Back to Journals » Infection and Drug Resistance » Volume 17
Diagnosis of a Familial Psittacosis Outbreak with Clinical Analysis and Metagenomic Next-Generation Sequencing Under COVID-19: A Case Series
Authors Wang J, Jia P, Zhang D, Zhao Y, Sui X, Jin Z, Song W
Received 19 October 2023
Accepted for publication 5 March 2024
Published 18 March 2024 Volume 2024:17 Pages 1099—1105
DOI https://doi.org/10.2147/IDR.S440400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Jiaru Wang,1 Peiyao Jia,2 Dong Zhang,2 Ying Zhao,2,3 Xin Sui,1 Zhengyu Jin,1,* Wei Song1,*
1Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China; 3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Song, Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, No. 1 Shuaifuyuan Wangfujing Dongcheng District, Beijing, 100730, People’s Republic of China, Tel/Fax +86 10 69159525, Email [email protected]
Purpose: To explore the clinical characteristics, diagnosis, and treatment of family outbreak of psittacosis and to improve the success rate of treatment.
Patients and Methods: The clinical characteristics, diagnosis, treatment, and outcome of family outbreak of psittacosis, which consists three patients, diagnosed by clinical analysis and metagenomic next-generation sequencing (mNGS) in our hospital were analyzed retrospectively.
Results: We report on three instances of clustered atypical pneumonia, which were caused by Chlamydia psittaci during the COVID-19 pandemic. All patients exhibited symptoms of fever and cough, while one patient also experienced gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Laboratory tests indicated no significant increase in leukocytes and neutrophils, but a mild increase in C-reactive protein was observed in all three patients. Chest computed tomography (CT) scans revealed a consolidation shadow in a unilateral lung lobe in all three patients. Both patients were treated with empirical moxifloxacin, yielding unsatisfactory outcomes. mNGS was conducted on sputum samples from one adult patient, revealing the presence of Chlamydia psittaci. Additional doxycycline was prescribed immediately, and then the patients’ temperatures were stabilized, and the lesion in chest CT was absorbed. The pediatric patient exhibited less severe symptoms compared to the adult patients and exhibited a favorable response to azithromycin administration.
Conclusion: This study reports a cluster of a family outbreak of atypical pneumonia caused by C. psittaci in China. The occurrence of a family outbreak during the COVID-19 pandemic may be attributed to familial aggregation resulting from the epidemic. The three cases reported in this study did not experience severe complications, which can be attributed to the prompt medical intervention and swift diagnosis. This finding implies the need to enhance patients’ awareness and vigilance towards their health. Additionally, mNGS emerges as a valuable technique for accurately identifying pathogens causing pulmonary infections.
Keywords: psittacosis, outbreak, COVID-19, metagenomic next-generation sequencing, pneumonia
Introduction
Chlamydia psittaci is an intracellular bacterium of Chlamydiaceae family.1 Psittacosis, a zoonotic disease caused by C. psittaci, primarily affects individuals with close contact with birds, particularly poultry. The disease typically manifests in humans following exposure to infected poultry or their excreta and has an incubation period of 1–4 weeks. Psittacosis is usually sporadic,2,3 but human-to-human transmission has also been reported.4,5 There were a few reports of human psittacosis outbreaks in European countries, United States, and Australia etc.6–10 However, there were fewer reports on the clustered onset of C. psittaci in China.11 The course of C. psittaci can vary from acute to chronic, with clinical presentations ranging from asymptomatic to severe pneumonia. Additionally, patients may have multiple extrapulmonary complications, including acute kidney injury, rhabdomyolysis, and meningitis,12,13 which can be life-threatening if not managed promptly. However, rapid diagnosis of psittacosis in the early disease stages is difficult because of the similarity of the clinical presentation with other common community-acquired pneumonia.
Isolating and cultivating C. psittaci from sputum or alveolar lavage fluid are crucial diagnostic steps. However, culturing C. psittaci is a time-intensive endeavor and necessitates highly graded biosafety facilities and serological outcomes in patients are typically nonspecific, rendering precise diagnosis challenging. Metagenomic next-generation sequencing (mNGS) is a high-throughput sequencing technique that enables direct comparison of DNA/RNA in extracted clinical samples with a pathogen database. This method exhibits the ability to efficiently and objectively identify uncommon pathogenic microorganisms in clinical specimens, thereby presenting substantial potential for comprehensive infection diagnosis, particularly in instances of severe pneumonia.14
In this study, we present a comprehensive analysis of the clinical manifestations observed in a familial outbreak of psittacosis during the COVID-19 pandemic. The occurrence of family outbreaks during the COVID-19 pandemic may be associated with the clustering of cases within families as a result of the epidemic. The diagnosis of psittacosis was confirmed using mNGS, highlighting the utility of this technique in accurately identifying pulmonary pathogens causing infections.
