Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Detection and Severity Identification of Neonatal Seizure Using Deep Convolutional Neural Networks from Multichannel EEG Signal
Authors Debelo BS, Thamineni BL, Dasari HK, Dawud AA
Received 21 July 2023
Accepted for publication 26 October 2023
Published 1 November 2023 Volume 2023:14 Pages 405—417
DOI https://doi.org/10.2147/PHMT.S427773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Biniam Seifu Debelo,1 Bheema Lingaiah Thamineni,2 Hanumesh Kumar Dasari,3 Ahmed Ali Dawud2
1Department of Biomedical Engineering, Nigist Eleni Mohamed Memorial Compressive Specialized Hospital, Wachamo University, Hosanna, Ethiopia; 2School of Biomedical Engineering, Jimma Institute of Technology, Jimma University, Jimma, Ethiopia; 3Department of Electronics and Communication, Rayalaseema University, Kurnool, AP, India
Correspondence: Ahmed Ali Dawud, School of Biomedical Engineering, Jimma Institute of Technology, Jimma University, Jimma, Ethiopia, Email [email protected]
Introduction: One of the most frequent neurological conditions in newborns is neonatal seizures, which may indicate severe neurological dysfunction. These seizures may have very subtle or very modest clinical indications because patterns like oscillatory (spike) trains begin with relatively low amplitude and gradually increase over time. This becomes very challenging and erroneous if clinical observation is the primary basis for identifying newborn seizures. In this study, a diagnosis system using deep convolutional neural networks is proposed to determine and classify the severity level of neonatal seizures using multichannel neonatal EEG data.
Methods: Datasets from publicly accessible online sources were used to compile clinical multichannel EEG datasets. Various preprocessing steps were taken, including the conversion of 2D time series data to equivalent waveform pictures. The proposed models have undergone training, and evaluations of their performance were conducted.
Results: The proposed CNN was used to perform binary classification with an accuracy of 92.6%, F1-score of 92.7%, specificity of 92.8%, and precision of 92.6%. To detect newborn seizures, this model is utilized. Using the proposed CNN model, multiclassification was performed with accuracy rates of 88.6%, specificity rates of 92.18%, F1-score rates of 85.61%, and precision rates of 88.9%. The results demonstrated that the suggested strategy can assist medical professionals in making accurate diagnoses close to healthcare institutions.
Conclusion: The developed system was capable of detecting neonatal seizures and has the potential to be used as a decision-making tool in resource-limited areas with a scarcity of expert neurologists.
Keywords: AlexNet, CNN, multichannel EEG, neonatal seizure, severity identification
Introduction
Seizures in newborns are a typical occurrence in intensive care units, affecting 1–7 out of 1000 full-term babies (37 to 42 weeks gestation), and are more common in preterm neonates (less than 37 weeks gestation) by approximately 57–132 out of 1000.1 The frequency of epileptic episodes increases as gestational age (GA) and birth weight decrease from the time of birth to the completion of the neonatal life stages.2 Hypoxia-ischemia, hemorrhage, intracerebral infarction, trauma, infections, aberrant cerebral development, drug withdrawal, fetal distress, toxicity, and other conditions are among the causes of neonatal seizures.2,3
The newborn stage is the most susceptible to seizure development of all life stages, especially in the first 1–2 days to weeks after delivery. Neonatal seizures can be challenging to recognize since they might be brief or fragmentary, the infant’s brain is still developing, and it is unable to produce coordinated reactions shown in older children.4,5 Seizures in newborns put them in danger of death, while those who survive are at risk for neurological issues, developmental delays, epilepsy in later life, and cognitive impairment.6 As a result, organized approaches for deciding the best diagnosis and treatment methodology for neonatal seizure management are critical. Differential diagnosis,7 pathology testing,8 neuroimaging,9 and neurophysiology10 tests are frequently used to diagnose neonatal seizures. On the other hand, these diagnostic methods are expensive and time-consuming. The majority of them necessitates the knowledge and excellent visual acuity of numerous experts. Medical imaging methods that are sophisticated and robust are complex, involve exposure to radioligands, and are available only at centralized health facilities.
