Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
“Diagnostic and Prognostic Biomarkers of Luminal Breast Cancer: Where are We Now?”
Authors Höller A, Nguyen-Sträuli BD , Frauchiger-Heuer H, Ring A
Received 1 April 2023
Accepted for publication 12 July 2023
Published 28 July 2023 Volume 2023:15 Pages 525—540
DOI https://doi.org/10.2147/BCTT.S340741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert Clarke
Anna Höller,1,* Bich Doan Nguyen-Sträuli,1,2,* Heike Frauchiger-Heuer,1,* Alexander Ring1– 3,*
1Department of Gynecology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; 2Department of Biology, Institute of Molecular Health Sciences, Swiss Federal Institute of Technology (ETH) Zurich, Zurich, Switzerland; 3Department of Medical Oncology and Hematology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
*These authors contributed equally to this work
Correspondence: Heike Frauchiger-Heuer, Tel +41 44 255 42 37, Email [email protected]; Alexander Ring, Tel +41 76 327 8287, Email [email protected]
Abstract: Luminal breast cancers are hormone receptor (estrogen and/or progesterone) positive that are further divided into HER2-negative luminal A and HER2-positive luminal B subtypes. According to currently accepted convention, they represent the most common subtypes of breast cancer, accounting for approximately 70% of cases. Biomarkers play a critical role in the functional characterization, prognostication, and therapeutic prediction, rendering them indispensable for the clinical management of invasive breast cancer. Traditional biomarkers include clinicopathological parameters, which are increasingly extended by genetic and other molecular markers, enabling the comprehensive characterization of patients with luminal breast cancer. Liquid biopsies capturing and analyzing circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) are emerging technologies that envision personalized management through precision oncology. This article reviews key biomarkers in luminal breast cancer and ongoing developments.
Keywords: breast cancer, luminal A and B, biomarker, circulating tumor cells, circulating tumor DNA
Introduction
Breast cancer is the most common non-cutaneous cancer among women, with an estimated 2.1 million new cases and 627,000 deaths globally in 2020.1 Luminal breast cancer is the most common subtype of breast cancer, accounting for approximately 70% of cases,2 and is considered less aggressive compared to other subtypes (ie, human epidermal growth factor receptor 2 (HER2) positive and triple negative). Luminal A breast cancers are characterized by the expression of estrogen receptor (ER) and progesterone receptors (PR), low proliferation and a better prognosis, while luminal B breast cancers also express HER2 and are characterized by high proliferation and a poorer prognosis.3
Early diagnosis and appropriate treatment are key factors in improving patient outcomes and survival.4,5 Several biomarkers are used to guide treatment decisions in luminal breast cancer. Biomarkers are molecular, cellular, or functional characteristics that can be used to identify the presence or progression of a disease or predict response to therapy, and have played a crucial role in the clinical management of breast cancer.6,7
In this review, we present a comprehensive overview of the current state of knowledge on diagnostic, prognostic and predictive biomarkers in luminal breast cancer, highlighting key biomarkers and their performance characteristics and limitations. We also evaluate the potential clinical utility of emerging biomarkers in the management of luminal breast cancer, current gaps or hurdles for implementation and suggest potential directions for further research.
Current Landscape of Established Biomarkers – a Chronological Perspective
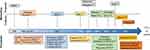
In luminal breast cancer, biomarkers are indispensable for diagnosis, prognosis, and treatment selection, and have led to significant improvements in patient outcomes. Broadly speaking, biomarker can be divided into diagnostic biomarkers that help to establish the presence of cancer, and to determine subtype or stage, prognostic biomarkers that are used to estimate patient outcomes, and predictive biomarkers which are used as a measure of expected response to guide treatment decisions.6,7 While valid as a general systematic classification, the lines between prognostic and predictive are often blurred. Several types of clinical, histological and molecular biomarkers have been developed and are currently used for the diagnosis, classification and treatment decision in breast cancer (Figure 1, Table 1).
 |
Table 1 Selected Biomarkers in Breast Cancer |
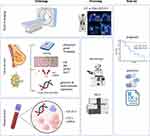
The assessment of a minimal subset of biomarkers at first diagnosis is indispensable for patient management in luminal breast cancer (Figure 2). Whenever feasible, measurements should be repeated on recurrent lesions. All laboratories should use validated assays and established criteria for soundness of results (such as the REMARK criteria),41 and should perform regular quality audits for accreditation by external organizations.
The TNM Classification and Grading
The TNM classification and grading system for breast cancer is used to determine the disease stage based on tumor spread, lymph node involvement, and distant metastasis.42 Both imaging and biopsies are used for staging (Figures 1 and 2). Tumor size (T) is important for prognosis and treatment options, where larger tumors have a worse prognosis and usually require more aggressive treatment.42,43 Nodal status (N) indicates the extent of breast cancer spreading to lymph nodes.42,44 Positive lymph nodes indicate a more aggressive biology, and diagnosis of lymph node involvement is often achieved on surgical specimen. The extent of lymph node involvement determines the surgical dissection, ranging from sentinel lymph node (SN) to axillary lymph node dissection (ALND).45 Concepts for de-escalation are being studied46,47 (NCT03513614). Distant metastasis (M) refers to the presence or absence of hematogenous distant metastases. It has strong implications for prognosis and treatment decisions. Distant or organ metastasis renders most patients incurable, with reduced 5-year survival probability.48 Palliative treatment options are available for metastatic disease. Staging with CT or PET is reserved for patients with high-risk early-stage disease or clinical suspicion49–51 (Figure 2). Tumor grade is a prognostic biomarker that defines cancer cell differentiation52,53 (Figure 2). Grade represents a semi-quantitative evaluation of morphological characteristics, including tubule or gland formation, nuclear pleomorphism, and mitotic count. The Nottingham Grading System (NGS) is widely used, assigning a grade from 1 to,54,55 and has been validated as an independent prognostic factor,22,26,27 regardless of stage, tumor size, and lymph node involvement.58
Hormone Receptors
Measuring the expression of the steroid hormone receptors (HR) for estrogen and progesterone is recommended by all breast cancer treatment guideline and constitutes routine clinical practice (Figure 2 and Table 1).48,59–61
The estrogen receptor counts among the most important biomarkers in breast cancer. It was identified in the late 1960s and has been used in clinical practice since the mid 1970s9–11 (Figure 1). ER is expressed in approximately 80% of breast cancers and is considered as a strong prognostic factor and primary indicator of response to endocrine therapy (ET) (ie, selective estrogen receptor modulators – SERMs (tamoxifen), third-generation aromatase inhibitors (anastrozole, letrozole, exemestane), LH-RH agonists (leuprolide, goserelin), pure estrogen receptor down regulators – SERDs (fulvestrant))62–65 (Figure 1). A meta-analysis of individual data from 20 randomized clinical trials including 21,457 patients found that in early-stage disease, adjuvant tamoxifen treatment reduced the 15-year recurrence rate by 39% and the mortality rate by 30%62 in HR positive breast cancer. On the molecular level, a distinction is made between ERα and ERβ receptors (encoded by the ESR1 and ESR2 genes, respectively),66 however only ERα evaluation is currently routinely clinically assessed.63
Progesterone receptor (PR) exists in two isoforms (alpha and beta), is a transcriptional target of ER and therefore strongly estrogen dependent, but also modulates ERα action in breast cancer.67,68 The proportion of PR-positive breast cancers is lower than ER-positive breast cancers, representing about 20% of cases.69 While both ER and PR are prognostic, the predictive value PR is controversial.62,70,71 ER- and PR-positive breast cancers tend to be less aggressive and are associated with a better prognosis compared to HR-negative breast cancers,63,72 though the impact of endocrine therapy on prognostic is difficult to exclude.