Case Presentation
Patient 1
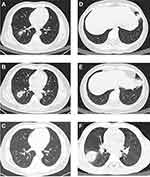
A 47-year-old male presented to the emergency department with fever, cough, dyspnea, and diarrhea. Notably, the patient had received a COVID-19 vaccination and reported owning a parrot. Laboratory findings indicated elevated levels of inflammatory markers, with a high-sensitive C-reactive protein (hsCPR) value of 33.4 mg/L (reference value <8 mg/L), but no significant changes in leukocyte or neutrophil counts (Table 1). Additionally, a chest computer tomography (CT) scan showed a scattered infiltrative patchy in the lower lobe of the right lung (Figure 1A). Following two days of empirical moxifloxacin treatment, hsCPR increased, and a repeat chest CT scan indicated significant expansion of the lesion (Figure 1B). His pCO2 levels also rose, and his pO2 levels, oxygen saturation, and oxyhemoglobin fraction significantly decreased. Considering the patient’s history of exposure to parrots, he was admitted to the hospital for atypical pneumonia and underwent mNGS of the sputum. 51 specific C. psittaci sequences that covered 0.57% of the total C. psittaci genome were detected by mNGS-DNA, and 3 specific C. psittaci sequences that covered 0.03% of the total C. psittaci genome were detected by mNGS-RNA (Table 2). Subsequently, sanger sequencing was applied to further identify C. psittaci subtype based on its outer membrane protein A gene (ompA) sequence. The following primers (Table 3) were used to amplify the ompA gene from the DNA nucleic acid extracted from sputum. After amplification (Figure 2A) and sequencing, the ompA gene sequence in this case was analyzed using CLC Sequence Viewer 8, indicating that ompA gene sequence amplified from patient 1 was completely consistent with type A (Figure 2B). Consequently, the patient was prescribed two weeks of doxycycline (100 mg twice a day). A follow-up assessment six months later demonstrated complete resolution of pneumonia on repeat CT scans (Figure 1C). No sequencing or treatment was performed on the parrot.
 |
Table 1 Clinical Characteristics and Laboratory Date of Three Patients |
 |
Table 2 mNGS Results of the Psittacosis Pneumonia Cases |
 |
Table 3 Amplification Primers of ompA Gene |
Patient 2
A 40-year-old woman presented to the emergency department with fever, cough, nausea, and loss of appetite. She disclosed that she had received a COVID-19 vaccination and was the spouse of patient 1. Laboratory findings indicated elevated inflammatory markers, including a hsCPR of 25.5 mg/L (with a reference value of <8 mg/L) and a slightly increased proportion of neutrophils and monocytes (Table 1). A chest CT scan revealed a patchy consolidation in the left lower lobe (Figure 1D). She was prescribed empiric moxifloxacin, but her condition did not satisfactorily improve. Subsequent hsCRP test conducted two days later revealed a continued elevation, while a chest CT scan indicated the presence of a progressively expanding lesion (Figure 1E). She was then prescribed two weeks of doxycycline (100 mg twice a day) due to her husband’s diagnosis of psittacosis through mNGS. A follow-up assessment six months later demonstrated the complete resolution of pneumonia on a CT scan. No sequencing or treatment was performed on the parrot.
Patient 3
A 7-year-old male patient presented to the emergency department with a fever. He had received the COVID-19 vaccination and was the offspring of patient 1 and patient 2. His symptoms were relatively mild when compared to those of patient 1 and patient 2, and his initial C-reactive protein (CPR) level was 5 mg/L (reference value <8 mg/L) (Table 1). However, his chest CT scan revealed a solid shadow in the lower lobe of the right lung which was actually more extensive than that of the parents (Figure 1F). He was prescribed azithromycin (240 mg twice a day) based on his parents’ test results.
Discussion
C. psittaci is a gram-negative, intracellular parasitic pathogen that primarily infects birds but shows occasional transmission to human.2 Notably, there were fewer reports on the clustered onset of C. psittaci in China,11 which might be associated with a lower prevalence of pathogen carriers among Asian parrots compared to European parrots15 This study reports a case of a family outbreak of atypical pneumonia caused by C. psittaci during the COVID-19 pandemic.