Recently, EEG-based diagnosis of neonatal seizures has become an alternative tool in neonatal intensive care units (NICUs).11 Despite the accessibility of EEG in most NICUs and the expansion of neonatal neurocritical care systems, reliable seizure diagnosis and treatment remain difficult. Recording EEG (continuous multichannel EEG) for long hours and aEEG (amplitude integrated EEG with video by an expert neurologist (neurophysiologist)) are two common methods for detecting and seeing neonatal seizures.12 However, such interpretation is exceedingly labor intensive, time consuming, and costly, and necessitates specialized expertise that is not always accessible in neonatal critical care units worldwide, especially in developing countries.
Artificial intelligence (AI)-based computer-aided diagnostics can reduce medical professionals’ workloads.13 EEG datasets should be used to diagnose neonatal seizures using a computer-aided diagnosis technique, according to several studies.14–16 According to Deburchgraeve et al.17 A heuristic model that replicates a human EEG reader is a good option. Over 217 hours, multichannel EEG recordings from 21 patients with seizures and 5 patients without seizures were used to test the complete approach. To identify and categorize neonatal seizures as suggested by several researchers, a support vector machine (SVM) using several feature-based studies has been used.18–20 Similarly, Temko et al21 designed a multichannel patient-independent neonatal seizure detection system based on the support vector machine (SVM) classifier with an average detection rate of 89%.
The creation of novel EEG classification algorithms that do not require a manual feature extraction stage has benefited from advances in deep learning-based research.22–24 Ansari et al.25 Proposed neonatal seizure detection using deep convolutional neural networks (CNNs). In this work, the random forest (RF) is used as an automatic feature selection as well as a classification tool. It uses a test set of multichannel EEG datasets from 22 babies, resulting in a false alarm rate of 0.9 Hr and a neonatal seizure detection rate of 77%. Samanta D et al.26 The proposed “neonatal seizure detection from raw multichannel EEG using a fully convolutional neural network”, claimed to improve by 56% compared to feature-based algorithms.
Previous studies have produced promising results on the ability to identify newborn seizures using EEG recordings. However, the majority of the studies relied on already established characteristics, required precise and strong expert labeling for each channel, had a high computational load, and used chaos theory for time-frequency analysis. Most of the research has used single-channel EEG signals; therefore, compared to multichannel EEG recorded data, some fundamental information on the diagnosis and management of neonatal seizure cases would be lost. Preterm neonates were also not included in the study, and a severity level identification study was not done. In this study, deep learning based neonatal seizure detection was proposed using binary classification as normal or abnormal classes and multiclassification for severity level identification of neonatal seizures as moderate (S_1), mild (S_2), or severe (S_3).
Materials and Methods
The system is proposed for applying deep learning to diagnose newborn seizures using multichannel EEG recordings. In this study, two CNN models a customized CNN and a pretrained AlexNet were proposed. It was trained using scaled raw waveform images made from multichannel EEG datasets that had been segmented. The main block diagram of the proposed system is shown in Figure 1 while Table 1 shows the list of materials used in this work.
 |
Table 1 The Materials Used in This Work |
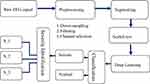 |
Figure 1 The overall methodology of the developed system. |
Data Collection
The multichannel neonatal EEG signal used in this study was collected from a publicly available dataset.27 The recording was collected using a 10–20 electrode placement system. The data were collected from 79 infants admitted to the Helsinki University Hospital NICU, Finland. Signals were sampled at 256 Hz and recorded for an average of 74 minutes. Three experts from different parts of the world independently noted the presence of seizures in the EEGs.28 An established definition of a seizure, consisting of persistent, recurrent evolving spike/sharp waves or rhythmic waveforms, with a definite beginning and ending, was used to annotate them. Each of the 79 element cell arrays in the MAT file that make up the file corresponds to a study neonate identification number. The databases annotated by these three specialists are also accessible as CSV files (A, B, and C).
According to the study, by far the common cause for neonatal seizure is hypoxic-ischemic encephalopathy (HIE); sometimes it’s called asphyxia. It is responsible for 80% of all seizure in the first two days.29 In Zenodo dataset clinical information data (data 3 in .csv format) out of 79 some neonates the severity of HIE or asphyxia were listed by experts as moderate, mild, or severe asphyxia. Because the severity of asphyxia is largely related to the severity of neonatal seizures, which has a direct impact on neonatal EEG. In order to categorize seizures as moderate, mild, or severe, the initial step is to choose neonatal data from clinical records for each category using expert opinions on asphyxia severity (moderate, mild, or severe). Second, for neonates selected according to clinical information, expert consent during annotation is also considered.