HR expression correlates with tumor grade in luminal BC, with low-grade tumors exhibiting higher ER and PR expression, whereas intermediate- and high-grade tumors may have lower levels of ER and may lack PR expression.73 The presence of both receptors can be detected using immunohistochemistry (IHC) techniques and is mandatory to diagnose and classify breast cancer subtypes (Table 1), including luminal A and luminal B (Figure 2). While traditionally ≥1% positive nuclei was used as a cut off for positivity for ER or PR, recent evidence suggests that tumors with low ER positivity (1–9%) should be considered separately as these tumors seem biologically and prognostically closer to ER-negative or basal-like cancers.74 The following subdivision is recommended: ER-/PR-positive: >10% positive tumor cells, ER-/PR-low positive: 1–9% positive tumor cells, ER-/PR-negative: <1% positive tumor cells.48,59–61,75 Further research is needed to translate these findings into robust clinical application. To avoid ambiguity and better distinguish borderline cases, additional scores have been developed such as the Allred score,76 which considers staining intensity in addition to the proportion of positive cells, or the immunoreactive score (IRS) according to Remmele and Stenger.77
Molecular investigations demonstrated brisk crosstalk of both hormone receptors with other receptor pathways. PR interaction with growth receptors leads to co-activation and co-regulation of common transcriptional targets.78 The cyclin D1/CDK4/6/RB/E2F1 and ER/PR pathways are tightly linked in luminal breast cancer and together with other findings, such as loss of ER expression, mutations in the ligand binding domain, overexpression of ER co-activators or downregulation of co-repressors, the interaction of ER with growth factor receptors, and/or the regulation of ER by miRNAs offer explanations for therapy resistance as well as opportunities for targeting.79,80 The immune system also effects ER activity, for example activation of breast cancer NFκB/STAT3 via tumor-associated macrophages can drive ER ligand-independent phosphorylation.81
Molecular markers, including gene signatures such as Prosigna/PAM50, Endopredict (described in more detail below), breast cancer index (BCI), HOXB13/IL17BR82 or the immunohistochemical 4 (IHC4) score83 enable a differentiation of patients based on relapse risk after endocrine therapy and are discussed further below.
Ki-67
Ki-67 was first discovered in 1983 as a nuclear protein expressed in proliferating cells and is widely used to predict patient prognosis and guide treatment decisions (Figures 1 and 2, Table 1).84–87 Ki-67 is expressed as a percentage or proportion score of tumor cells showing positive nuclear staining, independent of the intensity of coloration.87 A high Ki-67 index as determined by immunohistochemistry (IHC) is used to distinguish luminal B from A breast cancer.19,88,89 Results from various trials support the prognostic role of Ki67, with higher expression indicating a more aggressive cancer and a worse prognosis.38,90 Regarding predictive power, available data suggest that high Ki67 expression can provide important information in the neoadjuvant setting concerning pathological complete response.91 On the contrary, a predictive role in the adjuvant setting could not be established.91–94
Reproducibility issues are an important caveat and were addressed by the International Ki67 in Breast Cancer working group or the Swiss Working Group of Breast- and Gynecopathologists.19,95,96 This also partially explains why Ki67 is not yet standard in all hospitals and medical centers.
Human Epidermal Growth Factor Receptor HER2
The expression of HER2 promotes the growth and spread of breast cancer cells and was first described in 1987 by Slamon et al12,97 (Figures 1 and 2, Table 1). Determining HER2 status is crucial due to its impact on prognosis and treatment. Traditionally, HER2-positive breast cancer was defined overexpression according to ASCO/CAP guidelines, with an IHC score of 3+ or HER2 gene amplification.98 Approximately 15–20% of breast cancers show HER2 overexpression, predicting aggressive biology and response to HER2-targeted therapies (eg, trastuzumab, pertuzumab, lapatinib, trastuzumab-emtansine/T-DM1).25–28,97,99,100 In 2022, the DESTINY-Breast04 trial demonstrated benefit with trastuzumab deruxtecan/T-DXd in traditionally HER2-positive patients and those with IHC scores of 1+ or 2+28 T-DXd targets HER2 as an epitope to deliver high concentrations of chemotherapy causing cell death (bystander effect).101 Including HER2-low cases thereby expands the potential population for HER2-targeted therapies to around 75% of all breast cancer cases.28,102 Novel drugs leveraging the bystander effect are under investigation.103 For completion, we mention here circular HER2 RNA as a novel concept for predictive biomarkers.104
Importantly, co-expression of HER2 and HR has important implications for prognosis and response to receptor-targeted therapies. HER2/HR-positive tumors have a lower likelihood of response when treated with either ET or HER2-targeted therapy alone.105 Several studies demonstrated crosstalk between HR and HER2 modulating both anti-HER2–directed and endocrine therapy via compensatory escape pathways due to the bi-directional signaling.105
Further Molecular Predictive Biomarker
ET represents the corner stone for systemic treatment in luminal breast cancers. However, resistance to these agents poses a major obstacle, in particular in the metastatic setting.106 Acquired activating mutations in ESR1 under the selective pressure of ET lead to constitutive activation and diminished efficacy of AI treatment.107 Epigenetic changes and upregulation of alternative pathways further predicate resistance to ET in luminal breast cancer.106 The presence of FOXA1 is crucial for ER-chromatin interactions and gene expression alterations, while also impacting chromatin accessibility throughout the genome.108 Aberrant c-Myc expression contributes to ET resistance via decrease of the cell cycle regulator p21.109 Cyclin D1 activates ER-mediated transcription even in the absence of estrogen.110
Phosphatidylinositol 3-kinase (PI3K) is involved in AKT–mammalian target of rapamycin (mTOR) signaling, which is related to HR signaling in breast cancer.20,111 The aberrant activation of PI3K/AKT/mTOR signaling, driven by PI3K mutations, can lead to increased ER transcriptional activity and enhanced cell survival, thereby reducing the effectiveness of endocrine therapies.112 Mutations occur in up to 40% of breast cancers and predict response to PI3K inhibitors (ie, alpelisib).34,113 Related to this pathway, the inhibition of mTOR (everolimus) improves PFS (as demonstrated in the BOLERO-2 trial),114 and mTOR alterations can be assessed by next-generation sequencing such as FoundationOne CDx.
