Back to Journals » Drug Design, Development and Therapy » Volume 9
Design of a gelatin microparticle-containing self-microemulsifying formulation for enhanced oral bioavailability of dutasteride
Authors Baek I, Ha E, Yoo J , Jung Y, Kim M
Received 11 April 2015
Accepted for publication 13 May 2015
Published 23 June 2015 Volume 2015:9 Pages 3231—3238
DOI https://doi.org/10.2147/DDDT.S86458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shu-Feng Zhou
In-hwan Baek,1,* Eun-Sol Ha,2,* Jin-Wook Yoo,2 Yunjin Jung,2 Min-Soo Kim2
1College of Pharmacy, Kyungsung University, 2College of Pharmacy, Pusan National University, Busan, Republic of Korea
*These authors contributed equally to this work
Abstract: In this study, a gelatin microparticle-containing self-microemulsifying formulation (SMF) was developed using a spray-drying method to enhance the oral delivery of the poorly water-soluble therapeutic dutasteride. The effect of the amount of gelatin and the type and amount of hydrophilic additives, namely, Gelucire® 44/14, poloxamer 407, sodium lauryl sulfate, Soluplus®, Solutol™ HS15, and D-α-tocopheryl polyethylene glycol 1000 succinate, on the droplet size, dissolution, and oral absorption of dutasteride from the SMF was investigated. Upon dispersion of the gelatin microparticle-containing SMF in water after spray-drying, the mean droplet size of the aqueous dispersion was in the range of 110–137 nm. The in vitro dissolution and recrystallization results showed that gelatin could be used as a solid carrier and recrystallization inhibitor for the SMF of dutasteride. Furthermore, combination of the gelatin microparticle-containing SMF and Soluplus enhanced the dissolution properties and oral absorption of dutasteride. The results of our study suggest that the gelatin microparticle-containing SMF in combination with Soluplus could be useful to enhance the oral absorption of dutasteride.
Keywords: dissolution, solubility, bioavailability, dutasteride
Introduction
Self-microemulsifying formulations (SMFs) consisting of oil, surfactant, and/or cosurfactant (cosolvent) provide a good solvent system for poorly water-soluble active pharmaceutical ingredients and a large oil/water (o/w) interface area, thereby incorporating active pharmaceutical ingredients into oil droplets.1,2 Presently, SMFs have become an enhanced strategy for the improvement of the oral delivery of various active pharmaceutical ingredients.3,4 Importantly, the liquid state of an SMF must be solidified for the development of a solid dosage form with enhanced stability, reproducibility, and improved patient compliance.5 The extrusion/spheronization or wet granulation method has been employed to prepare solid forms of SMFs based on the adsorption principle using an adsorbent such as colloidal silica (Aerosil® 200) and magnesium aluminometasilicate (Neusilin®).6,7 The spray-drying process is a one-step method using a solid carrier such as alginate, hydroxypropylmethyl cellulose, lactose, or sodium carboxymethylcellulose to form microspheres, microparticles, and microcapsules.8–12
The present study was conducted to develop a gelatin microparticle-containing SMF for enhancing the oral delivery of the poorly water-soluble therapeutic dutasteride. Dutasteride is widely used for the treatment of symptomatic benign prostatic hyperplasia by oral administration as a soft capsule formulated with monoglycerides and diglycerides of caprylic/capric acid. However, the oral bioavailability of the commercial product in humans is only 40%–60% due to its extremely low aqueous solubility (below 0.038 ng/mL at 25°C).13 In our previous study, we developed and optimized a dutasteride-loaded SMF containing Capryol™ 90, Cremophor® EL, and Transcutol® HP, based on a D-optimal mixture design.14 In the present study, a gelatin microparticle-containing SMF was developed using a spray-drying method. The effect of the amount of gelatin, the type and amount of the hydrophilic additives, namely, Gelucire® 44/14, poloxamer 407, sodium lauryl sulfate, Soluplus®, Solutol™ HS15, and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), on the droplet size, dissolution, and oral absorption of dutasteride from an SMF was investigated.
