Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Cutaneous Mycobacterium Abscessus Infection Following Plastic Surgery: Three Case Reports
Authors Shen H, Zhang Q, Peng L, Ma W, Guo J
Received 30 October 2023
Accepted for publication 6 March 2024
Published 14 March 2024 Volume 2024:17 Pages 637—647
DOI https://doi.org/10.2147/CCID.S445175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Hongwei Shen,1,* Qiaomin Zhang,1,* Liang Peng,2 Wen Ma,1 Jingdong Guo2
1Clinical Laboratory, Shenzhen Hospital of Southern Medical University, Shenzhen, Guangdong, People’s Republic of China; 2Department of Burns and Plastic Surgery, Shenzhen Hospital of Southern Medical University, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingdong Guo; Hongwei Shen, Email [email protected]; [email protected]
Aim: Mycobacterium abscessus is ubiquitous in the environment and seldom causes infections in immunocompetent individuals. However, skin and soft tissue infections caused by M. abscessus have been reported in recent years. Additionally, the cutaneous infections or outbreaks post cosmetic surgery caused by M. abscessus have been increasing due to the popularity of plastic surgery. The main modes of transmission are through contaminated saline, disinfectants, or surgery equipment, as well as close contact between patients. This article describes three patients who were admitted to our hospital between November 2019 and October 2020. They presented with long-term non-healing wounds caused by M. abscessus infection after undergoing plastic surgery. Symptoms presented by the three patients included swelling, ulceration, secretion, and pain. After identification of M. abscessus with Ziehl-Neelsen staining and MALDI-TOF MS system, the patients were treated with surgical debridement and clarithromycin.
Conclusion: It is important to note that a long-term wound that does not heal, especially after plastic surgery, should raise suspicion for M. abscessus infection. The infection mechanism in these three patients may have been due to exposure to surgical equipment that was not properly sterilized or due to poor sterile technique by the plastic surgeon. To prevent such infections, it is important to ensure proper sterilization of surgical equipment and saline.
Keywords: Mycobacterium abscessus, cutaneous infection, plastic surgery, treatment, antibiotic resistance
Introduction
Skin and soft tissue infections caused by nontuberculous mycobacteria are on the rise. These ubiquitous acid-fast bacilli are found in the environment and primarily cause cutaneous infections after trauma, surgery, or cosmetic procedures. Abscesses, sporotrichoid nodules, or ulcers are common skin findings. Significant species implicated in these infections include Mycobacterium marinum and the rapidly growing mycobacterium: Mycobacterium fortuitum, M. abscessus, and Mycobacterium chelonae.1 The three rapidly growing pathogenic species exhibit significant differences in their antimicrobial susceptibilities, making the identification of species crucial for clinical purposes.2
M. abscessus seldom leads to severe infections in immunocompetent individuals, but it has been reported to cause cutaneous infections in cases of trauma, tattoos, skin injuries, and surgery.3 It can also result in pulmonary or systemic infection in patients who are immunosuppressed.4 According to a 2021 review,5 the most common rapidly growing mycobacteria species causing skin and soft tissue infections was M. abscessus (184/475, 38.7%). Cases of skin and soft tissue infections caused by M. abscessus have been reported in various regions, including the USA, Taiwan, India, Switzerland, France, and Germany.5 In China, there have been sporadic reports of M. abscessus soft tissue infections following injections or plastic surgery.6–8 A total of 955 cases of M. abscessus soft tissue infections were reported in 60 studies by 2020.9 The infections were caused by trauma (76%), injections (54%), acupuncture (26%), and plastic surgery (15%). While 95% of patients fully recovered after treatment, two cases resulted in disseminated infections and death.9 It is worth noting that plastic surgery may increase the risk of M. abscessus infection.
Cutaneous infections caused by M. abscessus have been reported through various routes, including tattooing,10 liposuction,11 skin transplantation,12 rhytidectomy,13 hip augmentation,14 hair transplant,15 botulinum toxin injection,16 abdominal plastic surgery, and skin rejuvenation.17 Outbreaks of invasive infection have been reported in children’s dental clinics and skin infections caused by acupuncture in traditional Chinese medical clinics.18 These infections were most common in departments specializing in cosmetic surgery, liposuction, and intravenous cell therapy. The transmission routes included contaminated saline, disinfectants, surgical instruments, and close contact with the patient.14
Due to M. abscessus resistance to various antibiotics, conventional antibiotic treatment may not prove effective and diagnosis and treatment are highly dependent on etiological findings. This article reports three cases of long-term M. abscessus cutaneous infection after plastic surgery. Results are presented to contribute to epidemiologic data and clinical treatments for M. abscessus soft tissue infection in future.