These data can be utilized as a reference dataset of neonatal seizures, to perform interobserver agreement studies, and to develop automated seizure detection algorithms and other EEG-based analyses.27 Figure 2 illustrates the database’s general organizational structure.
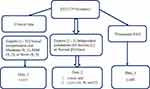 |
Figure 2 The overall structure of dataset and annotation.27 |
Preprocessing
Segmenting or windowing, bandpass filtering, downsampling, and minimizing weak signals and/or undesirable channels are the key preprocessing stages in this study. With the help of the MATLAB module EEGLAB, these preprocessing processes were carried out. Each class’s raw waveform of the preprocessed neonatal EEG is prepared and saved in an image (.jpg) format. The images were then resized to a set number of pixels to fit the input size of the pretrained AlexNet models and the customized CNN models. Figure 3 details the overall preprocessing block diagram.
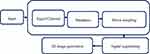 |
Figure 3 The overall preprocessing of the algorithm. |
Signal/Channel Selection and Bad Signal Removal
Neonatal EEG has in Zenodo dataset has 19 channels but, along with 19 channels there are respiratory and EKG (ECG) channels. Because respiratory and ECG channels have spike waves, artefacts, and noises that have an effect on visualization during scaled image preparation for the proposed model training and testing processes. In order to concentrate on 19 channels (bi-polar and referential electrodes), channel selection therefore entails the removal of respiratory and ECG channels.
Bandpass Filtering
The multi-channel EEG signal is band-pass filtered between 0.1 Hz and 15 Hz in the proposed work because newborn seizures occur between 0.1 and 12 Hz in frequency.30
Down Sampling
To simplify the CNN network and account for the differences in neonatal seizure characteristics, the 256 Hz multichannel EEG signal was down-sampled to 32 Hz. This was necessary due to the very low amplitude (<100μV/cm) and low frequency (0.1 to 12 Hz) of neonatal seizures compared to adults.30,31
Signal Segmenting
The raw continuous EEG data is divided into predetermined time periods using one of the signal processing techniques called signal segmentation. EEG signals were first captured at 256 Hz sampling rate. EEG shows sudden, repetitive, stereotyped, evolving waveforms that last at least 10 seconds and have a clear beginning, middle, and end as seizures.32
In this work each of these segments includes 10 seconds of EEG data prepared using manual segmentation from all available channels. The manual segmentation of multichannel EEG signal has been performed based on expert annotated data.
Proposed Models
Pretrained AlexNet
AlexNet is a deep CNN architecture created by Alex Krizhevsky in collaboration with S. Ilya and H. Geoffrey.28 The architecture was created in 2012 and represented a significant step forward in CNN development. The use of several GPUs for training and using an enhanced version of the image are two of the main benefits of adopting the AlexNet architecture. Key developments were the ReLU activation function, overlapping pools, and dropout. Therefore, employing these techniques reduces the difficulty of neuronal adaptation and enables the model to identify different aspects. The first phase in learning new proposed works is transfer learning, which is carried out using the existing network. Using transfer learning to fine-tune the network is typically quicker and less difficult than starting from scratch. We must replace the last three fully linked layers, the softmax layer, and the classification layer to use the current AlexNet network. A 227 * 227-pixel input image is required by the model. The proposed AlexNet architecture for binary classification is shown in Figure 4.
 |
Figure 4 The proposed AlexNet architecture for binary classification. |
Customized Deep CNN
To identify seizures in newborns, a deep convolutional neural network (CNN) trained entirely from scratch was developed in this section. Five convolutional layers, 3 * 3-fold filters, and 1 * 1-fold stride make up the completed CNN. The network computed the final output probabilities for each class using the ReLU activation function, maximum pooling layer, and fully connected layer. A softmax layer was utilized to normalize the fully linked layer’s outputs, and the cross-entropy loss function was employed for learning. Gradient descent with momentum and a learning rate was set at 0.001 for training the suggested deep CNN. The maximum number of epochs is set at 30. Figure 5 shows the structure of customized Deep CNN.