Materials and methods
Dutasteride and gelatin (type A) were obtained from Dr Reddy’s Laboratories Ltd and Geltech Co Ltd, respectively. Cremophor EL (polyoxyl 35 hydrogenated castor oil), poloxamer 407 (ethylene oxide-propylene oxide block copolymer), and Soluplus (polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer) were kindly donated by BASF Co Ltd. Capryol 90 (propylene glycol monocaprylate) and Transcutol HP (purified diethylene glycol monoethyl ether) were kindly donated by Gattefosse. TPGS and finasteride (internal standard) were purchased from Eastman Chemical Company and Sigma Chemical Company, respectively. Acetonitrile, ethanol, and methanol were of high-performance liquid chromatography (HPLC) grade.
Preparation of gelatin microparticle-containing SMF of dutasteride
The gelatin microparticle-containing SMF was developed by a spray-drying method using a Buchi 191 nozzle type mini spray-dryer. First, dutasteride was completely dissolved in a mixture of Capryol 90, Cremophor EL, and Transcutol HP. This combination was selected based on our previous study.14 This solution was added to a gelatin solution with or without the hydrophilic additive Gelucire 44/14, poloxamer 407, sodium lauryl sulfate, Soluplus, Solutol HS15, or TPGS. The detailed composition of the solutions for fabrication of the gelatin microparticles is described in Table 1. The resulting solution was delivered to the inner line of the nozzle at a flow rate of 3–6 mL/min using a peristaltic pump and was solidified under the following conditions: inlet temperature, 110°C–130°C; outlet temperature, 65°C–80°C; and atomization air pressure, 5 kPa.
Characterization of gelatin microparticle-containing SMF of dutasteride
The morphology of the gelatin microparticles was visualized using a scanning electron microscope from JEOL Ltd. The particle size and size distribution of the gelatin microparticles were characterized using a Helos laser diffraction spectrometer from SYMPATEC Ltd. For measurement of the droplet size in water, the gelatin microparticle-containing SMF was dispersed by gentle mixing in 25 mL of distilled water at 37°C. The resulting emulsion was incubated for 60 minutes at room temperature before samples were taken for measurement of droplet size using an ELSZ-1000 dynamic light scattering device from Otsuka Electronics. The drug content in the gelatin microparticles was determined by HPLC analysis. About 20 mg of the gelatin microparticles were dissolved in 50% methanol solution. All samples were analyzed using a Waters HPLC system equipped with a Waters 600 controller pump, a Waters 717 Plus autosampler, and a Waters 486 tunable absorbance detector. The mobile phase was acetonitrile/water (60:40, v/v) at a flow rate of 1.0 mL/min. All chromatographic analyses were performed on a Luna C18 analytical column (5 μm, 4.6 mm ×250 mm) from Phenomenex with detection at 210 nm. The dissolution profiles of dutasteride from the gelatin microparticles were determined at 37°C and 50 rpm in 300 mL of pH 1.2 dissolution medium using a USP rotating paddle apparatus from Electrolab. At predetermined intervals, 3 mL samples were collected for analysis and replaced with 3 mL of fresh dissolution medium after each sample collection. samples from various time points were filtered using a 0.11 μm syringe filter followed by dilution with methanol, and the amount of drug dissolved in each sample was determined by HPLC.
Effect of gelatin on recrystallization of dutasteride in a supersaturated solution
To investigate the effect of gelatin on the recrystallization of a supersaturated dutasteride solution, dutasteride was dissolved in methanol at a concentration of 3 mg/mL. A 1 mL aliquot of the dutasteride solution was added to 300 mL of pH 1.2 dissolution medium containing various gelatin concentrations and agitated with a USP rotating paddle apparatus set at 37°C and 100 rpm. Samples (2 mL) were removed at various time intervals and filtered using a 1.2 μm syringe filter. The filtered samples were diluted with methanol and the concentration of dutasteride was determined by HPLC.
