initiation of adjuvant systemic and radiation therapy in patients who undergo BCS.13,14 To curtail these detrimental effects, numerous evidence-based practices exist to minimize the variation in re-excision rates observed across the U.S. (0 to 70%), but implementation has been suboptimal.11,13–21 The assessment of margin status may aid in lowering re-excision rates as the decision to perform a re-excision is dependent on the patient’s pathological margin status.22,23 Multiple studies have been conducted demonstrating various techniques and modalities for assessing margin status in order to reduce re-excision rates.24–26 However, these studies have yet to specifically investigate the relationship between margin location and re-excision rates. Prior studies have suggested that breast cancer spreads along the ductal system toward the nipple, which plays a crucial role in this context. This notion is supported by the pathological spread of Paget’s disease of the nipple. Nevertheless, when surgeons perform BCS, they typically do not account for the potential spread of cancer towards the nipple which might later result in re-excisions.
Therefore, the primary aim of our study was to examine whether positive margins towards the nipple (nipple-ward positive margins) are associated with a higher likelihood of re-excision in our patient population. Our hypothesis was that patients undergoing lumpectomy for either DCIS or invasive breast cancer would necessitate re-excision surgery because of the presence of positive margins and that a significant proportion of these positive margins would be oriented towards the nipple.
Methods
Study Design
A retrospective chart review study design was established, and the study protocol was approved by the Institutional Review Board (IRB; no. 2022–14501), and the requirement for informed consent was waived due to the retrospective design of the study and the use of de-identified patients’ data in accordance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). A total of 98 patients’ data were identified from the Montefiore Medical Center breast cancer patients entered in the American Society of Breast Surgeons Mastery of Breast Surgery Program between November 2017 and September 2022 who had undergone a re-excision procedure following positive margin report after lumpectomy at our institute.
The inclusion criteria consisted of 1) age ≥18 years, 2) women who underwent a re-excision procedure at our institute. The exclusion criteria were 1) age ≤18 years, 2) pregnant women and 3) women who underwent an initial lumpectomy and/or re-excision at another institute.
Population Demographics
After acquiring initial list of re-excised patients from the Breast Surgery Mastery, the 98 patients’ data were extracted from our institute's electronic medical record. Demographics such as age, BMI, ethnicity, cancer-stage, surgical procedure, and radiological and histopathological reports were all recorded (Table 1).
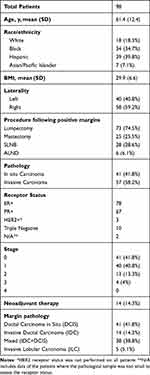 |
Table 1 Patients’ Demographic and Pathological Information Along with the Type of Surgery Performed as the Re-Excision Procedure |
Identification of Nipple-Ward Margin
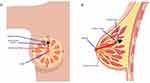
The primary endpoint of the study was the identification of nipple-ward margins and their contribution to the re-excision rate. Nipple-ward margins refer to tumors that appear to be spreading toward the nipple. To determine the direction of cancer spread, we used a combined analysis of radiological and pathological reports. Initially, the tumor’s location was determined using mammography, ultrasound, or MRI, which were performed before the initial lumpectomy. These radiological reports indicate the tumor’s position in relation to the nipple, denoting it in a clockwise orientation. Subsequently, we examined the postoperative pathological report to identify which margins (superior, inferior, medial, lateral, anterior, posterior) were positive, leading to the patient’s need for re-excision surgery. By correlating the tumor’s location with the margin pathology, we established whether the tumor was indeed positive in the nipple-ward direction. At our institution, positive margins were defined as cancer present within 2 mm of the excised tissue. An example of the tumor present at the 12 o’clock position in relation to the nipple with inferior margins positive spreading towards the nipple (nipple-ward) has been illustrated in Figures 1 and 2.