Cyclin-dependent kinases 4 and 6 (CDK4/6) are important regulators of cell-cycle progression and have emerged as valuable predictive biomarkers in the metastatic setting.115 Several pivotal randomized prospective trials (PALOMA-3, MONALEESA-7 and MONARCH-2) demonstrated both PFS and OS benefit among both premenopausal and postmenopausal women and established CDK inhibitors (ie, palbociclib, ribociclib, or abemaciclib) in combination with ET (AI or SERD) as the gold standard in metastatic hormone receptor-positive tumors.35–37,115–118 Later this approach was also proven effective in the (neo-) adjuvant setting.119,120
Breast cancer type 1 susceptibility protein 1 (BRCA1), BRCA2 and partner and localizer of BRCA2 (PALB2) are involved in the repair of double strand breaks in DNA.121 Germline mutations in BRCA1/2 occur in 5% of unselected patients and are culprits in the hereditary breast-ovarian cancer syndrome.122,123 Therapeutically, these alterations predict a higher likelihood of response to platinum-based chemotherapy and are used in the adjuvant setting to predict response to poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors such as olaparib and talazoparib in germline mutation carriers.29,30,113
The biomarkers urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1) have demonstrated promising prognostic and predictive validity for adjuvant chemotherapy in lymph node-negative HR-positive early breast cancer124,125 (Table 1).
Among serum biomarker the cancer antigen CA 15–3 is the most widely used serum biomarker in breast cancer but is only established to monitor disease course and treatment efficacy in advanced stages and not for initial diagnosis or early detection due to low sensitivity126,127 (Figure 2).
Gene Expression Signatures
Gene expression profiling was the key to refine breast cancer into molecular subtypes, including luminal A and B, HER2-enriched, basal, and normal like, which have different clinical characteristics and treatment responses.2,14 Further development and validation of multiparametric gene expression profiles (MammaPrint, Oncotype DX, Endopredict, and Prosigna/PAM50) established their role as predictive tools able to stratify early-stage HR-positive patients according to relapse risk and benefit of additional (chemo)therapy (Figures 1 and 2, Table 1).15,31,33,128–130 The signatures can help to identify patients with ER-positive, HER2-negative and lymph node–negative or positive (1–3 lymph nodes) breast cancer that may benefit from or could avoid (neo-)adjuvant chemotherapy.16,59 The most widely used signatures, Oncotype DX and MammaPrint, were initially validated retrospectively including in the NSABP B14/B20,131,132 SWOG-8814,133 TransATAC134 or RASTER135 trials. Several landmark prospective trials subsequently confirmed their prognostic and predictive power. Oncotype DX uses 21 genes and is the only NCCN accredited multigene test.17,32 A score of 26 or higher correlates with higher risk of distant recurrence in both pre- and post-menopausal women with N0 or N1 lymph node status, and addition of chemotherapy to endocrine therapy is recommended.18,32,33,129 The MammaPrint 70-gene signature was first developed and validated as a prognostic tool.31,136,137 The MINDACT trial subsequently showed that almost half of all women (46%) at high clinical high risk but low genomic risk for relapse may forgo adjuvant chemotherapy.138 The GEICAM 9906, ABCSG-6 and −8 trials validated EndoPredict as an independent prognostic parameter in node-positive, luminal BC patients treated with adjuvant chemotherapy followed by hormone therapy.139,140 The Prosigna assay was validated to predict potential benefit from extended endocrine therapy or the addition of chemotherapy.140,141
A couple of aspects are noteworthy regarding gene expression signatures: 1) all use different technologies and show little overlap in regard of genes tested and, more importantly, a considerable level of discordance within the same patient.142 Biologically and technically, it is not surprising that tests measuring fundamentally different genes with different technologies give dissimilar results. Clinically, the discordance might be explained by the absence of any clinical or molecular agreement as to the true boundary between a luminal A and luminal B cancer. 2) Oncotype DX, Prosigna, EndoPredict and BCI do not correlate with tumor size or nodal status, but with tumor grade, perhaps reflecting the proliferative status of tumors as the major criterion for response to chemotherapy.143
Emerging Biomarkers and Personalized Oncology
In recent years, there have been significant advances in the discovery and validation of new biomarkers in breast cancer. One prominent example is liquid biopsies, which involve the analysis of blood and other body fluids to identify cellular or molecular changes associated with breast cancer.144,145 Circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) represent the most advanced among these emerging biomarkers, holding significant potential as non-invasive diagnostic tools for breast cancer and will be reviewed in more detail below.75,144 Other circulating biomarkers include microRNAs, non-coding RNA molecules or exosomes, which have been comprehensively reviewed elsewhere.146–148
CTCs
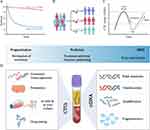
Pathological analysis of tissue biopsies is routinely utilized to predict therapy response and guide further drug selection48 (Figure 2). However, inter- and intra-tumor heterogeneity, poor accessibility of metastatic lesions, and challenges in repeatedly asking a patient to undergo invasive or even surgical procedures during disease progression can hamper precise therapy decisions. In this context, CTCs have emerged as promising liquid analytes (Figure 3). They are living cells that have broken off from a primary tumor or from a metastatic lesion and are shed into the bloodstream to seed distant metastasis.149 One of the main advantages is their minimally invasive detection in peripheral blood which allows serial blood sampling and longitudinal monitoring during tumor progression. Further, CTCs as metastatic precursors might hold the potential to capture all biologically and therapeutically relevant aspects of a cancer, which might not be fully reflected by tissue biopsies that only provide a snapshot of mutations present at a given time and location. Further, tissue biopsies might not address evolutionary changes within the tumor and its metastases which can alter the genetic landscape and its responsiveness to therapies during cancer progression.150 As of May 1st, 2023 there are 215 trials on the ClinicalTrials.gov database related to CTCs in breast cancer, indicating the immense interest in developing CTCs into both prognostic and predictive biomarkers.