Pharmacokinetic study
The animal study protocol was in compliance with the institutional guidelines for the care and use of laboratory animals and was approved by the ethics committee of Kyungsung University (Busan, Korea). Fifteen male Sprague-Dawley rats (250±10 g) from Orient Bio Inc were divided into three treatment groups of five rats each. Prior to the study, the rats were fasted for 18 hours and each group received a physical mixture or gelatin microparticles at dutasteride doses of 2 mg/kg (dose/rat weight) for each formulation by oral administration. The physical mixture of dutasteride with Soluplus as surfactant was prepared by simple mixing at a drug to surfactant ratio of 1:5. The physical mixture and the gelatin microparticles were dispersed in 1 mL of water immediately prior to oral dosing. Approximately 0.35 mL blood samples were withdrawn from the retro-orbital plexus of the rats at 0, 0.5, 1, 2, 3, 4, 8, 12, and 24 hours after dosing and collected in heparinized tubes. Plasma samples were obtained by centrifugation at 10,000 rpm for 5 minutes (4°C). Plasma dutasteride concentrations were determined by liquid chromatography with tandem mass spectrometry according to our previously reported method.15 Pharmacokinetic analysis of the data was carried out using WinNonlin Standard Edition version 5.3 software. The area under the curve (AUC0→24h) was calculated according to the trapezoidal method. The peak plasma concentration (Cmax) and time to reach Cmax of dutasteride in plasma were taken directly from the data. Statistical analysis of the dissolution data and pharmacokinetic parameters was performed using a one-way analysis of variance test followed by the Student–Newman–Keuls and least-squares difference tests with Statistical Package for the Social Sciences version 21.0 software.
Results and discussion
In this study, a gelatin microparticle-containing SMF was developed using a spray-drying method. The effect of the amount and type of gelatin and the amount of the hydrophilic additives, Gelucire 44/14, poloxamer 407, sodium lauryl sulfate, Soluplus, Solutol HS15, and TPGS on the droplet size, dissolution, and oral absorption of dutasteride from the SMF was investigated. The gelatin microparticle-containing SMF was well developed by spray-drying as observed by scanning electron microscopy (Figure 1). As shown in Table 2, all particles showed irregular spherical sizes with similar volumes and mean particle size (9–11 μm) without significant differences between the gelatin microparticles (P>0.05). This result indicates that the morphology of the gelatin microparticle-containing SMF was not influenced by the amount or type of gelatin or the amount of surfactants used. The drug content in each gelatin microparticle was almost equal to that of the theoretical values as shown by HPLC analysis. Further, no indication of chemical degradation of the drug was observed during the spray-drying process. Upon dispersion of the gelatin microparticle-containing SMF in water after spray-drying, the mean droplet size of the aqueous dispersion was 110–137 nm (Table 2). In addition, the low polydispersity index of less than 0.3 in all formulations indicated a narrow and homogeneous size distribution of the droplets. Generally, the droplet size is a critical factor in the self-emulsification process because a smaller droplet size yields a larger interfacial surface area for drug absorption and allows a faster rate of drug release.16,17 In this study, no significant difference was found between the tested compositions (P>0.05).