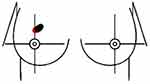 |
Figure 1 Example of positive inferior margin defined as positive nipple-ward margin for a lesion at 12:00. |
Study Stratification
Additionally, because the lumpectomy specimen is three-dimensional, and margins are reported in this three-dimensional way, it was crucial to classify positive margins as either nipple-ward or non-nipple-ward. In cases where some patients had multiple positive margins in the pathological report, we decided to assign them to the nipple-ward positive group only if they had a maximum of three positive margins, with at least one in the nipple-ward direction. This process led to the categorization of patients into four groups and assigned them a number based on their margin status: 0 for a single margin that was not nipple-ward, 1 for a single nipple-ward margin, 2 for cases with multiple margins (less than 4) with at least one in the nipple-ward direction, and 3 for situations with multiple margins (4 or more) that were not nipple-ward. This has been further illustrated in Table 2.
 |
Table 2 Nipple-Ward Margin Stratification |
Statistical Analysis
To test our hypothesis, less than 25% of positive margins served as the threshold for this null hypothesis. In other words, demonstrating a significant correlation between nipple-ward margins and re-excision rates required more than 25% of positive margins to be oriented in the nipple-ward direction. A Chi-square test with a 95% confidence interval was applied, with p-values less than 0.05 indicating statistical significance. Additionally, a comprehensive analysis of the overall demographic characteristics of the study cohort was conducted using nonparametric tests.
Results
Between 2017 and 2022, a total of 98 patients were identified who underwent lumpectomy as well as re-excision procedure at our institute. Their mean age and BMI along with standard deviation accounted for 61.4±12.4 years and 29.9±6.6 kg/m2, respectively. Majority of the patients in our study belonged to ethnic minorities accounting for 81.9% (Table 1). Based on pathology, 41 patients were diagnosed with in situ carcinoma, whereas 57 patients had invasive carcinoma. Additionally, 82.6% of patients had early-stage disease (Stage 0 = 41 patients, Stage 1 = 40 patients), and 31/98 had a family history of breast cancer with 4 patients testing positive for BRCA. Demographic data of the patients are further illustrated in Table 2.
Nipple-Ward Results
Nipple-ward margins contributed significantly to re-excision rates p < 0.001 compared to non-nipple-ward margins. Overall, 48% of patients (n = 47) had positive nipple-ward margins, with 44.7% (n = 21) falling under the category of DCIS. Despite DCIS representing the majority of cases with nipple-ward positive margins, this association was not statistically significant (p = 0.093). However, when analyzing the role of DCIS using the null hypothesis (considering more than 25% of DCIS cases), it was found to be a significant contributor to nipple-ward positive margins (p < 0.001).
Stratification based on the number of positive margins showed that 45 cases were identified as single-margin positive, 26 (55.8%) of which were nipple-ward. The remaining 53 patients had multiple positive margins, with 21 (39.6%) cases being in the nipple-ward direction Table 2.
Margin Pathology and Surgery
In terms of margin pathology, DCIS was present in 41 margins, invasive ductal carcinoma was reported on 14 margins, invasive lobular carcinoma on 5, and mixed pathology was reported on 38 margins. Initially, all 98 patients underwent BCS, of which 31 had sentinel lymph node biopsy (SLNB), and 1 patient had axillary lymph node dissection (ALND). For re-excision surgery, 73 patients were treated with lumpectomy, while 25 patients were treated with mastectomy. Moreover, during re-excision surgery, 28 patients had SLNB, and 6 underwent ALND Table 1.
Discussion
The success of BCS hinges on achieving clear resection margins to minimize re-excision rates. Our study highlights the pivotal role of nipple-ward positive margins in necessitating re-excision surgeries. In our study, we aimed to explore the relationship between positive margins oriented toward the nipple and the need for re-excision in our patient population. Our hypothesis was that patients undergoing lumpectomy would require re-excision due to the presence of positive nipple-ward margins. In line with our hypothesis, we discovered that nipple-ward margins significantly correlated with higher re-excision rates compared to non-nipple-ward margins (p < 0.001). Nearly half of our study patients (48%) exhibited positive nipple-ward margins, with a significant portion (57.8%) of single-margin positive cases oriented toward the nipple. This correlation highlights the need for a deeper understanding of margin orientation, particularly the impact of nipple-ward margins on re-excision rates.