Numerous studies have already confirmed the clinical prognostic validity of CTCs, foremost in metastatic breast cancer,151–155 but also in early-stage disease.156–158 A large meta-analysis of N = 2436 patients with breast cancer by Cristofanilli et al suggests a classification of metastatic disease into aggressive ≥5 CTCs vs indolent (<5 CTCs), which is supported by robust OS data.159 While CTCs are not yet routinely used in the clinical management of breast cancer, they have been incorporated into the latest version of the WHO Classification of Tumors: Breast Tumors and AJCC Cancer Staging Manual.42
The predictive value of CTCs, including potential de-escalation of systemic treatment, has been investigated, with mixed results. In the early-stage setting, the GeparQuattro and REMAGUS 02 trials showed no association between pathological response of the primary tumor and changes in CTC numbers before and after neoadjuvant chemotherapy.160,161 Nonetheless, the presence of CTCs post therapy was an independent prognostic factor for early relapse,151,161 suggesting that the detection of persistent CTCs after completion of treatment might provide superior information on therapy response and risk of relapse than the observed chemosensitivity of the primary tumor. In one Phase II-study, trastuzumab decreased the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells.162
In the metastatic setting, early switching to an alternate treatment regime in breast cancer patients where a positive CTC count was recorded after one cycle of first-line chemotherapy showed no impact on overall survival.163 Two proof-of-concept studies investigated HER2-targeted therapies (T-DM1 and lapatinib) in metastatic breast cancer patients with HER2- negative primary tumors and HER-2 positive CTCs. They revealed only marginal benefit in a subset of patients with a HER2-negative primary tumor presents with HER2-positive CTCs during disease progression.164,165 On the contrary, the STIC CTC trial demonstrated that CTCs were helpful to distinguish a subset of HR-positive, HER2-negative patients who benefit from chemotherapy rather than endocrine therapy.166 The ongoing DETECT III study (NCT01619111) compares standard therapy ± lapatinib in HER2-negative metastatic breast cancer and HER2-positive CTCs.167 A recent meta-analysis of N = 1944 breast cancer patients showed the CTC counts before and after treatment have a strong and independent predictive value for survival outcome.151
Several challenges and opportunities remain. Currently, only two FDA-approved devices (CELLSEARCH® and Parsortix®) can be used to monitor the presence and number of CTCs in a person’s blood.168,169 CELLSEARCH® uses antigen-dependent immunomagnetic positive selection to capture CTCs based on EpCAM expression, while Parsortix® uses antigen-independent, size-based microfluidics technology. The main shortcoming of these technologies is limited detection due to the rare nature of CTCs in peripheral blood (roughly one CTC in one billion blood cells) and their short circulation time (10–30 minutes).149 Efficient, robust and reliable CTC enrichment is critical for reproducible downstream analysis and clinical applications.149,170 Hence, novel approaches to overcome that limitation have been developed including implantable devices such as direct intravascular coated guidewires,171 cytapheresis172 which allows cell fraction enrichment from large blood volumes, or the concept of gaining access to tumor draining vessels to enhance CTC enrichment.173,174 Although important, these technologies are not yet routine, and the latter scenario will not be suitable for patients with advanced-stage disease who do not qualify for surgery. Recently, a study showed that temporal dynamics of CTC intravasation vary dramatically based on circadian rhythm, both in mouse models and in patients with breast cancer, emphasizing the time-critical aspect of biomarker assessment.175
Several high-profile studies have elucidated molecular aspects of CTCs and the role of CTC clusters for metastasis, including the importance of hypoxia, cell–cell junctions, and heterotypic clustering with other cell types in circulation (such as neutrophils),176–179 or demonstrated the feasibility of drug testing on CTCs.180 These findings have not yet found their way into clinical application but hold significant potential as therapeutic targets (Figure 3D).
ctDNA
All cells in the human body release DNA into the bloodstream as circulating cell free DNA (cfDNA). The fraction that is released by cancer cells is called ctDNA and represents a sensitive method to detect and monitor tumor-specific aberration via minimally invasive blood draws as liquid biopsies (Figures 2 and 3).144 Several studies demonstrated the potential role of ctDNA as a clinically relevant tool for early detection, diagnosis, prediction of pathologic complete response (pCR), monitoring of minimal residual disease or relapse and to guide targeted therapies or detect resistance (eg, ESR1 mutations, PI3K mutations).181–187 In early-stage breast cancer, sub analysis of the NeoALTTO trial demonstrated that ctDNA detection can stratify patients with HER-amplified tumors that are at risk for failure to achieve pCR after NACT.183 The neoadjuvant I-SPY 2 trial demonstrated that persistent ctDNA was a predictor of poor response and metastatic recurrence, while clearance of ctDNA after NACT predicted improved survival independent of pCR.188 The CHiRP trial demonstrated the ability of ctDNA to detect MRD in patients with late adjuvant HR+ breast cancer with a median lead time of 12.4 months before overt disease recurrence.189 Ongoing trials are intended to further validate the role of ctDNA as a prognostic and predictive tool in breast cancer. The TRAK-ER trial investigates whether therapy escalation in ER+ patients with ctDNA-based molecular relapse can prevent clinical relapse (NCT04985266). The STRIVE trial is testing whether ctDNA be used for early detection of breast cancer (and other solid tumors) that will occur within one year (NCT03085888). In the advanced setting, both the PlasmaMATCH and SOLAR-1 trial demonstrated the accuracy of ctDNA-based predictive biomarker assessment for mutation-directed therapy.34,184 SOLAR-1 showed that PIK3CA-targeted therapy prolonged survival in patients with HR-positive, HER2- advanced breast cancer.34 Targeted assays such as FoundationOne Liquid CDx or the Guardant360 have been prospectively validated and received FDA approval as companion diagnostics.34,184 Other approaches such as fragmentomics combined with mutation-based analysis hold significant potential to improve diagnosis and management of breast cancer190 (Figure 3D).