Materials and Methods
Culture and Identification of M. Abscessus
Samples of drainage fluid and wound secretion were cultured on blood agar, chocolate agar, and MacConkey agar (Autobio, China) and then incubated in a CO2 incubator (Thermo Fisher Scientific, USA) at 35°C for several days. Ziehl-Neelsen staining was performed for preliminary examination. The M. abscessus strain was identified using the Autof ms 1000 MALDI-TOF MS system (Autobio, China) according to manufacturer’s instructions.
Antimicrobial Susceptibility Testing
The susceptibility of the M. abscessus isolate to 12 antimicrobials was tested using the Sensititre RAPMYCO2 plate (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. After incubation, the minimal inhibitory concentration (MIC) value was measured and the results were interpreted as susceptible, intermediate, or resistant based on the Clinical and Laboratory Standards Institute guideline M24-A2.19 Quality control was performed using M. peregrinum ATCC 700686.
Case 1
Clinical Information
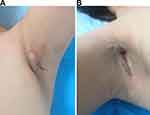
A 32-year-old female underwent bilateral augmentation mammoplasty with silicone implants in November 2019. Subsequently, she developed a wound infection on the left side with yellowish-white purulent discharge, although no pathogenic organisms were isolated from the discharge. Despite removing the bilateral breast implants in December 2019, the bilateral axillary wound continued to exude and did not heal. Ten days following the removal procedure, red and blue light anti-inflammatory was administered. In addition to increasing blood supply to the wound surface and reducing excessive inflammatory response, red and blue lights have been shown to inhibit multiple bacteria, accelerate wound healing, and reduce levels of pathogenic bacteria on the wound surface.20 However, there was a persistent bilateral axillary wound dehiscence with a purulent yellow-green discharge. The wound exhibited erosive changes, and a 4 cm subcutaneous cavity was detected in the direction from the right armpit towards the breast (Figure 1).
Chest MRI revealed an encapsulated effusion in the posterior space of both pectoralis major muscles. Following admission, the patient underwent debridement, deformity correction, and pedicled composite tissue flap plasty on January 22, 2020. Necrotic tissue at the wound’s edge was excised, though no pathogens could be isolated from the tissue sample.
The patient’s right wound exhibited inadequate healing post-operation, and mild light yellow exudates were observed upon wound compression. Levofloxacin (0.5g QD, inv) was administered as an empirical treatment. On February 5, a chest MRI revealed multiple lesions near the upper marginal muscular space and beneath the armpit. A second debridement of the right armpit and negative pressure drainage for the ventricular septal defect (VSD) were performed on February 6 due to unsatisfactory results from dressing changes. Cefoperazone/sulbactam (1.5g BID, inv) and metronidazole (100mL, 3 days) were used for antibiotic therapy.
The patient’s bilateral wounds in the axillary region exhibited poor healing progress after the second operation, particularly on the right side. Observations revealed the presence of pale red, bloody drainage fluid and some erosion on the left armpit. Additionally, the patient experienced swelling, pain, and ulceration with purulent secretions on the left breast fold (Figure 2). To address the non-healing wound that persisted unexpectedly, the third axillary debridement and VSD negative pressure drainage were performed on March 4. The drainage fluid on the right exhibited dark red coloration with flocculent necrosis, while the left was clear. After obtaining culture results, levofloxacin (0.5g QD, inv) and vancomycin (0.5g BID, inv) were prescribed as antibiotics.
Twenty days after the third surgery, the drainage tubes were removed, revealing a small amount of clear yellowish exudate at the drainage site. Prolonged hospitalization led to damage of the pectoralis major muscle and limitations in both upper limb mobility. Shortly after, a dark red area with a diameter of approximately 3cm surfaced in the right armpit (Figure 3). On March 13, the wound secretion was subjected to Ziehl-Neelsen staining, which resulted in a grade of +++. Considering the possibility of skin tuberculosis, treatment with moxifloxacin and anti-tuberculosis quadruple drugs was initiated. However, the patient’s wound still healed poorly and the symptoms did not improve.
 |
Figure 3 Dark redness, swelling, and pain are present on the right side following the third surgical drainage procedure. |
Etiological Examination
The drainage fluid and wound secretion samples were taken and examined for pathogenic organisms during or after the debridement surgery. Sequentially, Staphylococcus lugdunensis, Enterococcus faecalis, Staphylococcus aureus, and Corynebacterium jejuni were isolated, and antibiotics were modified based on culture results. On March 15 and 31, the drainage fluid on the right side tested positive for Ziehl-Neelsen staining (Table 1). Small colonies were identified in drainage fluid sample after 3 days of culture. After 4 days of culture, rough and colorless colonies of M. abscessus were isolated (Figure 4). Antimicrobial susceptibility testing showed resistance to multiple antibiotics including imipenem, levofloxacin, and cefoxitin. A combination therapy of clarithromycin and tigecycline was recommended.