In current clinical practice, positive or close resection margins after BCS increase the risk of local recurrence (LR), which in turn is associated with distant recurrence and poor survival.27–29 According to the current guidelines published by the Society of Surgical Oncology and the American Society of Radiation Oncology, no ink on the tumor is considered an adequate margin for stage I and II invasive disease.22,23 Based on these guidelines, patients having positive margins undergo re-excision procedure; however, what remains notably absent in the current discourse is a comprehensive consideration of margin orientation, which is a significant factor influencing the need for re-excision. Furthermore, the efficacy of these re-excision surgeries continues to be a subject of debate. Prior literature has demonstrated an absence of residual disease on their re-excision procedure in 37–70% of the patients.30–33 Contrastingly, patients with negative but close margins have reported residual disease in 55% of cases.34,35 At our institution, we have formally defined positive margins as the presence of tumor cells within a 2 mm proximity to the excised tissue. Based on these criteria, 98 patients in our study had undergone a re-excision procedure and found that 36 (36.7%) patients had no residual disease on their re-excision pathology. This complexity surrounding margin status and the variable outcomes of re-excision procedures remains a subject of controversy and challenge, which could have been addressed by considering the role of margin orientation, particularly the nipple-ward margins, at the time of initial BCS.
Apart from pathological outcomes, re-excision following BCS has been linked to detrimental physical, psychological, and financial consequences for the patient. Returning to the operating room has been linked to increased surgical morbidity, extended hospital stays, and a higher risk of complications like infections, hematomas, seromas, and fat necrosis. Prior studies have shown that the risk of infection doubles in the first 3 months for patients undergoing additional breast surgery compared to the ones who underwent a single BCS.36 The 2-year infection rate in patients undergoing additional surgery is similar to those undergoing mastectomy (15.3% vs 15.7%, respectively) defying the purpose for undergoing BCS.36 Additionally, patients undergoing re-excision delays the course for initiation of adjuvant therapy. Vandergrift et al37 demonstrated that patients undergoing re-excision procedure had a delay in initiation of adjuvant therapy by 2.1 weeks. Additionally, when patients were informed of their positive margin status and the need for additional surgery, 35% of patients chose mastectomy over re-excision.38 In line with this trend, our study reveals that 25.5% of our patients ultimately underwent mastectomy as their re-excision procedure after being diagnosed with positive margins. This underscores the importance of considering nipple-ward margins during the initial BCS, as addressing this factor could potentially have prevented these patients from choosing mastectomy, which, in essence, contradicts the purpose of performing BCS. Moreover, the financial burden of performing a repeat breast surgery has shown to increase the cost to the healthcare system and patient by $16,072 to $26,026.36,39,40
Taking additional margins during the initial surgery has emerged as a popular perspective that could mitigate the problem of re-excision and decrease the likelihood of positive margins and improve outcomes. Multiple studies and trials have been conducted highlighting the benefit of shaving additional margins at the time of BCS. In a retrospective review, Kobbermann et al41 demonstrated that cavity shaving (CS) was associated with lower re-excision rates when compared to partial mastectomy (22% vs 42%, P = 0.01). Similarly, another retrospective study found that CS resulted in twofold decline in the re-excision rate when compared to partial mastectomy (24% vs 47%, P < 0.001).42 Cao et al43 and Tengher-Barna et al44 demonstrated that CS resulted in negative margins in 59% of 103 and 42% of 47 patients, respectively, who had positive margins on their initial specimen. Furthermore, Chagpar et al45 conducted a randomized control trial with 235 patients comparing BCS vs BCS with CS and found a difference in final histological margin positivity rates between the two groups (BCS with CS vs single BCS) after randomization of 19% and 34%, respectively, p = 0.01. In contrast, Chen et al46 in a randomized control trial demonstrated no significant reduction in re-excision rate 26.4% vs 23.3% after BCS or BCS with CS (p = 0.64). Several studies on additional shaving revealed only a tendency for CS to reduce residual tumor or avoid re-excision, but no statistical significance.47 Additionally, CS has been shown to be futile in patients with invasive lobular carcinoma (ILC) or ductal carcinoma in situ (DCIS).48 Multiple hypotheses for failure in patients with ILC and DCIS have been reported, ranging from insufficient volume of tissue resected to differences in epidemiological characteristics in different populations.47 Nevertheless, it is worth noting that none of these hypotheses have attributed the failure of CS to margin orientation. In our institutional practice, we incorporate CS during BCS, yet we continue to have patients in need of re-excision procedures. It becomes evident that the utility of CS could be significantly enhanced by taking into account margin orientation, particularly in the context of nipple-ward margins during the procedure. Adopting a targeted approach that includes an additional shave directed toward the nipple may hold the potential to substantially decrease the re-excision rate.