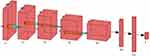 |
Figure 5 The proposed customized Deep CNN architecture for multi classification. |
Materials
The performances of the proposed models were evaluated using a confusion matrix that calculates FNtrue positive/TP, true negative/TN, false positive/FP and false negative/FN values. Furthermore, calculating and finding the values of F1-Score, Recall, Precision, Specificity, and Accuracy are also performance measurement tools used in this work.
Results
Preprocessing
The neonatal multichannel EEG signal was bandpass filtered in this proposed experiment between 0.1 Hz and 15 Hz. The deep CNN networks’ complexity was reduced throughout training time by downsampling the multi-channel EEG to 32 Hz.21 The Nyquist sampling theorem was used to conduct downsampling. The sampling frequency should be at least twice as high as the signal’s maximum frequency. By establishing nominal sample ranges, this theory assists in preventing aliasing.33 Furthermore, removing bad signals and/or unwanted channels, EEG signal segmenting to 10 sec, and scaled color image generation were the next preprocessing steps performed in this study. During preprocessing stages for the neonatal EEG in the case with a small number of channels, we used zero vectors to prepare homogeneous input images with identical sizes.
Input Data Distribution for the Proposed Models
To train the proposed models, the preprocessed continuous EEG data were segmented or windowed, and then the raw waveform was saved in the .jpg image format. The image was rescaled to 256-by-256 for the custom CNN and 227-by-227 for the pretrained AlexNet models. The number of image data to train, validate, and test the proposed models during binary and severity classification is described in Table 2 and Table 3 respectively.
 |
Table 2 The Training, Test, and Validation Image Data for Binary Classification |
 |
Table 3 The Training, Test, and Validation Image Data for Severity Classification |
Results of Binary Classification
Before beginning the training approach, a total of 1209 image data points for the normal classes and 1201 pictures for the seizure classes were prepared for this classification activity (seizure or normal classes). The data were split into 80% for training and 20% for the validation set, and data equivalent to the validation set were also prepared as the test set. During binary classification using AlexNet, out of 242 validation images in the normal class, 218 image data were correctly classified as normal types, while 24 images were wrongly classified as seizure class. Similarly from the total validation set of 240 image data in the seizure class, 229 were classified as seizure and 11 images were incorrectly classified as normal. Finally, the average test accuracy of the model for binary classification was calculated using the average of the performances of the two classes. As a result, an accuracy rate of 92.7%, aprecision rate of 92.9%, a recall rate of 92.6%, an F1-score rate of 92.7%, and a specificity rate of 92.9% were achieved. However, using custom CNN, out of 242 validation data in the normal class, 215 image data were correctly classified as normal types, while 27 images were wrongly classified as seizure class. Similarly, from the total test set of 240 image data in the seizure class, 222 were correctly and 18 images were incorrectly classified. As a result, an accuracy rate of 90.66%, a precision rate of 90.65%, a recall rate of 90.75%, an F1-score rate of 90.7%, and a specificity rate of 90.65% were achieved. Figure 6 shows the curves of accuracy and loss during binary classification using AlexNet, Figure 6a shows the accuracy and loss curve of training and validation during binary classification using AlexNet, and Figure 6b shows the accuracy and loss curve of training and validation during binary classification using custom CNN. As shown in Figures 7a and b, the confusion matrix is used to describe the binary classifier performance for both AlexNet and custom CNN, while Table 4 summarizes the rates of accuracy, F1-score, sensitivity, and specificity.
 |
Table 4 The Overall Results of Neonatal Seizure Detection (Binary Classification) |
 |
Figure 6 The training curves of accuracy and loss during binary classification using AlexNet (a) and custom CNN (b). |
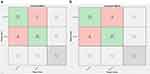 |
Figure 7 Confusion matrix of binary classification using AlexNet (a) and custom CNN (b). |
Multiclass Classification
Figure 7 shows the curves of accuracy and loss during multiclassification, Figure 8a shows the accuracy and loss curve of training and validation during multiclassification using the AlexNet model, and Figure 8b shows the accuracy and loss curve of training and validation during multiclassification using the custom CNN model.