 |
Table 1 Infection-Related Laboratory Test Results |
Case Follow-Up
The patient was readmitted on April 10 due to non-healing wounds located in both axillary and inframammary folds. An area measuring 4 cm in diameter and dark red in color was observed under the right axilla. Additionally, the breast MRI disclosed that there was encapsulated effusion in the posterior region of both pectoralis major muscles. The treatment regimen was altered to include rifampicin + ethambutol + clarithromycin + linezolid on April 14 subsequent to the culture outcomes. Incision and drainage were performed on the right precordial abscess and bilateral axillary chronic ulcer due to bilateral axillary erosion ulcer. A chest CT scan revealed the presence of inflammatory granulomas in the lungs, ruling out tuberculosis. The treatment plan was modified on April 19 based on antimicrobial susceptibility outcomes to include clarithromycin (0.5g BID, oral) and tigecycline (50mg Q12H, inv). However, tigecycline was discontinued due to nausea and vomiting side-effects. The patient was discharged on April 29 after the wound on the right submammary fold had healed without bleeding, exudation, or secretions.
On May 13, the patient was admitted to the hospital for the third time due to redness, swelling, and pain in the left armpit. A 2 cm diameter red and swollen area was observed in the left armpit, and a small amount of pale yellowish exudate was noted in the right armpit wound. Treatment for the left armpit chronic ulcer wound repair and incision and drainage of the abscess were carried out. The individual was discharged on May 16 after showing improvement. She received 6 months of continued oral treatment with clarithromycin (0.5g BID, oral) before the wound fully healed.
Case 2
A 51-year-old female underwent a facial rhytidectomy in August 2020. Four days after the surgery, she experienced local redness, swelling, and pus. Antibiotic treatment with Levofloxacin and Omeprazole was administered after incision and drainage. Due to inadequate improvement, the patient was admitted to our hospital on October 18, 2020.
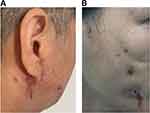
During the admission examinations, significant swelling of the bilateral cheeks, ears, and neck was observed (Figure 5A). The patient had multiple masses that were tender upon palpation, with a sense of fluctuation. Multiple visible cracks were present on the face and neck, with white pus discharge. A dark red, uneven scar was visible on the face and ear. The mouth appeared slightly crooked on the left side while smiling. The craniocerebral CT revealed swelling with air in the bilateral maxillofacial area and subcutaneous tissue of the cheek, as well as thickening and local damage to the maxillofacial skin, suggesting an infectious lesion and local abscess.
Laboratory tests revealed a high-sensitivity C-reactive protein (hCRP) level of 9.08 mg/L and a procalcitonin (PCT) level of 0.187 ng/mL. The patient’s clinical symptoms and epidemiological history indicated a strong likelihood of non-tuberculous mycobacterial infection. Therefore, empiric treatment was initiated with imipenem, amikacin, and doxycycline, while wound secretions were collected for bacterial culture.
The patient underwent surgical procedures for facial ulcer debridement, foreign body removal, and facial defect flap transfer repair on October 21. A week later, the wound secretion specimen showed the isolation of M. abscessus. The isolate showed sensitivity to clarithromycin, amikacin, and linezolid, as well as intermediate susceptibility to cefoxitin while being resistant to ciprofloxacin, doxycycline, tobramycin, and trimethoprim/sulfamethoxazole. After analyzing the antibiotic susceptibility, the medication was altered to include amikacin, clarithromycin, and linezolid.
However, the patient’s wound healed poorly, and there was white purulent exudation with bleeding at the incision on the left side of the neck. On November 10, wound resection and debridement of subcutaneous tissue on the head, face, and neck skin were performed. The drainage fluid on the left was pale yellow with floccules, and the fluid on the right was clear. The dressing was changed regularly after the operation, and the sutures were removed 9 days later. Due to side effects of tinnitus, amikacin was discontinued. Subcutaneous tissue from the face and neck underwent its third debridement and drainage procedure on December 2. After 20 days, the incision sites on the face and neck had healed well, resulting in the patient’s discharge. Further recovery took place over a duration of 6 months, aided by oral administration of clarithromycin.