In this study, it took only three days for the diagnoses to be confirmed after the onset of symptoms. Since late 2019, COVID-19 has emerged as a highly prevalent respiratory pathogen in humans, prompting patients to have heightened concern regarding respiratory symptoms such as fever, coughs, and dyspnea. In this context, the three patients in our study sought immediate medical attention at a nearby emergency department after experiencing a family-wide fever cluster. After inquiring about the patients’ history of exposure to a parrot, the attending emergency department physician arranged for mNGS testing, enabling a swift and accurate diagnosis of the ailment.
The diagnosis of psittacosis pneumonia has traditionally relied on a history of contact with infected birds and the detection of pathogens in bronchial secretions. The primary diagnostic tests for psittacosis include serological assays, pathogenic cultures, and polymerase chain reactions (PCRs). Serologic testing is not a viable option for diagnosing psittacosis during the acute phase, and pathogenic culture is susceptible to specimen contamination and has a low positivity rate. And although PCR detection is both accurate and expeditious, its reliance on identifying a specific pathogen renders it susceptible to overlooking singular pathogens.16 In contrast, mNGS is a non-culture-based, next-generation pathogen detection technology that boasts rapid and efficient detection of clinical samples, a high rate of positive pathogen detection, swift detection speed, broad coverage, and heightened sensitivity. Therefore, mNGS has widespread application in identifying unknown pathogens, including genetic diseases, tumors, infectious diseases, and in noninvasive prenatal screening.17 If intracellular bacteria or rare pathogens are detected, they should be considered as causative agents even if the overall sequence number is low.
All three patients in the current study presented fever and weakness, two adult patients also experienced gastrointestinal symptoms, including nausea, poor appetite, and diarrhea, which was similar with previously reported cases.18 The laboratory data of patients showed normal or slightly elevated neutrophilic granulocyte percentage and PCT, along with high hsCRP levels. This might be due to the fact that C. psittaci is more pathogenic and reproduces faster than other chlamydiae,19 resulting in a more severe inflammatory response caused by C. psittaci. Chest CT scans revealed patchy, solid lesions in a single lung lobe in all three patients, consistent with prior research. Notably, one of the patients was a 7-year-old child who displayed milder symptoms than his parents but actually had a more extensive lesion on chest CT, suggesting a combination of mild clinical symptoms but more severe imaging signs in pediatric pneumonia.
Previous research has indicated that tetracycline is the preferred therapeutic option for treating C. psittaci infections.20 Azithromycin has also demonstrated efficacy in this condition. Conversely, quinolones have limited inhibitory effects and are only suitable as a secondary treatment option.21 Macrolide antibiotics, including azithromycin, are deemed optimal for patients who cannot tolerate tetracyclines. In cases of severe, life-threatening infections, a combination of tetracycline, macrolides, and quinolones may be necessary. Following administration of tetracycline antibiotics, a reaction typically occurs within 24–48 hours, often manifesting as a decrease in body temperature. Treatment regimens are recommended to be at least 14 days, and preferably 21 days, to minimize the risk of recurrence. In the present case, the two adult patients were administered moxifloxacin for two days without significant reductions in body temperature, and their chest CT scans revealed increases in pulmonary consolidation. Upon confirmation of C. psittaci infection, the two adult patients were treated with doxycycline, and the pediatric patient was treated with azithromycin. Following these treatments, all patients’ temperatures stabilized immediately. A follow-up assessment six months later demonstrated complete absorption of the pulmonary lesion on CT scans.
In our study, it was unfortunate for these three patients in this family outbreak. The implementation of social isolation and the imposition of recreational limitations as a result of the COVID-19 pandemic may result in a heightened desire for pet ownership. Concurrently, the prevalence of COVID-19 has led to a reduction in non-essential travel, thereby increasing the likelihood of familial clustering and the potential for a family-wide outbreak of psittacosis. On the contrary, fortuitously, the COVID-19 pandemic has instilled a heightened sense of health consciousness among patients, specifically regarding respiratory symptoms. In our study, the three patients promptly sought medical intervention within a mere 12-hour timeframe upon encountering a fever cluster affecting their entire family. This expeditious response allowed for extended periods of diagnosis and treatment, thereby facilitating the acquisition of an accurate diagnosis and prompt treatment within a maximum of 3 days from the onset of symptoms. Consequently, this timely intervention may potentially avert the occurrence of more severe complications.