 |
Figure 8 The training curves of accuracy and loss of multiclassification using AlexNet (a) and custom CNN (b). |
A confusion matrix is provided in Figure 9a to show how the AlexNet model for multiclassification performed during training. Seventy-three of the 82 images in the S_1 class were properly identified as S_1 types, whereas 7 images were predicted to belong to the S_2 class and 2 to the S_3 class. Fifty-five of the 68 images in the S_2 class were correctly identified as belonging to that category, whereas 10 were incorrectly assigned to the S_1 class and three to the S_3 class. Forty-six of the 52 images in the S_3 classes were accurately categorized as S_3, whereas 2 were incorrectly categorized as S_1 and 4 as S_2 classes.
 |
Figure 9 The confusion matrix of multiclassification using AlexNet (a) and custom CNN (b). |
On the other hand, the training result of the custom CNN model for multiclassification is indicated using a confusion matrix shown in Figure 9b. Out of the 82 images in the S_1 class, 74 images were correctly classified as S_1 types, while 7 of the images were predicted as S_2 and 1 image as S_3 classes. Out of 68 images in the S_2 class, 56 images were correctly classified as S_2 types, while 10 images were wrongly classified as S_1 classes and 2 images as S_3 classes. Out of 52 images in S_3 classes, 49 images were correctly classified as S_3, while 1 image was wrongly classified as S_1 and 2 images as S_2 classes.
From the results found using the confusion matrix, the precision, recall, specificity, F1-score, recall rate, and test accuracy results were calculated. Table 5 shows the severity of classification results using the custom CNN model.
 |
Table 5 The Overall Result of Severity Classification |
Discussion
The major aim of this study was to classify neonatal seizures into normal or abnormal/seizure classes and to determine the severity level of resulting seizures. To achieve this, two different classifying models were developed: custom CNN and AlexNet. Better results were achieved by training a fine-tuned AlexNet for binary classification and custom CNN for severity classification. To classify the severity level of neonatal seizures, the first step was classifying a given image of a neonatal EEG data segment into (normal or seizure). This was achieved by using the developed binary class classifying model. The binary classifying models’ performance was tested using the test dataset, and a remarkable result was achieved with an average accuracy rate of 92.6%, a precision rate of 92.6%, a recall rate of 92.9%, an F1-score rate of 92.59%, and a specificity rate of 92.9%. After binary class classification, the identified seizure type was further classified into its subtypes (severity levels). After model training for seizure severity classification, the performance was measured using a confusion matrix, accuracy, precision, recall, and specificity. An average test accuracy of 88.1%, precision of 88.06%, recall of 83.67%, F1 score of 85.61%, and specificity of 92.18% were achieved.
Generally, based on the test results, the final model of this research work is prepared by taking the best performing networks. As a result, AlexNet performs better during binary classification, and the network will be used as the final binary classifier. The custom CNN performs better results during multiclassification; therefore, it will be used as the final multiclassifier. In other words, the network of binary classification by pretrained AlexNet and multiclassification by custom CNN is taken as the final model of this work. The performances of the final AlexNet and custom CNN models are summarized in Table 6.
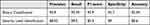 |
Table 6 Summary of the Performance of AlexNet for Binary Classification and Custom CNN for Multiclass Classification |
Comparing the result of this research with the studies conducted utilizing the Zenodo dataset34,35 as a reference point, as illustrated in Table 7, we can conclude that the developed system can classify neonatal EEG datasets in the form of images with better classification accuracy. Furthermore, the developed system has overcome the gap in further classification of neonatal EEG (seizure classes) into severity levels (S_1, S_2, and S_3).
 |
Table 7 Comparing This Work to Earlier State of the Art |
Conclusion
AlexNet, a pretrained CNN, and a customized CNN that was trained from scratch are the two models that are used to detect neonatal seizures and categorize the severity levels of seizures. During training, AlexNet performed better in binary classifications (normal or seizure), whereas custom CNN performed better in multiclass or severity level classifications (S_1, S_2, S_3). Moreover, in the case of binary classification, the system can classify a given image or segment of the EEG waveform into its class normal or seizure with an accuracy of 92.7% and in the case of severity-level classification with an average accuracy of 88.6%. As a result, this developed system can be employed as a decision support system in the diagnosis of neonatal seizures, which will have a significant impact by assisting neurologists or other medical practitioners, especially in low-resource nations where both expertise and funds are limited.
Data Sharing Statement
Link to the datasets (for Data_1, Data_2, and Data_3): https://doi.org/10.5281/zenodo.2547147.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research has been approved by Wachamo University, College of Health Science (IRB: 35/2018) and the study was conducted following the Declaration of Helsinki (as revised in 2013).