Case 3
A 37-year-old woman received facial line carving and autologous fat grafting at a cosmetic clinic 6 months prior to admission. Four months prior to admission, she underwent multiple injections of lysozyme and hormones into the lower eyelid. Two months prior to admission, she developed localized facial redness and swelling, and subsequently underwent a foreign body removal procedure. Despite treatment with cephalosporin, imipenem, levofloxacin, and penicillin in multiple institutions, there was no significant improvement in the local redness, swelling, or pus (Figure 5B). On October 15, 2020, the patient was admitted to the Department of Burn and Plastic Surgery at our hospital.
Admission examination revealed multiple localized masses on both cheeks with evident redness, swelling, and fluctuation. Additionally, four incisions on the right side of the face were unhealed with minimal discharge, and there was a slight deviation in the left angle of the mouth during smiling with visible tooth exposure. M. abscessus was isolated from the wound secretion specimen at another hospital, but its antimicrobial susceptibility was unknown. Empiric treatment was initiated with imipenem, amikacin, levofloxacin, and doxycycline.
On October 21, surgery was conducted to repair a chronic facial ulcer and remove a foreign body. Following the surgery, wound secretion was collected for bacterial culture and antibiotic susceptibility testing. The M. abscessus isolate exhibited sensitivity to clarithromycin, amikacin, cefoxitin, doxycycline, and linezolid. As such, treatment consisted of a combination of linezolid, amikacin, and doxycycline. Most of the symptoms had improved 1 month later; however, the patient’s left cheek exhibited local redness and swelling with a visible break observed. White purulent secretions and a small amount of exudative bleeding were found from the break, thus requiring a second debridement on December 2. Following 12 days, the antibiotics regimen was altered to include clarithromycin, doxycycline, and levofloxacin. The patient was discharged with antibiotics and her wound healed after 5 months of antibiotic therapy.
Discussion
The initial M. abscessus isolate was obtained from a patient with a knee abscess in 1952.21 M. abscessus and M. chelonei were initially classified as the same species and were later identified as an independent species in 1992. Mycobacterium massiliense and Mycobacterium bolletii, two new subspecies, were subsequently discovered and combined with M. abscessus to form the Mycobacterium abscessus complex (MABC).22 MABC is present widely in the environment, including in water tanks, hot water pipes, swimming pools, medical equipments such as endoscopes, and indoor dust.23 MABC is resistant to high concentrations of chlorine and other disinfectants, such as alkaline glutaraldehyde. Furthermore, its high-temperature resistance and biofilm formation enable it to survive in aquatic environments and infect wounds that are exposed to improperly disinfected surgical equipment or contaminated water from showers and faucets.24 Cases of M. abscessus infection have been reported after plastic surgery procedures such as silicone injection, liposuction, abdominoplasty, bilateral mastopexy, botulinum toxin injections, and breast augmentation.25 The infection is most likely caused by a lack of proper surgical equipment sterilization or poor sterile technique by the surgeon.26 Although, in our three cases, M. abscessus was not isolated from the environment of the cosmetic institution, it is suspected that the infection was a result of inadequate disinfection of medical equipment or contaminated sterile saline, based on their epidemiological history.
Skin infections caused by M. abscessus are commonly present with erythema, purplish-red nodules, and painless abscesses. Clinical symptoms encompass cellulitis, papular lesions, purple discolored nodules, abscesses, drainage sinus, subcutaneous nodules (nodular pseudoerythema), and ulcers.27 Soft tissue infections caused by M. abscessus generally result in multiple skin lesions, while sporadic Mycobacterium infections tend to result in a single skin lesion.28
In our cases, patients presented with non-healing wounds accompanied by facial redness, purulent discharge, exudate, swelling, pain, and ulceration, which are common symptoms of M. abscessus infection. The increasing prevalence of plastic surgery has led to a rise in related infections, making it an issue that requires attention. A thorough physical examination of patients with a history of plastic surgery and persistent non-healing wounds should include a careful evaluation of any abnormal clinical manifestations. Recurrent or unhealed subcutaneous abscesses, even after adequate debridement and antibiotic treatment, should be monitored for timely diagnosis and treatment of M. abscessus infection. To prevent infection, it is essential to properly sterilize all surgical equipment and saline, adequately manage postoperative wounds, and provide patients with education on wound care.14
Due to the limited number of cases and clinical trials, recommended treatment options for M. abscessus soft tissue infection primarily follow guidelines for lung infections, with multiple antibiotic options available for clinical use. The inherent multidrug resistance induced resistance to macrolides and disparities between in vitro drug sensitivity testing and clinical responses all contribute to the complexity of treatment. The in vitro susceptibility testing results of M. abscessus to macrolides and tigecycline are relatively reliable, followed by clofazimine, bedaquiline, amikacin, imipenem, and cefoxitin.29 There was only one prospective non-random observational study on the treatment of soft tissue infections caused by M. abscessus.30 This study compared the therapeutic effects of oral clarithromycin+moxifloxacin with that of clarithromycin+amikacin from 2007 to 2008. The former treatment had a shorter time of clinical improvement (17 weeks) compared to the latter (20 weeks). However, the study only recruited 52 patients, and patients who received oral clarithromycin+amikacin had more severe symptoms, which might have introduced bias into the results.