Limitations of Current Biomarkers and Future Developments
Survival rates of early-stage breast cancer are excellent, reaching 90% within 5 years.191 Yet roughly one-third of patients experience distant recurrence up to 32 years after initial diagnosis and standard of care treatments.192,193 The prognosis of metastatic disease is drastically reduced to 30% 5-year relative survival.191 We suggest two major shortcomings of current biomarkers and treatments that represent urgent clinical needs to further improve outcomes.
Firstly, the inability of current biomarkers and treatment strategies to efficiently detect and eradicate micro-metastatic disease. Estimates suggest that up to 75% of BC patients harbor micro-metastases or disseminated tumor cells (DTCs) at the time of diagnosis.194,195 DTCs may enter a (initially indolent) state of dormancy that protects them from detection and eradication with current clinical standard of care strategies.196,197 Novel strategies for detection (including liquid biopsies) and targeting of dormant disease (eg, enforcing a dormant state, awakening or targeted eradication of dormant tumor cells) are subject to intensive research efforts.197,198
Secondly, the dearth of therapies that specifically target the metastatic processes such as intra- and extravasation, circulating tumor cell dissemination, homing of tumor cells at distant sites.94 To address this shortcoming, CTCs as the progeny of metastatic tumors could serve as ideal prognostic and predictive biomarkers and targeting of CTCs could directly interrupt the metastatic progression.149 The finding that dissociation of CTC clusters via Na+/K+-ATPase inhibitors (eg, digoxin) dramatically reduces distant metastasis in pre-clinical models underlines this notion and is currently being tested in a phase I/II clinical trial (NCT03928210).
Conclusion
The increasing combination of traditional histopathological and molecular biomarkers, such as genomic alterations and transcriptional signatures, continues to improve clinical management and outcomes in patients with luminal breast cancers. However, there are still limitations to the clinical utility of current biomarkers regarding early detection and follow-up with minimal harm to patients. Emerging technologies such as CTC and ctDNA analysis as liquid biopsies have the potential to improve upon these shortcomings and foster the implementation of precision oncology in the management of luminal breast cancer. The discovery and validation of new biomarkers is an active area of research that will improve our understanding of breast cancer and guide highly personalized treatment approaches in the future.
Disclosure
Anna Höller and Bich Doan Nguyen-Sträuli are co-first authors for this study. Heike Frauchiger-Heuer and Alexander Ring are co-last authors for this study. The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi:10.3322/caac.21708
2. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi:10.1038/35021093
3. Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. 2014;5:412–424. doi:10.5306/wjco.v5.i3.412
4. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi:10.1016/s0140-6736(99)02143-1
5. Waks AG, Winer EP. Breast Cancer Treatment: a Review. JAMA. 2019;321:288–300. doi:10.1001/jama.2018.19323
6. Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–392. doi:10.1023/a:1014778713034
7. Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17:R245–262. doi:10.1677/ERC-10-0136
8. Denoix PF, Baclesse F. Presentation of the project for clinical classification of malignant breast tumors by the international union for cancer control. Mem Acad Chir. 1956;82:407–410.
9. Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A. 1967;57:1740–1743. doi:10.1073/pnas.57.6.1740
10. Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. doi:10.1126/science.182.4108.126
11. Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat. 2010;120:293–308. doi:10.1007/s10549-010-0746-x
12. Slamon DJ, Clark GM, Wong SG, et al. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/ neu Oncogene. Science. 1987;235:177–182. doi:10.1126/science.3798106
13. Kolata G. Breaking ranks, lab offers test to assess risk of breast cancer. N Y Times Web. 1996;A1–A15.
14. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci US A. 2001;98:10869–10874. doi:10.1073/pnas.191367098
15. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi:10.1200/JCO.2008.18.1370
16. Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi:10.1056/NEJMra0801289
17. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi:10.1056/NEJMoa041588
18. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi:10.1056/NEJMoa1510764
19. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi:10.1093/jnci/djr393
20. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi:10.1038/nature11412
21. Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–275. doi:10.1038/bjc.1971.33
22. Baum M. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi:10.1016/s0140-6736(02)09088-8
23. Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant,formerly ICI 182,780,is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi:10.1200/JCO.2002.10.057
24. Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi:10.1200/JCO.1998.16.8.2659
25. Geyer CE, Cameron D, Lindquist D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi:10.1056/NEJMoa064320
26. Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi:10.1056/NEJMoa1113216
27. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi:10.1056/NEJMoa1209124
28. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi:10.1056/NEJMoa2203690
29. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi:10.1056/NEJMoa1706450
30. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi:10.1056/NEJMoa1802905
31. Piccart M, Sørlie T, Perou CM, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. doi:10.1016/S1470-2045(21)00007-3
32. Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol. 2019;37:1956–1964. doi:10.1200/JCO.19.00945
33. Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385:2336–2347. doi:10.1056/NEJMoa2108873
34. Andre F. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929–1940. doi:10.1056/NEJMoa1813904
35. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
36. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375:1738–1748. doi:10.1056/NEJMoa1609709
37. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi:10.1200/JCO.2017.73.7585
38. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi:10.1093/jnci/djp335
39. Atkins D. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi:10.1136/bmj.328.7454.1490
40. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17:59–67. doi:10.3122/jabfm.17.1.59
41. McShane LM, Chauhan SP, Ward TC, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97:1180–1184. doi:10.1093/jnci/dji237
42. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–1785. doi:10.1245/s10434-018-6486-6
43 Narod SA. Tumour size predicts long-term survival among women with lymph node-positive breast cancer. Curr Oncol. 2012;19:249–253. doi:10.3747/co.19.1043
44. Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat. 2018;170:647–656. doi:10.1007/s10549-018-4796-9
45. Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–350. doi:10.1053/ejso.2002.1385
46. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi:10.1016/S1470-2045(13)70035-4
47. Hersh EH, King TA. De-escalating axillary surgery in early-stage breast cancer. Breast. 2022;62(1):S43–S49. doi:10.1016/j.breast.2021.11.018
48. Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi:10.1016/j.annonc.2021.09.019
49. Groheux D, Cochet A, Humbert O, et al. 18 F-FDG PET/CT for Staging and Restaging of Breast Cancer. J Nucl Med. 2016;57(1):17S–26S. doi:10.2967/jnumed.115.157859
50. Groheux D, Hindie E. Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging. 2021;9:221–231. doi:10.1007/s40336-021-00426-z
51. Arnaout A, Varela NP, Allarakhia M, et al. Baseline staging imaging for distant metastasis in women with stages I, II, and III breast cancer. Curr Oncol. 2020;27:e123–e145. doi:10.3747/co.27.6147
52. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:154–161.