Acknowledgments
The authors of this study gratefully acknowledge Helsinki University Hospital, Finland, for sharing the largest open database of EEG signals. Moreover, the authors extend gratitude to Jimma University School of Biomedical Engineering and Wachamo University Hospital’s pediatrics department staff.
Disclosure
The authors declare that they have no competing interests.
References
1. Paterson A. Neonatal sepsis and its complications. Pediatr Radiol. 2014;44:S279–S280. doi:10.1007/s00247-014-2968-2
2. Padiyar S, Nusairat L, Kadri A, Abu-Shaweesh J, Aly H. Neonatal seizures in the US National Inpatient Population: prevalence and outcomes. Pediatr Neonatol. 2020;61(3):300–305. doi:10.1016/j.pedneo.2019.12.006
3. Anil Kumar P, Ramgopal Rao V. Study of clinical profile of neonatal seizures in level III NICU. Asian J Clin Pediatr Neonatol. 2020;8(4):30–35. doi:10.47009/ajcpn.2020.8.4.7
4. Agrawal A, Singh S, Kolhapure S, Kandeil W, Pai R, Singhal T. Neonatal pertussis, an under-recognized health burden and rationale for maternal immunization: a systematic review of South and South-East Asian Countries. Infect Dis Ther. 2019;8(2):139–153. doi:10.1007/s40121-019-0245-2
5. Ziobro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies, and management. Semin Neurol. 2020;40(2):246–256. doi:10.1055/s-0040-1702943
6. Tadic BV, Kravljanac R, Sretenovic V, Vukomanovic V. Long-term outcome in children with neonatal seizures: a tertiary center experience in cohort of 168 patients. Epilepsy Behav. 2018;84:107–113. doi:10.1016/j.yebeh.2018.05.002
7. Sheth RD. Patterns specific to pediatric EEG. J Clin Neurophysiol. 2019;36(4):289–293. doi:10.1097/WNP.0000000000000600
8. Soul JS. Acute symptomatic seizures in term neonates: etiologies and treatments. Semin Fetal Neonatal Med. 2018;23(3):183–190. doi:10.1016/j.siny.2018.02.002
9. Pan W, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Simultaneous fMRI and electrophysiology in the rodent brain. J Vis Exp. 2010;42. doi:10.3791/1901
10. Abend NS, Wusthoff CJ. Neonatal seizures and status epilepticus. J Clin Neurophysiol. 2012;29(5):441–448. doi:10.1097/WNP.0b013e31826bd90d
11. Pisani F, Pavlidis E. The role of electroencephalogram in neonatal seizure detection. Expert Rev Neurother. 2018;18(2):95–100. doi:10.1080/14737175.2018.1413352
12. Shellhaas RA. Continuous long-term electroencephalography: the gold standard for neonatal seizure diagnosis. Semin Fetal Neonatal Med. 2015;20(3):149–153. doi:10.1016/j.siny.2015.01.005
13. Secinaro S, Calandra D, Secinaro A, Muthurangu V, Biancone P. The role of artificial intelligence in healthcare: a structured literature review. BMC Med Inform Decis Mak. 2021;21(1):1–23. doi:10.1186/s12911-021-01488-9
14. O’Shea A, Lightbody G, Boylan G, Temko A. Neonatal seizure detection from raw multi-channel EEG using a fully convolutional architecture. Neural Networks. 2020;123:12–25. doi:10.1016/j.neunet.2019.11.023
15. van Rooij LGM, Hellström-Westas L, De Vries LS. Treatment of neonatal seizures. Semin Fetal Neonatal Med. 2013;18(4):209–215. doi:10.1016/j.siny.2013.01.001
16. Temko A, Marnane W, Boylan G, Lightbody G. Clinical implementation of a neonatal seizure detection algorithm. Decis Support Syst. 2015;70:86–96. doi:10.1016/j.dss.2014.12.006
17. Deburchgraeve W, Cherian PJ, De Vos M, et al. Automated neonatal seizure detection mimicking a human observer reading EEG. Clin Neurophysiol. 2008;119(11):2447–2454. doi:10.1016/j.clinph.2008.07.281