The standard approach to treating M. abscessus infection is through debridement coupled with antimicrobials. Given the high resistance of M. abscessus to antibiotics, a combined therapy is required. However, the most effective treatment regimen and course is not entirely clear.31 For a duration of at least 4.5 months, oral clarithromycin or moxifloxacin should be maintained, with an average course of treatment lasting 6–8 months.32
In our study, the second patient underwent three surgeries with debridement and was treated with amikacin, clarithromycin, and linezolid during hospitalization. Additionally, oral administration of clarithromycin aided in her total recovery. For a third patient, the antibiotics was changed from a combination of linezolid, amikacin, and doxycycline to clarithromycin, doxycycline, and levofloxacin. Treatment for M. abscessus infection is complex. Physicians should exercise caution and adjust or alter therapy in a timely manner based on patient reactions.
The treatment for M. abscessus infection is constrained by multiple factors such as drug tolerance, toxicity, and drug interferences. Cefoxitin and imipenem exhibit potent bacteriostasis activity against M. abscessus in vitro. However, when used alone, they lack bactericidal effect. Hence, a combined therapy is proposed to accomplish synergistic bactericidal activity.33 Although tigecycline exhibited effective bactericidal activity in vitro and in vivo, 90% of patients experienced adverse reactions including nausea and vomiting.34 Studies indicate that treatment with azithromycin, amikacin, and imipenem significantly improved bacterial clearance rates among patients with pulmonary infections caused by M. abscesses.35 Furthermore, drugs that have lower toxicity, such as clofazimine,36 exhibit a synergistic effect with amikacin, omycycline,37 and tedizolomide.38 These medications show promise in treating skin and soft tissue infections and can be considered for use in treating M. abscessus infections.
Due to the slower growth of M. abscessus compared to general pathogenic bacteria, it can be easily overlooked by traditional bacterial culture. Case 1 was hospitalized for over 100 days before a diagnosis was made. Throughout her hospitalization, samples of wound secretions were collected for etiological examination, and antibiotics were changed several times in response to the results. Despite undergoing five debridement operations and receiving multiple antibiotics, the wound still healed poorly. Long-term bed confinement causes difficulty in lifting the upper limbs and imposes significant physiological, psychological, and financial burdens. Valuable evidence for targeted treatment and prevention of adverse effects resulting from long-term anti-tuberculosis treatment was only provided after the Ziehl-Neelsen staining results showed positive and M. abscesses were isolated. Similar cases have also been reported in Switzerland39 and Denmark.40 M. abscessus was isolated from the wound specimens 10 years and 2 years after breast augmentation, respectively, indicating that diagnosing and treating such cases is extremely challenging.
For skin and soft tissue infections that occur after trauma, surgery, and plastic surgery, particularly those that fail to respond to traditional antibiotic treatment, and exhibit symptoms such as abscesses, ulcers, and nodules resembling sporotrichosis,1 it is crucial to widen the pathogen screening scope beyond routine pathogenic examinations. The identification of subspecies in M. boletii and M. abscessus is vital for guiding clinical treatment due to the presence of the inducible macrolide resistance gene, erm,41 while M. massiliense lacks erm.41 Commonly used methods for subspecies identification include MALDI-TOF mass spectrometry42 and molecular techniques such as sequencing 16S rRNA, hsp65, and rpoB regions.43 In our study, the identification of M. abscessus in three cases depended on MALDI-TOF MS system. However, the growth of M. abscessus takes several days. To increase the efficiency of treating M. abscessus infection, it is more desirable to combine MALDI-TOF mass spectrometry with molecular techniques. The secretion from the wound can be sequenced for testing the16S rRNA, hsp65, and rpoB regions.