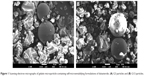  | Figure 1 Scanning electron micrographs of gelatin microparticle-containing self-microemulsifying formulations of dutasteride. (A) G3 particles and (B) G13 particles. |
The dissolution profiles of the gelatin microparticle-containing SMF were evaluated in pH 1.2 dissolution media (G1–G4). To compare the difference between the dissolution profiles obtained from various compositions, the dissolution profiles were characterized using the percent dissolution efficiency as defined by Khan and Rhodes.18 The dissolution efficiency for all prepared gelatin microparticle-containing SMFs was calculated from the area under the dissolution curves at 120 minutes and expressed as a percentage of the area of the rectangle described by 100% dissolution within the same time period (Table 2). The effect of the amount of gelatin on the dissolution of dutasteride from the gelatin microparticle-containing SMF is shown in Figure 2. For G1–G4 particles, the release of dutasteride from the gelatin microparticles had a maximum dissolution of more than 90% within 30 minutes. The drug release from G1 and G2 gradually decreased to about 60% at 120 minutes. Interestingly, the drug release at 120 minutes increased with increasing amount of gelatin, although no significant differences between G3 and G4 particles were found. Since the enhanced drug release with increasing amount of gelatin might be attributed to inhibition of drug precipitation from the supersaturated state induced by the SMF, the inhibitory effect of gelatin on recrystallization of dutasteride was investigated in pH 1.2 dissolution medium. As shown in Figure 3, dutasteride rapidly precipitated from methanol in pH 1.2 dissolution medium without gelatin. However, recrystallization of dutasteride was significantly inhibited by gelatin at 1 mg/mL and 5 mg/mL. In fact, gelatin, as a precipitation inhibitor, provided prolonged supersaturation of dutasteride, which suggested that mechanisms including inhibition of nucleation and/or crystal growth from a highly supersaturated state were responsible for the stabilization effects.19,20 Therefore, gelatin can be used as a solid carrier and recrystallization inhibitor for a SMF of poorly water-soluble dutasteride.
Although gelatin inhibited the precipitation of dutasteride, the release of dutasteride from gelatin microparticles (G3 and G4) had a maximum dissolution of over 90% within 30 minutes and gradually decreased to about 71% at 120 minutes. Therefore, the effect of the hydrophilic additives Gelucire 44/14, poloxamer 407, sodium lauryl sulfate, Soluplus, Solutol HS15, and TPGS on the dissolution of dutasteride from the gelatin microparticle-containing SMF was investigated based on the G3 composition for further enhancement of drug release. As shown in Figure 4, the dissolution profiles of dutasteride were significantly changed by addition of a hydrophilic additive. In particular, Gelucire 44/14 showed a decrease in maximum dissolution. Poloxamer 407, sodium lauryl sulfate, and Solutol HS15 did not influence drug release at 120 minutes. However, addition of Soluplus to the gelatin microparticle-containing SMF significantly enhanced the drug release at 120 minutes. Among the hydrophilic additives tested, the percent dissolution efficiency ranked by the Student–Newman–Keuls test was increased as follows: Gelucire 44/14 < no additive = poloxamer 407 = sodium lauryl sulfate = Solutol HS15 < TPGS < Soluplus (Table 2). In this study, the most effective hydrophilic additive was Soluplus, followed by TPGS. Therefore, the effect of the amount of Soluplus on dissolution of dutasteride was further evaluated based on the G3 composition. As shown in Figure 5, Soluplus had a synergistic effect on the dissolution of dutasteride from the gelatin microparticle-containing SMF in an amount-dependent dissolution manner. In particular, the complete dissolution of dutasteride was observed in G13 particles (containing 25 mg Soluplus) and dutasteride dissolution exceeded 95% at 120 minutes. Furthermore, the dissolution profiles of G13 particles were evaluated in different types of dissolution medium (pH 1.2, pH 4.0, pH 6.8, and water). As shown in Figure 6, G13 particles showed a higher dissolution of over 90% in all types of dissolution medium, indicating that dutasteride release from G13 particles was not influenced by the pH of the dissolution medium. In fact, dutasteride has nonionizable groups with the pH-independent solubility profiles (pH 1–12).21 However, the physical mixture exhibited an extremely low dissolution of less than 5% in all types of dissolution medium due to the poor solubility of dutasteride.