18. Gotman J, Flanagan D, Zhang J, Rosenblatt B. Automatic seizure detection in the newborn: methods and initial evaluation. Electroencephalogr Clin Neurophysiol. 1997;103(3):356–362. doi:10.1016/S0013-4694(97)00003-9
19. Govindan RB, Massaro A, Chang T, Vezina G, du Plessis A. A novel technique for quantitative bedside monitoring of neurovascular coupling. J Neurosci Methods. 2016;259:135–142. doi:10.1016/j.jneumeth.2015.11.025
20. Greene BR, Faul S, Marnane WP, Lightbody G, Korotchikova I, Boylan GB. A comparison of quantitative EEG features for neonatal seizure detection. Clin Neurophysiol. 2008;119(6):1248–1261. doi:10.1016/j.clinph.2008.02.001
21. O’Shea A, Lightbody G, Boylan G, Temko A. Neonatal seizure detection using convolutional neural networks. IEEE Int Workshop Mach Learn Signal Process. 2017;2017:1–6. doi:10.1109/MLSP.2017.8168193
22. Cecotti H, Gräser A. Convolutional neural networks for P300 detection with application to brain-computer interfaces. IEEE Trans Pattern Anal Mach Intell. 2011;33(3):433–445. doi:10.1109/TPAMI.2010.125
23. Truong ND, Nguyen AD, Kuhlmann L, et al. Convolutional neural networks for seizure prediction using intracranial and scalp electroencephalogram. Neural Networks. 2018;105:104–111. doi:10.1016/j.neunet.2018.04.018
24. Roy Y, Banville H, Albuquerque I, Gramfort A, Falk TH, Faubert J. Deep learning-based electroencephalography analysis: a systematic review. J Neural Eng. 2019;16(5):051001. doi:10.1088/1741-2552/ab260c
25. Ansari AH, Cherian PJ, Caicedo A, Naulaers G, De Vos M, Van Huffel S. Neonatal seizure detection using deep convolutional neural networks. Int J Neural Syst. 2019;29(4):1850011. doi:10.1142/S0129065718500119
26. Samanta D. Recent advances in the diagnosis and treatment of neonatal seizures. Neuropediatrics. 2021;52(2):73–83. doi:10.1055/s-0040-1721702
27. Stevenson NJ, Tapani K, Lauronen L, Vanhatalo S. A dataset of neonatal EEG recordings with seizure annotations. Sci Data. 2019;6(1). doi:10.1038/sdata.2019.39
28. Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Commun ACM. 2017;60(6):84–90. doi:10.1145/3065386
29. Panayiotopoulos C. Clinical Aspects of the Diagnosis of Epileptic Seizures and Epileptic Syndromes. Bladon Medical Publishing; 2005.
30. Palmu K, Wikström S, Hippeläinen E, Boylan G, Hellström-Westas L, Vanhatalo S. Detection of “EEG bursts” in the early preterm EEG: visual vs. automated detection. Clin Neurophysiol. 2010;121(7):1015–1022. doi:10.1016/j.clinph.2010.02.010
31. Clancy RR. The contribution of EEG to the understanding of neonatal seizures. Epilepsia. 1996;37(s1). doi:10.1111/j.1528-1157.1996.tb06022.x
32. Hahn JS. Neonatal and Pediatric Electroencephalography. In: Aminoff’s Electrodiagnosis in Clinical Neurology. Churchill Livingstone; 2012. doi10.1016/B978-1-4557-0308-1.00004-2
33. Vogrin SJ, Lau S, Cook MJ. High temporal resolution EEG in EEG-fMRI investigation of epileptiform brain activity. Clin EEG Neurosci. 2013;44(4):E68–E69.
34. Tapani KT, Vanhatalo S, Stevenson NJ. Time-varying EEG correlations improve automated neonatal seizure detection. Int J Neural Syst. 2019;29(4):1850030. doi:10.1142/S0129065718500302
35. Stevenson NJ, Lauronen L, Vanhatalo S. The effect of reducing EEG electrode number on the visual interpretation of the human expert for neonatal seizure detection. Clin Neurophysiol. 2018;129(1):265–270. doi:10.1016/j.clinph.2017.10.031
36. Zeedan A, Al-Fakhroo K, Barakeh A. EEG-based seizure detection using feed- forward and LSTM neural networks based on a neonates dataset; 2022:0–12. doi:10.36227/techrxiv.20728411.v1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