53. Ellis IO, GALEA M, BROUGHTON N, et al. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology. 1992;20:479–489. doi:10.1111/j.1365-2559.1992.tb01032.x
54. Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. doi:10.1007/BF01840834
55. Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. doi:10.1186/bcr2607
56. Sundquist M, Thorstenson S, Brudin L, Nordenskjold B. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Study Group. Breast Cancer Res Treat. 1999;53:1–8. doi:10.1023/a:1006052115874
57. Rakha EA, El-Sayed ME, Lee AHS, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–3158. doi:10.1200/JCO.2007.15.5986
58. Henson DE, Ries L, Freedman LS, Carriaga M. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer. 1991;68:2142–2149. doi:10.1002/1097-0142(19911115)68:10<2142::aid-cncr2820681010>3.0.co;2-d
59. Duffy MJ, Harbeck N, Nap M, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 2017;75:284–298. doi:10.1016/j.ejca.2017.01.017
60. Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer,Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:691–722. doi:10.6004/jnccn.2022.0030
61. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30:1194–1220. doi:10.1093/annonc/mdz173
62. Early Breast Cancer Trialists’ Collaborative, G, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi:10.1016/S0140-6736(11)60993-8
63. Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. doi:10.1002/(SICI)1097-0215(20000320)89:2<111::AID-IJC2>3.0.CO;2-W
64. Ruhstaller T, Giobbie-Hurder A, Colleoni M, et al. Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women With Hormone Receptor-Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial. J Clin Oncol. 2019;37:105–114. doi:10.1200/JCO.18.00440
65. Howell A. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi:10.1016/S0140-6736(04)17666-6
66. Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi:10.1016/j.steroids.2014.06.012
67. Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi:10.1002/j.1460-2075.1990.tb08280.x
68. Yu WC, Leung BS, Gao YL. Effects of 17 beta-estradiol on progesterone receptors and the uptake of thymidine in human breast cancer cell line CAMA-1. Cancer Res. 1981;41:5004–5009.
69 Hilton HN, Clarke CL, Graham JD. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol. 2018;466:2–14. doi:10.1016/j.mce.2017.08.011
70. Hammond ME, Hayes DF, Dowsett M, et al. American society of clinical oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi:10.1200/JCO.2009.25.6529
71. Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006;17:818–826. doi:10.1093/annonc/mdl016
72. Ravdin PM, Green S, Dorr TM, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10:1284–1291. doi:10.1200/JCO.1992.10.8.1284
73. Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–1109. doi:10.1200/JCO.1984.2.10.1102
74. Reinert T, Cascelli F, Resende CAAD, et al. Clinical implication of low estrogen receptor (ER-low) expression in breast cancer. Front Endocrinol (Lausanne). 2022;13:1015388. doi:10.3389/fendo.2022.1015388
75. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38:1346–1366. doi:10.1200/JCO.19.02309
76. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi:10.1200/JCO.1999.17.5.1474
77. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140.
78. Pecci A, Ogara MF, Sanz RT, Vicent GP. Choosing the right partner in hormone-dependent gene regulation: Glucocorticoid and progesterone receptors crosstalk in breast cancer cells. Front Endocrinol (Lausanne). 2022;13:1037177. doi:10.3389/fendo.2022.1037177
79. Belachew EB, Sewasew DT. Molecular mechanisms of endocrine resistance in estrogen-positive breast cancer. Front Endocrinol (Lausanne). 2021;12:599586. doi:10.3389/fendo.2021.599586
80. Roskoski R Jr. Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol Res. 2016;107:249–275. doi:10.1016/j.phrs.2016.03.012
81. Andrade de Oliveira K, Sengupta S, Yadav AK, Clarke R. The complex nature of heterogeneity and its roles in breast cancer biology and therapeutic responsiveness. Front Endocrinol (Lausanne). 2023;14:1083048. doi:10.3389/fendo.2023.1083048
82. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst. 2013;105:1036–1042. doi:10.1093/jnci/djt146
83. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067–1076. doi:10.1016/S1470-2045(13)70387-5
84. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi:10.1002/ijc.2910310104
85. Lopez F, Belloc F, Lacombe F, et al. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42–49. doi:10.1002/cyto.990120107
86. Guarneri V, Piacentini F, Ficarra G, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol. 2009;20:1193–1198. doi:10.1093/annonc/mdn761
87. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi:10.1200/JCO.2005.07.501
88. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi:10.1093/annonc/mdr304
89. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi:10.1093/annonc/mdt303
90. Luporsi E, André F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi:10.1007/s10549-011-1837-z
91. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi:10.1016/S1470-2045(09)70262-1
92. Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi:10.1200/JCO.2008.17.0829
93. Dumontet C, Krajewska M, Treilleux I, et al. BCIRG 001 molecular analysis: prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res. 2010;16:3988–3997. doi:10.1158/1078-0432.CCR-10-0079
94. Anderson RL, Balasas T, Callaghan J, et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 2019;16:185–204. doi:10.1038/s41571-018-0134-8
95. Polley MY, Leung SCY, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28:778–786. doi:10.1038/modpathol.2015.38
96. Varga Z, Diebold J, Dommann-Scherrer C, et al. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS One. 2012;7:e37379. doi:10.1371/journal.pone.0037379
97. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/ neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi:10.1126/science.2470152
98. Wolff AC, Elizabeth Hale Hammond M, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–2122. doi:10.1200/JCO.2018.77.8738
99. Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi:10.1200/JCO.2002.20.3.719
100. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi:10.1056/NEJM200103153441101
101. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi:10.1111/cas.12966
102. Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–1962. doi:10.1200/JCO.19.02488
103. Zhang X, Huang AC, Chen F, et al. Novel development strategies and challenges for anti-Her2 antibody-drug conjugates. Antib Ther. 2022;5:18–29. doi:10.1093/abt/tbac001
104. Li J, Ma M, Yang X, et al. Circular HER2 RNA positive triple negative breast cancer is sensitive to Pertuzumab. Mol Cancer. 2020;19:142. doi:10.1186/s12943-020-01259-6