Accurate identification of MABC subspecies is crucial for appropriate treatment due to their varying antimicrobial resistance properties. While MALDI-TOF MS is the main identification method in clinical laboratories for most NTMs, it remains a challenge to differentiate closely related species such as MABC subspecies. However, a combination of MALDI-TOF MS and machine learning has shown a high accuracy in identifying MABC subspecies, although the geographic origin of the strains can impact the protein spectra.44,45
In addition to identifying subspecies, it is necessary to genotype macrolide susceptibility to guide precise treatment for clinical M. abscessus infections. The real-time multiplex assay allows for both the distinguishing of MABC subspecies and the determination of its susceptibility to macrolides. Compared to the 3 to 14 days needed for clarithromycin phenotypic susceptibility testing, this assay can provide clinically significant treatment information in less than 3 hr.46 Multiple PCR methods have been established and have shown excellent capacity for identifying MABC subspecies and detecting clarithromycin resistance due to erm and 23S rrl genes, particularly when mixed genetic profiles are present.46–49
Despite the advantages of PCR methods, genetic tests require additional reagents and reaction time for subspecies identification and detection of antibiotic resistance. In contrast, using machine learning algorithm to detect MABC subspecies based on MALDI-TOF spectra does not require any additional cost or reaction time. Therefore, the combination of MALDI-TOF MS and real-time PCR offers high cost-effectiveness, making it a more favourable option for real-world deployment. Expanding diagnostic capabilities in health-care facilities would greatly improve patient care by enabling the administration of appropriate antibiotic treatment and antimicrobial stewardship programs for M. abscessus infections.
Conclusion
This article details three cases of M. abscessus cutaneous infection after plastic surgery, and presents etiological evidence for treatment. Medical professionals should consider this rare organism as a possible cause for long-term non-healing wounds following postoperative incision and immediately perform etiological examination to determine diagnosis. For treatment, surgery combined with long-term antibiotics is recommended. Additional attention should be given to surgical instruments and saline disinfection in the plastic surgery operating room. Due to the relatively low incidence of M. abscessus cutaneous infection and the prolonged course of treatment, further study of its pathogenicity and treatment guidelines are necessary for long-term follow-up.
Ethical Approval
All aspects of the study were performed in accordance with national ethics regulations and approved by the Ethics Committee of Shenzhen Hospital of Southern Medical University. Written informed consent was obtained from each patient for publication of this case reports and any accompanying images.
Acknowledgments
We would like to thank the staff at Clinical Laboratory and Department of Burn and Plastic Surgery in Shenzhen Hospital of Southern Medical University for their contribution to this study.
Funding
Natural Science Foundation of Guangdong Province (2020A1515010008) and Shenzhen Science and Technology Innovation Committee (JCYJ20210324130801004 and JCYJ20210324120801005) provided grants for the study design, isolation and antibiotic susceptibility, and data collection and analysis. Research Foundation of Shenzhen Hospital, Southern Medical University (PY2020YM02), and Research Promotion Funds for the Key Discipline Construction Program (ZDXKKYTS007) provided funding in the writing and revision of the manuscript. Shenzhen Science and Technology Innovation Committee (JCYJ20230807142202004) supported the publication fees for this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria skin and soft tissue infections. Dermatologic Clinics. 2015;33(3):563–577. doi:10.1016/j.det.2015.03.017
2. Rodriguez-Garcia R, Espina Angulo MJ, Escudero Augusto D. Cutaneous infection with Mycobacterium abscessus. Intensive Care Med. 2018;44(12):2292–2293. doi:10.1007/s00134-018-5284-8
3. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
4. Lopeman RC, Harrison J, Desai M, Cox JAG. Mycobacterium abscessus: environmental bacterium turned clinical nightmare. Microorganisms. 2019;7(3):90. doi:10.3390/microorganisms7030090