The oral absorption of dutasteride from the gelatin microparticles was evaluated in Sprague-Dawley rats. Figure 7 shows the plasma concentration-time profile of dutasteride after oral administration of the gelatin microparticle-containing SMF (G3 and G13) and the physical mixture at a drug dose of 2 mg/kg. The pharmacokinetic parameters (AUC0→24h, Cmax, and time to reach Cmax) are presented in Table 3. As shown in Figure 7, the plasma concentration of dutasteride after administration of G3 and G13 particles was dramatically higher than that after administration of the physical mixture, with sustained increases up to 6 hours. However, the G3 and G13 particles exhibited a rapid drug absorption rate. In addition, the G13 particles caused a higher plasma concentration of dutasteride over 12 hours than the G3 particles. Expectedly, the pharmacokinetic parameters (AUC0→24h and Cmax) for the G13 particles were higher than that for the other formulations (P<0.05). In particular, the G13 particles exhibited a higher bioavailability than the physical mixture and the G3 particles, with approximately 4.5-fold and 1.3-fold increases in AUC0→24h. Therefore, the oral absorption of dutasteride was significantly increased by the gelatin microparticle-containing SMF combined with Soluplus.
In general, when SMFs consisting of oil, surfactant, and/or cosurfactant (cosolvent) are placed in an in vitro dissolution medium or in gastrointestinal fluid in vivo, homogeneous oil-in-water microemulsions form spontaneously with mild agitation. However, a gradual decrease in the amount of drug dissolved occurs due to a reduction in the solubilizing capacity by dilution of the SMFs in the in vitro dissolution medium or gastrointestinal fluid.22 Therefore, precipitation of the dissolved drug in a supersaturated state as induced by SMFs is prevented. Hydrophilic additives can control the precipitation of poorly water-soluble active pharmaceutical ingredients from a supersaturated state. Inhibition of precipitation from a supersaturated state by a SMF may be due to inhibition of drug nucleation and crystal growth by blocking the active surface and providing steric stabilization, and to specific interactions between the drug and hydrophilic additives such as hydrophobic interactions or hydrogen bonding.23–25 In this study, gelatin acted as a solid carrier and recrystallization inhibitor for the SMF of dutasteride. The dissolution and oral bioavailability of dutasteride from the gelatin microparticle-containing SMF was further enhanced by addition of the surfactant Soluplus. This effect might have been due to the increased dissolution property via the prolonged supersaturated state of dutasteride as induced by the combination of gelatin and Soluplus.
Conclusion
The present study was carried out to develop an oral drug delivery system for dutasteride using a gelatin microparticle-containing SMF. Gelatin played a critical role in solidification of the liquid SMF as well as in inhibition of drug precipitation from the supersaturated state induced by the SMF. Based on the in vitro dissolution data and the in vivo pharmacokinetic parameters, the enhanced dissolution properties of dutasteride led to increased oral absorption by the combination of the gelatin microparticle-containing SMF and Soluplus. Taken together, the gelatin microparticle-containing SMF is an effective oral drug delivery system for the poorly water-soluble therapeutic dutasteride.
Acknowledgment
This work was supported by a grant from the National Research Foundation of Korea funded by the Korea government (2009-0083538).
Disclosure
The authors report no conflicts of interest in this work.