105. Pegram M, Jackisch C, Johnston SRD. Estrogen/HER2 receptor crosstalk in breast cancer: combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer. 2023;9:45. doi:10.1038/s41523-023-00533-2
106. Burstein HJ. Systemic Therapy for Estrogen Receptor-Positive, HER2-Negative Breast Cancer. N Engl J Med. 2020;383:2557–2570. doi:10.1056/NEJMra1307118
107. Turner NC, Swift C, Kilburn L, et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor-Positive Breast Cancer: a Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin Cancer Res. 2020;26:5172–5177. doi:10.1158/1078-0432.CCR-20-0224
108. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi:10.1038/ng.730
109. Mukherjee S, Conrad SE. c-Myc suppresses p21WAF1/CIP1 expression during estrogen signaling and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2005;280:17617–17625. doi:10.1074/jbc.M502278200
110. Zwijsen RM, Wientjens E, Klompmaker R, et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi:10.1016/s0092-8674(00)81879-6
111. Sabine VS, Crozier C, Brookes CL, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014;32:2951–2958. doi:10.1200/JCO.2013.53.8272
112. Bosch A, Li Z, Bergamaschi A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra251. doi:10.1126/scitranslmed.aaa4442
113. Henry NL, Somerfield MR, Dayao Z, et al. Biomarkers for systemic therapy in metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2022;40:3205–3221. doi:10.1200/JCO.22.01063
114. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi:10.1056/NEJMoa1109653
115. Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395:817–827. doi:10.1016/S0140-6736(20)30165-3
116. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi:10.1056/NEJMoa1810527
117. Im SA, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi:10.1056/NEJMoa1903765
118. Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a Randomized clinical trial. JAMA Oncol. 2020;6:116–124. doi:10.1001/jamaoncol.2019.4782
119. Cottu P, D’Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29:2334–2340. doi:10.1093/annonc/mdy448
120. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38:3987–3998. doi:10.1200/JCO.20.02514
121. Trenner A, Sartori AA. Harnessing DNA double-strand break repair for cancer treatment. Front Oncol. 2019;9:1388. doi:10.3389/fonc.2019.01388
122. Kurian AW, Gong GD, John EM, et al. Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2009;18:1084–1091. doi:10.1158/1055-9965.EPI-08-1090
123. Malone KE, Daling JR, Doody DR, et al. Prevalence and Predictors of BRCA1 and BRCA2 Mutations in a Population-Based Study of Breast Cancer in White and Black American Women Ages 35 to 64 Years. Cancer Res. 2006;66:8297–8308. doi:10.1158/0008-5472.CAN-06-0503
124. Harbeck N, Schmitt M, Meisner C, et al. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur J Cancer. 2013;49:1825–1835. doi:10.1016/j.ejca.2013.01.007
125. Harbeck N, Kates RE, Look MP, et al. Enhanced benefit from adjuvant chemotherapy in breast cancer patients classified high-risk according to urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (n = 3424). Cancer Res. 2002;62(62):4617–4622.
126. Cheung KL, Graves CR, Robertson JF. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev. 2000;26:91–102. doi:10.1053/ctrv.1999.0151
127. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi:10.1200/JCO.2007.14.2364
128. Kwa M, Makris A, Esteva FJ. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017;14:595–610. doi:10.1038/nrclinonc.2017.74
129. Sparano JA, Bornschein R, Brown K, et al. adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi:10.1056/NEJMoa1804710
130. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–2964. doi:10.1038/bjc.2013.671
131. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi:10.1200/JCO.2005.04.7985
132. Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi:10.1200/JCO.2009.23.7610
133. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi:10.1016/S1470-2045(09)70314-6
134. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi:10.1200/JCO.2009.24.4798
135. Drukker CA, Bueno‐de‐Mesquita JM, Retèl VP, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer. 2013;133:929–936. doi:10.1002/ijc.28082
136. van ‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi:10.1038/415530a
137. van de Vijver MJ, He YD, van ‘t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi:10.1056/NEJMoa021967
138. Cardoso F, Van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi:10.1056/NEJMoa1602253
139. Martin M, Brase JC, Calvo L, et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 2014;16:R38. doi:10.1186/bcr3642
140. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi:10.1093/annonc/mdt494
141. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33:916–922. doi:10.1200/JCO.2014.55.6894
142. Bartlett JM, Bayani J, Marshall A, et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: no test is more equal than the others. J Natl Cancer Inst. 2016;108:djw050. doi:10.1093/jnci/djw050
143. Buus R, Sestak I, Kronenwett R, et al. Molecular Drivers of Onco type DX, Prosigna, EndoPredict, and the Breast Cancer Index: a TransATAC Study. J Clin Oncol. 2021;39:126–135. doi:10.1200/JCO.20.00853
144. Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297–312. doi:10.1038/s41571-020-00457-x
145. Alix-Panabieres C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858–873. doi:10.1158/2159-8290.CD-20-1311
146. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. doi:10.1126/science.aau6977
147. Hamam R, Kassem M, Zaher W, et al. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8:e3045. doi:10.1038/cddis.2017.440
148. Amelio I, Bernassola F, Candi E. Emerging roles of long non-coding RNAs in breast cancer biology and management. Semin Cancer Biol. 2021;72:36–45. doi:10.1016/j.semcancer.2020.06.019
149. Ring A, Nguyen-Strauli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2022;22:1–17. doi:10.1038/s41568-022-00536-4
150. Martinez-Jimenez F, Movasati A, Brunner SR, et al. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature. 2023;618:333–341. doi:10.1038/s41586-023-06054-z
151. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi:10.1016/S1470-2045(14)70069-5
152. Cristofanilli M, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi:10.1056/NEJMoa040766
153. Allard WJ, Repollet M. Connelly MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi:10.1158/1078-0432.CCR-04-0378
154. Hayes DF, Miller EM, GV MJ. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi:10.1158/1078-0432.CCR-05-2821
155. Giuliano M, Giordano A, Jackson S, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13:R67. doi:10.1186/bcr2907
156. Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106. doi:10.1093/jnci/dju066