Although anatomical and pathological indicators point to the possibility of the spread of cancer in the nipple-ward direction, it is rarely discussed in mainstream research. The female breast is composed of collecting ducts that coalesce and open at the nipple. Breast cancer tends to develop in the terminal ducts and spreads up the ductal tree to the nipple.49 A 3-dimensional reconstructions study of cancers within quadrantectomy specimens from patients revealed three types of invasive breast cancer that spread along the mammary glandular tree: central, peripheral, and extensive. The central type, in which the lesion spreads from the invasive tumor to the nipple, was the most common, accounting for 69% of cases.50 Standard BCS does not take into account the anatomy of the ductal system and the tendency for ductal carcinoma to spread within individual ductal trees towards the nipple. In our study, 48% (n = 47) of the patients had cancer spread in the nipple-ward direction, with DCIS accounting for 44.7% (n = 21). However, DCIS, as the sole factor contributing to nipple-ward positive margins, did not achieve statistical significance (p = 0.093). Nevertheless, when we applied our null hypothesis, considering 25% or less for DCIS, the results were statistically significant (p < 0.001), suggesting that more than 25% of DCIS cases were oriented in the nipple-ward direction, leading to re-excision surgery. This might be one of the reasons for failure of CS in patients with DCIS who have cancer spread in the nipple-ward direction.
Pathologically, Paget disease is widely accepted to be associated with some form of insidious breast cancer, most commonly DCIS or invasive ductal carcinoma (IDC).51 The malignant ductal epithelial cells are thought to migrate toward the skin via the lactiferous ducts and ductules.51 The pathophysiology behind the migration to nipple is hypothesized as epidermotropic theory, wherein the Paget cell arises from an underlying mammary adenocarcinoma, with neoplastic ductal epithelial cells migrating through the breast ductal system and reaching the nipple epidermis.51 Furthermore, the theory also proposes that Paget cells migrate from the duct system to the nipple epidermis via a motility factor that acts via the HER2 receptor.51 Fu et al52 supported the epidermotropic theory further by demonstrating a correlation between Paget cells and ductal carcinoma cells or intraductal cells using molecular markers. Our study hypothesis was in accordance with the above proposed theory. To our knowledge, no previous study has focused on the role of nipple-ward margins contributing to the rate of re-excision. In our study, DCIS was diagnosed in 41.8% (n = 41) of the patients of which 51.2% (n = 21) had the spread of cancer in the nipple-ward direction. Additionally, patients who had single margin positive on their post-surgical pathology accounted for 45.9% (n = 45) of which 57.8% (n = 26) had cancer spread in the nipple-ward direction. Thus, our study findings add to limited literature in breast cancer patients with positive nipple-ward margins. To support the widespread acceptance of the nipple-ward margin theory in breast cancer patients with DCIS and IDC, further clinical studies are needed.