References
Sprunk A, Strachan CJ, Graf A. Rational formulation development and in vitro assessment of SMEDDS for oral delivery of poorly water soluble drugs. Eur J Pharm Sci. 2012;46:508–515. | ||
Cho W, Kim MS, Kim JS, et al. Optimized formulation of solid self-microemulsifying sirolimus delivery systems. Int J Nanomedicine. 2013;8:1673–1682. | ||
Pandey S, Das U, Patil A. Formulation and ex-vivo evaluation of metronidazole microemulsion loaded hydrogel for prevention of periodontitis. J Pharm Invest. 2014;44:225–236. | ||
Yadav P, Yadav E, Verma A, Amin S. In vitro characterization and pharmacodynamic evaluation of furosemide loaded self nano emulsifying drug delivery system (SNEDDS). J Pharm Invest. 2014;44:443–453. | ||
Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov Today. 2008;13:606–612. | ||
Qureshi MJ, Mallikarjun C, Kian WG. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: a study in diet induced hyperlipidemic rabbits. Asian J Pharm Sci. 2015;10:40–56. | ||
Krupa A, Jachowicz R, Kurek M, Figiel W, Kwiecień M. Preparation of solid self-emulsifying drug delivery systems using magnesium aluminometasilicates and fluid-bed coating process. Powder Technol. 2014;266:329–339. | ||
Qi X, Qin J, Ma N, Chou X, Wu Z. Solid self-microemulsifying dispersible tablets of celastrol: formulation development, characterization and bioavailability evaluation. Int J Pharm. 2014;472:40–47. | ||
Baek IH, Kim JS, Ha ES, et al. Oral absorption of a valsartan-loaded spray-dried emulsion based on hydroxypropylmethyl cellulose. Int J Biol Macromol. 2014;69:222–228. | ||
de la Torre-Iglesias PM, García-Rodriguez JJ, Torrado G, Torrado S, Torrado-Santiago S, Bolás-Fernández F. Enhanced bioavailability and anthelmintic efficacy of mebendazole in redispersible microparticles with low-substituted hydroxypropylcellulose. Drug Des Devel Ther. 2014;8:1467–1479. | ||
Mooranian A, Negrulj R, Chen-Tan N, et al. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des Devel Ther. 2014;8:1221–1230. | ||
Sakr FM, Gado AM, Mohammed HR, Adam AN. Preparation and evaluation of a multimodal minoxidil microemulsion versus minoxidil alone in the treatment of androgenic alopecia of mixed etiology: a pilot study. Drug Des Devel Ther. 2013;7:413–423. | ||
Lee DH, Yeom DW, Song YS, et al. Improved oral absorption of dutasteride via Soluplus®-based supersaturable self-emulsifying drug delivery system (S-SEDDS). Int J Pharm. 2014;478:341–347. | ||
Choo GH, Park SJ, Hwang SJ, Kim MS. Formulation and in vivo evaluation of a self-microemulsifying drug delivery system of dutasteride. Drug Res. 2013;63:203–209. | ||
Baek IH, Kim MS. Improved supersaturation and oral absorption of dutasteride by amorphous solid dispersions. Chem Pharm Bull. 2012;60:1468–1473. | ||
Sha X, Wu J, Chen Y, Fang X. Self-microemulsifying drug-delivery system for improved oral bioavailability of probucol: preparation and evaluation. Int J Nanomedicine. 2012;7:705–712. | ||
Liu Y, Zhang P, Feng N, Zhang X, Wu S, Zhao J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2009;365:136–142. | ||
Khan KA, Rhodes CT. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm Acta Helv. 1972;47:594–607. | ||
Warren DB, Benameur H, Porter CJ, Pouton CW. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target. 2010;18:704–731. | ||
Kim MS. Influence of hydrophilic additives on the supersaturation and bioavailability of dutasteride-loaded hydroxypropyl-β-cyclodextrin nanostructures. Int J Nanomedicine. 2013;8:2029–2039. | ||
Kim NA, Choi DH, Lim JY, et al. Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical characterization. Arch Pharm Res. 2014;37:214–224. | ||
Song WH, Park JH, Yeom DW, et al. Enhanced dissolution of celecoxib by supersaturating self-emulsifying drug delivery system (S-SEDDS) formulation. Arch Pharm Res. 2013;36:69–78. | ||
Kim MS, Kim JS, Cho W, et al. Supersaturatable formulations for the enhanced oral absorption of sirolimus. Int J Pharm. 2013;445:108–116. | ||
Yu H, Xia D, Zhu Q, Zhu C, Chen D, Gan Y. Supersaturated polymeric micelles for oral cyclosporine A delivery. Eur J Pharm Biopharm. 2013;85:1325–1336. | ||
Ha ES, Baek IH, Cho W, Hwang SJ, Kim MS. Preparation and evaluation of solid dispersion of atorvastatin calcium with Soluplus® by spray drying technique. Chem Pharm Bull. 2014;62:545–551. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