157. Ignatiadis M, Litière S, Rothe F, et al. Trastuzumab versus observation for HER2 nonamplified early breast cancer with circulating tumor cells (EORTC 90091-10093, BIG 1-12, Treat CTC): a randomized phase II trial. Ann Oncol. 2018;29:1777–1783. doi:10.1093/annonc/mdy211
158. Janni WJ, Rack B, Terstappen LWMM, et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res. 2016;22:2583–2593. doi:10.1158/1078-0432.CCR-15-1603
159. Cristofanilli M, Pierga J-Y, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39–45. doi:10.1016/j.critrevonc.2018.12.004
160. Riethdorf S, Müller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16:2634–2645. doi:10.1158/1078-0432.CCR-09-2042
161. Pierga JY, Bidard F-C, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14:7004–7010. doi:10.1158/1078-0432.CCR-08-0030
162. Georgoulias V, Bozionelou V, Agelaki S, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23:1744–1750. doi:10.1093/annonc/mds020
163. Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–3489. doi:10.1200/JCO.2014.56.2561
164. Jacot W, Cottu P, Berger F, et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial. Breast Cancer Res. 2019;21:121. doi:10.1186/s13058-019-1215-z
165. Pestrin M, Bessi S, Puglisi F, et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat. 2012;134:283–289. doi:10.1007/s10549-012-2045-1
166. Bidard FC, Jacot W, Kiavue N, et al. Efficacy of circulating tumor cell count-driven vs clinician-driven first-line therapy choice in hormone receptor-positive, ERBB2-negative metastatic breast cancer: the STIC CTC randomized clinical trial. JAMA Oncol. 2021;7:34–41. doi:10.1001/jamaoncol.2020.5660
167. Muller V, Banys-Paluchowski M, Friedl TWP, et al. Prognostic relevance of the HER2 status of circulating tumor cells in metastatic breast cancer patients screened for participation in the DETECT study program. ESMO Open. 2021;6:100299. doi:10.1016/j.esmoop.2021.100299
168. Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi:10.1158/1078-0432.CCR-06-1695
169. Miller MC, Robinson PS, Wagner C, et al. The Parsortix Cell Separation System-A versatile liquid biopsy platform. Cytometry A. 2018;93:1234–1239. doi:10.1002/cyto.a.23571
170. Lin D, Shen L, Luo M, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6:404. doi:10.1038/s41392-021-00817-8
171. Saucedo-Zeni N, Mewes S, Niestroj R, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. 2012;41:1241–1250. doi:10.3892/ijo.2012.1557
172. Eifler RL, Lind J, Falkenhagen D, et al. Enrichment of circulating tumor cells from a large blood volume using leukapheresis and elutriation: proof of concept. Cytometry B Clin Cytom. 2011;80B:100–111. doi:10.1002/cyto.b.20560
173. Crosbie PA, Shah R, Krysiak P, et al. Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. J Thorac Oncol. 2016;11:1793–1797. doi:10.1016/j.jtho.2016.06.017
174. Chemi F, Rothwell DG, McGranahan N, et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med. 2019;25:1534–1539. doi:10.1038/s41591-019-0593-1
175. Diamantopoulou Z, Castro-Giner F, Schwab FD, et al. The metastatic spread of breast cancer accelerates during sleep. Nature. 2022;607:156–162. doi:10.1038/s41586-022-04875-y
176. Donato C, Buczak K, Schmidt A, Aceto N. Mass spectrometry analysis of circulating breast cancer cells from a Xenograft mouse model. STAR Protoc. 2021;2:100480. doi:10.1016/j.xpro.2021.100480
177. Donato C, Kunz L, Castro-Giner F, et al. Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep. 2020;32:108105. doi:10.1016/j.celrep.2020.108105
178. Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112.e114. doi:10.1016/j.cell.2018.11.046
179. Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi:10.1038/s41586-019-0915-y
180. Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi:10.1126/science.1253533
181. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. doi:10.1126/scitranslmed.aab0021
182. McDonald BR, Contente-Cuomo T, Sammut S-J, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med. 2019;11. doi:10.1126/scitranslmed.aax7392
183. Rothe F, Silva MJ, Venet D, et al. Circulating Tumor DNA in HER2-Amplified Breast Cancer: A Translational Research Substudy of the NeoALTTO Phase III Trial. Clin Cancer Res. 2019;25:3581–3588. doi:10.1158/1078-0432.CCR-18-2521
184. Turner NC, Kingston B, Kilburn LS, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296–1308. doi:10.1016/S1470-2045(20)30444-7
185. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7:313ra182. doi:10.1126/scitranslmed.aac7551
186. Kodahl AR, Ehmsen S, Pallisgaard N, et al. Correlation between circulating cell-free PIK 3 CA tumor DNA levels and treatment response in patients with PIK 3 CA -mutated metastatic breast cancer. Mol Oncol. 2018;12:925–935. doi:10.1002/1878-0261.12305
187. Cullinane C, Fleming C, O’Leary DP, et al. Association of circulating tumor DNA with disease-free survival in breast cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2026921. doi:10.1001/jamanetworkopen.2020.26921
188. Magbanua MJM, Swigart LB, Wu H-T, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021;32:229–239. doi:10.1016/j.annonc.2020.11.007
189. Lipsyc-Sharf M, de Bruin EC, Santos K, et al. Circulating Tumor DNA and Late Recurrence in High-Risk Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J Clin Oncol. 2022;40:2408–2419. doi:10.1200/JCO.22.00908
190. Cristiano S, Leal A, Phallen J, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385–389. doi:10.1038/s41586-019-1272-6
191. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2019) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021. Underlying mortality data provided by NCHS. Available from: www.cdc.gov/nchs.
192. Tevaarwerk AJ, Gray RJ, Schneider BP, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119:1140–1148. doi:10.1002/cncr.27819
193. Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10–32 years after primary diagnosis. J Natl Cancer Inst. 2022;114:391–399. doi:10.1093/jnci/djab202
194. Hu Z, Ding J, Ma Z, et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. 2019;51:1113–1122. doi:10.1038/s41588-019-0423-x
195. Friberg S, Nystrom A. Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis. 2015;8:43–49. doi:10.4137/CGM.S31244
196. Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20:398–411. doi:10.1038/s41568-020-0263-0
197. Ring A, Spataro M, Wicki A, Aceto N. Clinical and biological aspects of disseminated tumor cells and dormancy in breast cancer. Front Cell Dev Biol. 2022;10:929893. doi:10.3389/fcell.2022.929893
198. Park SY, Nam JS. The force awakens: metastatic dormant cancer cells. Exp Mol Med. 2020;52:569–581. doi:10.1038/s12276-020-0423-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.