The use of various diagnostic and treatment modalities may also aid in the identification of nipple-ward positive cancers. A study by Dooley52 proposed the use of breast endoscopy during lumpectomy for intraoperative margin assessment, which can identify additional intraluminal lesions outside the anticipated surgical margin and guide excision to negative margins. Dooley’s study highlighted the use of ductoscopy in identifying nipple-ward positive margin where they identified 10 patients (4.98%) of 201 having cancer spread in the nipple-ward direction.52 Similarly, image acquisition techniques using 3D transmission ultrasound have demonstrated the ability to not only perform precise mapping of complex ductal branching anatomy but also to use the information to print an individualized model of the breast tissue.53 This model can then be used to guide optimal resection margins based on individual ductal lobular units, including the nipple ward extension, to achieve microscopically clear margins.53 Additionally, taking an elliptical resection in the nipple-ward direction rather than a traditional spherical resection might aid in reducing re-excision rates (Figure 3). Nonetheless, future studies with prospectively collected data defining the nipple-ward margin on excision can better delineate if taking an elliptical resection can decrease re-excision rates.
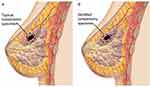 |
Figure 3 Lateral view of the breast highlighting: (A) traditional spherical resection of tumor, (B) recommended elliptical resection in the nipple-ward direction. |
Limitations
This study has a few limitations that should be considered. First, the number of patients included in our study is small which may not be sufficient enough to conclusively support our hypothesis. Second, our study identified the patients’ tumor location using mammography, ultrasound, and MRI and reported it in a clockwise fashion, which was then compared to their margin positivity; however, this correlation only illustrates a 2-dimensional aspect of the tumor in relation to the nipple. Finally, the distance between the tumor and the nipple was not taken into account in our study, which could be a major prognostic factor for cancer spread in the nipple-ward direction. Therefore, future prospective studies addressing these limitations with a larger sample size are required that would support the findings of our retrospective study.
Conclusion
Positive nipple-ward margins significantly contribute to a higher re-excision rate p < 0.001; 48% of re-excision surgeries had positive nipple-ward margins, and 57.8% of positive single-margin cases were nipple-ward. Taking an additional shave during initial lumpectomy decreases re-excision rates. However, planning a lumpectomy procedure with a more elliptical rather than a spherical resection with additional cavity shave (ie, larger volume) in the nipple-ward direction and minimizing the remaining cavity shaves so the total volume resected remains unchanged. Nevertheless, future studies with larger sample sizes are required to bolster our findings.
Synopsis
This retrospective chart review evaluates whether nipple-ward positive margins following breast conservation surgery contribute to a higher re-excision rate. Also, the review highlights the number of positive margins and their pathology contributing to the re-excision rate.
Consent
Written informed consent was not required from patients for publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. American Cancer Society. Cancer Facts & Figures 2021. American Cancer Society. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html.
2. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi:10.1056/NEJMoa022152
3. Christiansen P, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival-A population based study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2018;57(1):19–25. doi:10.1080/0284186X.2017.1403042
4. Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47(4):672–681. doi:10.1080/02841860801971439
5. Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010;17(2):558–563. doi:10.1245/s10434-009-0765-1
6. Blair SL, Thompson K, Rococco J, Malcarne V, Beitsch PD, Ollila DW. Attaining negative margins in breast-conservation operations: is there a consensus among breast surgeons? J Am Coll Surg. 2009;209(5):608–613. doi:10.1016/j.jamcollsurg.2009.07.026
7. Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241(4):629–639. doi:10.1097/01.sla.0000157272.04803.1b
8. Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–177. doi:10.1093/jncimonographs/lgq039
9. Morrow M, Van Zee KJ, Solin LJ, et al. Society of surgical oncology-American Society for radiation oncology-American society of clinical oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23(12):3801–3810. doi:10.1245/s10434-016-5449-z
10. Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16(10):2717–2730. doi:10.1245/s10434-009-0609-z
11. Cody HS, Van Zee KJ. Reexcision--the other breast cancer epidemic. N Engl J Med. 2015;373(6):568–569. doi:10.1056/NEJMe1507190
12. Wazer DE, DiPetrillo T, Schmidt-Ullrich R, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10(3):356–363. doi:10.1200/JCO.1992.10.3.356
13. Abe SE, Hill JS, Han Y, et al. Margin re-excision and local recurrence in invasive breast cancer: a cost analysis using a decision tree model. J Surg Oncol. 2015;112(4):443–448. doi:10.1002/jso.23990
14. Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg. 2014;149(12):1296–1305. doi:10.1001/jamasurg.2014.926
15. Jeevan R, Cromwell DA, Trivella M, et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ. 2012:
16. McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467–475. doi:10.1001/jama.2012.43
17. Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21(3):717–730. doi:10.1245/s10434-014-3480-5
18. Butler-Henderson K, Lee AH, Price RI, Waring K. Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast. 2014;23(2):112–119. doi:10.1016/j.breast.2014.01.002
19. Ahmed M, van Hemelrijck M, Douek M. Systematic review of radioguided versus wire-guided localization in the treatment of non-palpable breast cancers. Breast Cancer Res Treat. 2013;140(2):241–252. doi:10.1007/s10549-013-2547-5
20. Ahmed M, Douek M. Radioactive seed localisation (RSL) in the treatment of non-palpable breast cancers: systematic review and meta-analysis. Breast. 2013;22(4):383–388. doi:10.1016/j.breast.2013.04.016
21. Pouw B, de Wit-van der Veen LJ, Stokkel MP, Loo CE, Vrancken Peeters MJ, Valdés Olmos RA. Heading toward radioactive seed localization in non-palpable breast cancer surgery? A meta-analysis. J Surg Oncol. 2015;111(2):185–191. doi:10.1002/jso.23785
22. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507–1515. doi:10.1200/JCO.2013.53.3935
23. Morrow M, Van Zee KJ, Solin LJ, et al. Society of surgical oncology-American Society for Radiation Oncology-American Society of clinical oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016;34(33):4040–4046. doi:10.1200/JCO.2016.68.3573
24. Sandor MF, Schwalbach B, Hofmann V, et al. Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscope for intraoperative margin assessment - POLARHIS study. Breast. 2022;66:118–125. doi:10.1016/j.breast.2022.10.003
25. Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the national surgical quality improvement program data. Surgery. 2014;156(1):190–197. doi:10.1016/j.surg.2014.03.025
26. Gray RJ, Pockaj BA, Garvey E, Blair S. Intraoperative margin management in breast-conserving surgery: a systematic review of the literature. Ann Surg Oncol. 2018;25(1):18–27. doi:10.1245/s10434-016-5756-4
27. Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol. 2013;2013:793819. doi:10.1155/2013/793819
28. Nayyar A, Gallagher KK, McGuire KP. Definition and management of positive margins for invasive breast cancer. Surg Clin North Am. 2018;98(4):761–771. doi:10.1016/j.suc.2018.03.008
29. Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14(4):1458–1471. doi:10.1245/s10434-006-9236-0
30. Saarela AO, Paloneva TK, Rissanen TJ, Kiviniemi HO. Determinants of positive histologic margins and residual tumor after lumpectomy for early breast cancer: a prospective study with special reference to touch preparation cytology. J Surg Oncol. 1997;66(4):248–253. doi:10.1002/(sici)1096-9098(199712)66:4<248::aid-jso5>3.0.co;2-b
31. Scopa CD, Aroukatos P, Tsamandas AC, Aletra C. Evaluation of margin status in lumpectomy specimens and residual breast carcinoma. Breast J. 2006;12(2):150–153. doi:10.1111/j.1075-122X.2006.00223.x