Conclusion
This study reports a cluster of a family outbreak of atypical pneumonia caused by C. psittaci in China, which was very rare. The occurrence of a family outbreak during the COVID-19 pandemic may be attributed to familial aggregation resulting from the epidemic. Fortunately, the three cases reported in this study did not experience severe complications, which can be attributed to the prompt medical intervention and swift diagnosis. This finding implies the need to enhance patients’ awareness and vigilance towards their health. Additionally, mNGS emerges as a valuable technique for accurately identifying pathogens causing pulmonary infections.
Ethics Approval and Informed Consent
This case report was approved by the Institutional Review Board of the Peking Union Medical College Hospital (K23C2638). Written informed consent for publication of their details was obtained from the patient and guardians.
Acknowledgment
We extend our heartfelt appreciation to Ying Zhao for her invaluable assistance in the implementation of mNGS techniques.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2301002), National Natural Science Foundation of China (8217120884), and National High Level Hospital Clinical Research Funding (2022‐PUMCH‐B‐069, 2022‐PUMCH‐A‐034).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rybarczyk J, Versteele C, Lernout T, Vanrompay D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. 2020;75(1):42–48. doi:10.1080/17843286.2019.1590889
2. Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315(3):161–168. doi:10.1056/nejm198607173150305
3. Marrie TJ, Grayston JT, Wang SP, Kuo CC. Pneumonia associated with the TWAR strain of Chlamydia. Ann Intern Med. 1987;106(4):507–511. doi:10.7326/0003-4819-106-4-507
4. Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January–February 2013. Eurosurveillance. 2014;19(42):20937. doi:10.2807/1560-7917.ES2014.19.42.20937
5. Zhang Z, Zhou H, Cao H, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. 2022;3(7):e512–e520. doi:10.1016/S2666-5247(22)00064-7
6. Berk Y, Klaassen CH, Mouton JW, Meis JF. An outbreak of psittacosis at a bird-fanciers fair in the Netherlands. Ned Tijdschr Geneeskd. 2008;152(34):1889–1892.
7. Hedberg K, White KE, Forfang JC, et al. An outbreak of psittacosis in Minnesota Turkey industry workers: implications for modes of transmission and control. Am J Epidemiol. 1989;130(3):569–577. doi:10.1093/oxfordjournals.aje.a115371
8. Tiong A, Vu T, Counahan M, Leydon J, Tallis G, Lambert S. Multiple sites of exposure in an outbreak of ornithosis in workers at a poultry abattoir and farm. Epidemiol Infect. 2007;135(7):1184–1191. doi:10.1017/s095026880700790x
9. Gaede W, Reckling KF, Dresenkamp B, et al. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health. 2008;55(4):184–188. doi:10.1111/j.1863-2378.2008.01108.x
10. Belchior E, Barataud D, Ollivier R, et al. Psittacosis outbreak after participation in a bird fair, Western France, December 2008. Epidemiol Infect. 2011;139(10):1637–1641. doi:10.1017/s0950268811000409
11. Li N, Li S, Tan W, Wang H, Xu H, Wang D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerging Microbes Infect. 2021;10(1):1418–1428. doi:10.1080/22221751.2021.1948358
12. Shi Y, Chen J, Shi X, et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. 2021;21(1):1–8. doi:10.1186/s12879-021-06205-5
13. Zhang A, Xia X, Yuan X, et al. Severe Chlamydia psittaci pneumonia complicated by rhabdomyolysis: a case series. Infect Drug Resist. 2022;15:873–881. doi:10.2147/IDR.S355024
14. Langelier C, Kalantar KL, Moazed F, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A. 2018;115(52):E12353–E12362. doi:10.1073/pnas.1809700115
15. Cong W, Huang SY, Zhang XX, et al. Chlamydia psittaci exposure in pet birds. J Med Microbiol. 2014;63(Pt 4):578–581. doi:10.1099/jmm.0.070003-0
16. Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi:10.1007/s15010-020-01429-0
17. Langelier C, Zinter MS, Kalantar K, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med. 2018;197(4):524–528. doi:10.1164/rccm.201706-1097LE
18. de Gier B, Hogerwerf L, Dijkstra F, van der Hoek W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018;146(3):303–305. doi:10.1017/s0950268817003065
19. Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73(1):1–15. doi:10.1093/femspd/ftu007
20. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinl Infect Dis. 2007;44(Supplement_2):S27–72. doi:10.1086/511159
21. Paul L, Comstock J, Edes K, Schlaberg R. Gestational Psittacosis Resulting in Neonatal Death Identified by Next-Generation RNA Sequencing of Postmortem, Formalin-Fixed Lung Tissue. US: Oxford University Press; 2018:ofy172.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.