5. Kumar C, Shrivastava K, Singh A, Chauhan V, Varma-Basil M. Skin and soft-tissue infections due to rapidly growing mycobacteria: an overview. Int J Mycobacteriol. 2021;10(3):293–300. doi:10.4103/ijmy.ijmy_110_21
6. Mei Y, Zhang W, Shi Y, et al. Cutaneous tuberculosis and nontuberculous mycobacterial infections at a national specialized hospital in China. Acta Dermato-Ven. 2019;99(11):997–1003. doi:10.2340/00015555-3283
7. Liu Y, Chen Y. Surgical treatment for cutaneous Mycobacterium abscessus infection caused by injections of hyaluronic acid. Clin Cosmet Invest Dermatol. 2023;16:687–692. doi:10.2147/CCID.S394594
8. Zhang X, Feng Y, Li D, Han J, Shi D. Scalp infection caused by mycobacterium abscessus manifested as patchy alopecia in an female. Infect Drug Resist. 2023;16:5413–5419. doi:10.2147/IDR.S416974
9. Berkhout A, Curtis N, Gwee A, Harris C, Burgner D. Mycobacterium abscessus soft tissue infection review of published cases and challenges in treatment. Pediatr Infect Dis J. 2020;39(7):e130–e132. doi:10.1097/INF.0000000000002673
10. Kerkemeyer KL, Darby JD, Green J. Mycobacterium abscessus infection of a new tattoo in an Australian traveller returning from Bali, Indonesia. J Travel Med. 2020;27(6). doi:10.1093/jtm/taaa014
11. Hui SH, Noonan L, Chavada R. Post liposuction mycobacterium abscessus surgical site infection in a returned medical tourist complicated by a paradoxical reaction during treatment. Infectious Disease Reports. 2015;7(4):6304. doi:10.4081/idr.2015.6304
12. Summers NA, Kempker R, Palacio F. Mycobacterium abscessus subspecies massiliense infection after skin graft and cholecystectomy in a burn patient. Inter J Infect Dis. 2018;76:29–31. doi:10.1016/j.ijid.2018.08.016
13. Bowles P, Miller MC, Cartwright S, Jones M. Presentation of Mycobacterium abscessus infection following rhytidectomy to a UK plastic surgery unit. BMJ Case Rep. 2014;2014:bcr2014204000–bcr2014204000. doi:10.1136/bcr-2014-204000
14. Barone AAL, Grzelak MJ, Frost C, et al. Atypical mycobacterial infections after plastic surgery procedures abroad a multidisciplinary algorithm for diagnosis and treatment. Ann Plastic Surg. 2020;84(3):257–262. doi:10.1097/SAP.0000000000002061
15. Eustace K, Jolliffe V, Sahota A, Gholam K. Cutaneous Mycobacterium abscessus infection following hair transplant. Clin Exp Dermatol. 2016;41(7):768–770. doi:10.1111/ced.12900
16. Chen X, Jin Y, Torres KMT, et al. Mycobacterium abscessus cutaneous infection secondary to botulinum toxin injection: a report of 2 cases. JAAD Case Rep. 2019;5(11):982–984. doi:10.1016/j.jdcr.2019.09.017
17. Cai SS, Chopra K, Lifchez SD. Management of Mycobacterium abscessus infection after medical tourism in cosmetic surgery and a review of literature. Ann Plastic Surg. 2016;77(6):678–682. doi:10.1097/SAP.0000000000000745
18. Singh J, O’Donnell K, Nieves DJ, et al. Invasive Mycobacterium abscessus outbreak at a pediatric dental clinic. Open Forum Infect Diseases. 2021;8(6):doi:10.1093/ofid/ofab165
19. Woods GL, Brown-Elliott BA, Conville PS, et al. CLSI Standards: Guidelines for Health Care Excellence. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. Wayne (PA): Clinical and Laboratory Standards Institute; 2011.
20. Lv Y, Chen Z, Yang Z, et al. Evaluation of the red & blue LED effects on cutaneous refractory wound healing in male Sprague-Dawley rat using 3 different multi-drug resistant bacteria. Lasers Surg Med. 2022;54(5):725–736. doi:10.1002/lsm.23515
21. Brown-Elliott BA, Wallace RJ. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–746. doi:10.1128/CMR.15.4.716-746.2002
22. Victoria L, Gupta A, Gomez JL, Robledo J. Mycobacterium abscessus complex: a review of recent developments in an emerging pathogen. Front Cell Infect Microbiol. 2021;2021:11.
23. Thomson R, Tolson C, Sidjabat H, Huygens F, Hargreaves M. Mycobacterium abscessus isolated from municipal water - a potential source of human infection. BMC Infect Dis. 2013;13:13. doi:10.1186/1471-2334-13-13
24. Li T, Abebe LS, Cronk R, Bartram J. A systematic review of waterborne infections from nontuberculous mycobacteria in health care facility water systems. International Journal of Hygiene and Environmental Health. 2017;220(3):611–620. doi:10.1016/j.ijheh.2016.12.002
25. Moreno-Izquierdo C, Zurita J, Contreras-Yametti FI, Jara-Palacios MA. Mycobacterium abscessus subspecies abscessus infection associated with cosmetic surgical procedures: cases series. IDCases. 2020;22:e00992.
26. Bies JJ, Allen JC, Barsi ZE, et al. Brazilian butt lift gone wrong: a case series of non-tuberculous Mycobacterial gluteal infection. Cureus J Med Sci. 2023;15(12):1.