32. Rubio IT, Ahmed M, Kovacs T, Marco V. Margins in breast conserving surgery: a practice-changing process. Eur J Surg Oncol. 2016;42(5):631–640. doi:10.1016/j.ejso.2016.01.019
33. Chism DB, Freedman GM, Li T, Anderson PR. Re-excision of margins before breast radiation-diagnostic or therapeutic? Int J Radiat Oncol Biol Phys. 2006;65(5):1416–1421. doi:10.1016/j.ijrobp.2006.02.017
34. Vos EL, Gaal J, Verhoef C, Brouwer K, van Deurzen CHM, Koppert LB. Focally positive margins in breast conserving surgery: predictors, residual disease, and local recurrence. Eur J Surg Oncol. 2017;43(10):1846–1854. doi:10.1016/j.ejso.2017.06.007
35. Garvey EM, Senior DA, Pockaj BA, et al. Rates of residual disease with close but negative margins in breast cancer surgery. Breast. 2015;24(4):413–417. doi:10.1016/j.breast.2015.03.005
36. Metcalfe LN, Zysk AM, Yemul KS, et al. Beyond the margins-economic costs and complications associated with repeated breast-conserving surgeries. JAMA Surg. 2017;152(11):1084–1086. doi:10.1001/jamasurg.2017.2661
37. Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in national comprehensive cancer network institutions. J Natl Cancer Inst. 2013;105(2):104–112. doi:10.1093/jnci/djs506
38. Morrow M, Abrahamse P, Hofer TP, et al. Trends in reoperation after initial lumpectomy for breast cancer: addressing overtreatment in surgical management. JAMA Oncol. 2017;3(10):1352–1357. doi:10.1001/jamaoncol.2017.0774
39. Pataky RE, Baliski CR. Reoperation costs in attempted breast-conserving surgery: a decision analysis. Curr Oncol. 2016;23(5):314–321. doi:10.3747/co.23.2989
40. Singer L, Brown E, Lanni T. Margins in breast conserving surgery: the financial cost & potential savings associated with the new margin guidelines. Breast. 2016;28:1–4. doi:10.1016/j.breast.2016.04.007
41. Kobbermann A, Unzeitig A, Xie XJ, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol. 2011;18(5):1349–1355. doi:10.1245/s10434-010-1420-6
42. Unzeitig A, Kobbermann A, Xie XJ, et al. Influence of surgical technique on mastectomy and reexcision rates in breast-conserving therapy for cancer. Int J Surg Oncol. 2012;2012:725121. doi:10.1155/2012/725121
43. Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29(12):1625–1632. doi:10.1097/01.pas.0000180448.08203.70
44. Tengher-Barna I, Hequet D, Reboul-Marty J, et al. Prevalence and predictive factors for the detection of carcinoma in cavity margin performed at the time of breast lumpectomy. Mod Pathol. 2009;22(2):299–305. doi:10.1038/modpathol.2008.186
45. Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373(6):503–510. doi:10.1056/NEJMoa1504473
46. Chen K, Zhu L, Chen L, et al. Circumferential shaving of the cavity in breast-conserving surgery: a randomized controlled trial. Ann Surg Oncol. 2019;26(13):4256–4263. doi:10.1245/s10434-019-07725-w
47. Fernandez-Pacheco M, Ortmann O, Ignatov A, Inwald EC. Does cavity margin shaving reduce residual tumor and re-excision rates? A systematic review. Arch Gynecol Obstet. 2022. doi:10.1007/s00404-022-06512-5
48. Heiss N, Rousson V, Ifticene-Treboux A, Lehr HA, Delaloye JF. Risk factors for positive resection margins of breast cancer tumorectomy specimen following breast-conserving surgery. Horm Mol Biol Clin Investig. 2017;32:2.
49. Jenkinson AD, Al-Mufti RA, Mohsen Y, Berry MJ, Wells C, Carpenter R. Does intraductal breast cancer spread in a segmental distribution? An analysis of residual tumour burden following segmental mastectomy using tumour bed biopsies. Eur J Surg Oncol. 2001;27(1):21–25. doi:10.1053/ejso.2000.1051
50. Ohtake T, Yasuda M, Ito J, et al. Pathological aspects of the intraductal spread of breast cancer. Breast Cancer. 2013;20(1):34–40. doi:10.1007/s12282-011-0325-y
51. Yasir M, Khan M, Lotfollahzadeh S. Mammary paget disease. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563228/.
52. Fu W, Lobocki CA, Silberberg BK, Chelladurai M, Young SC. Molecular markers in Paget disease of the breast. J Surg Oncol. 2001;77(3):171–178. doi:10.1002/jso.1090
53. Wiskin JW, Borup DT, Iuanow E, Klock J, Lenox MW. 3-D nonlinear acoustic inverse scattering: algorithm and quantitative results. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(8):1161–1174. doi:10.1109/TUFFC.2017.2706189
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.