27. Franco-Paredes C, Marcos LA, Henao-Martinez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2019;32(1):1.
28. Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Exp Rev Anti-Infective Ther. 2016;14(12):1139–1154. doi:10.1080/14787210.2016.1238304
29. Cowman S, Burns K, Benson S, Wilson R, Loebinger MR. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect. 2016;72(3):324–331. doi:10.1016/j.jinf.2015.12.007
30. Choi WS, Kim MJ, Park DW, et al. Clarithromycin and amikacin vs. clarithromycin and moxifloxacin for the treatment of post-acupuncture cutaneous infections due to Mycobacterium abscessus: a prospective observational study. Clinical Microbiol Infect. 2011;17(7):1084–1090. doi:10.1111/j.1469-0691.2010.03395.x
31. Lamb GS, Starke JR. Mycobacterium abscessus infections in children: a review of current literature. J Pediatric Infect Dis Soc. 2018;7(3):e131–e44. doi:10.1093/jpids/piy047
32. Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Resp Crit Care Med. 2018;39(3):362–376. doi:10.1055/s-0038-1651494
33. Andrew EC, Connell T, Robinson P, et al. Pulmonary Mycobacterium abscessus complex in children with cystic fibrosis: a practical management guideline. J Paediatr Child Health. 2019;55(5):502–511. doi:10.1111/jpc.14427
34. Wallace RJ, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother. 2014;69(7):1945–1953. doi:10.1093/jac/dku062
35. Kwak N, Dalcolmo MP, Daley CL, et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J. 2019;54(1):1801991. doi:10.1183/13993003.01991-2018
36. Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest. 2017;152(4):800–809. doi:10.1016/j.chest.2017.04.175
37. Bax HI, de Vogel CP, Mouton JW, de Steenwinkel JEM. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother. 2019;74(10):2930–2933. doi:10.1093/jac/dkz267
38. Compain F, Soroka D, Heym B, et al. In vitro activity of tedizolid against the Mycobacterium abscessus complex. diagnostic microbiology and infectious disease. Diagnostic Microbiology and Infectious Disease. 2018;90(3):186–189. doi:10.1016/j.diagmicrobio.2017.11.001
39. Martinez AE, Gass SK, Meylan S, et al. Breast pain and fever in a 46-year-old immunosuppressed patient with breast implants. Internist. 2019;60(10):1102–1105.
40. Jensen E, Holst-Albrechtsen S, Christensen KO, Birk-Sorensen L, Juel J. Mycobacterium abscessus infection after cosmetic breast surgery in India. Ugeskrift for Laeger. 2018;180(7):1.
41. Griffith DE, Daley CL. Treatment of Mycobacterium abscessus pulmonary disease. Chest. 2022;161(1):64–75. doi:10.1016/j.chest.2021.07.035
42. Kehrmann J, Wessel S, Murali R, et al. Principal component analysis of MALDI TOF MS mass spectra separates M. abscessus (sensu stricto) from M. massiliense isolates. BMC Microbiol. 2016;16:doi:10.1186/s12866-016-0636-4
43. Zelazny AM, Root JM, Shea YR, et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol. 2009;47(7):1985–1995.
44. Rodriguez-Temporal D, Herrera L, Alcaide F, et al. Identification of Mycobacterium abscessus subspecies by maldi-tof mass spectrometry and machine learning. J Clin Microbiol. 2023;61(1):doi:10.1128/jcm.01110-22
45. Wang H-Y, Kuo C-H, Chung C-R, et al. Rapid and accurate discrimination of mycobacterium abscessus subspecies based on matrix-assisted laser desorption ionization-time of flight spectrum and machine learning algorithms. Biomedicines. 2023;11(1):doi:10.3390/biomedicines11041084
46. Marras SAE, Chen L, Shashkina E, et al. A molecular-beacon-based multiplex real-time pcr assay to distinguish Mycobacterium abscessus subspecies and determine macrolide susceptibility. J Clin Microbiol. 2021;59(8):doi:10.1128/JCM.00455-21
47. Sharma MK, La Y, Janella D, Soualhine H. A real-time PCR assay for rapid identification of inducible and acquired clarithromycin resistance in Mycobacterium abscessus. BMC Infect Dis. 2020;20(1). doi:10.1186/s12879-020-05686-0
48. Pedace CS, Goncalves MG, Souza AR, et al. Development of multiplex real- time PCR for detection of clarithromycin resistance genes for the Mycobacterium abscessus group. J Med Microbiol. 2023;72(3):doi:10.1099/jmm.0.001670
49. Shallom SJ, Zelazny AM. Detection of mixed populations of clarithromycin-susceptible and -resistant Mycobacterium abscessus strains. J Clin Microbiol. 2022;60(4). doi:10.1128/jcm.01694-21
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.