Back to Journals » International Journal of Nanomedicine » Volume 17
Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment
Authors Choukaife H , Seyam S , Alallam B, Doolaanea AA , Alfatama M
Received 26 May 2022
Accepted for publication 1 August 2022
Published 7 September 2022 Volume 2022:17 Pages 3933—3966
DOI https://doi.org/10.2147/IJN.S375229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Hazem Choukaife,1 Salma Seyam,1 Batoul Alallam,2 Abd Almonem Doolaanea,3 Mulham Alfatama1
1Faculty of Pharmacy, Universiti Sultan Zainal Abidin, Besut Campus, Terengganu, 22200, Malaysia; 2Advanced Medical and Dental Institute, Universiti Sains Malaysia, Kepala Batas, Penang, 13200, Malaysia; 3Department of Pharmaceutical Technology, Kulliyyah of Pharmacy, International Islamic University Malaysia, Kuantan, Pahang, 25200, Malaysia
Correspondence: Mulham Alfatama, Faculty of Pharmacy, Universiti Sultan Zainal Abidin, Besut Campus, Terengganu, 22200, Malaysia, Tel +60 142255604, Email [email protected]; [email protected]
Abstract: As per the WHO, colorectal cancer (CRC) caused around 935,173 deaths worldwide in 2020 in both sexes and at all ages. The available anticancer therapies including chemotherapy, radiotherapy and anticancer drugs are all associated with limited therapeutic efficacy, adverse effects and low chances. This has urged to emerge several novel therapeutic agents as potential therapies for CRC including synthetic and natural materials. Orally administrable and targeted drug delivery systems are attractive strategies for CRC therapy as they minimize the side effects, enhance the efficacy of anticancer drugs. Nevertheless, oral drug delivery till today faces several challenges like poor drug solubility, stability, and permeability. Various oral nano-based approaches and targeted drug delivery systems have been developed recently, as a result of the ability of nanoparticles to control the release of the encapsulant, drug targeting and reduce the number of dosages administered. The unique physicochemical properties of chitosan polymer assist to overcome oral drug delivery barriers and target the colon tumour cells. Chitosan-based nanocarriers offered additional improvements by enhancing the stability, targeting and bioavailability of several anti-colorectal cancer agents. Modified chitosan derivatives also facilitated CRC targeting through strengthening the protection of encapsulant against acidic and enzyme degradation of gastrointestinal track (GIT). This review aims to provide an overview of CRC pathology, therapy and the barriers against oral drug delivery. It also emphasizes the role of nanotechnology in oral drug targeted delivery system and the growing interest towards chitosan and its derivatives. The present review summarizes the relevant works to date that have studied the potential applications of chitosan-based nanocarrier towards CRC treatment.
Keywords: chitosan, colorectal cancer, nanocarriers, oral delivery, drug targeting, nanotechnology
Introduction
Colorectal cancer (CRC) is the third most prevailing cancer worldwide. In 2020, CRC caused 506,449 deaths in Asia, 244,824 in Europe, 63,987 in North America and 69,435 in Latin America and the Caribbean.1 The morbidity is expected to increase up to 1.1 million worldwide by 2030.2 CRC is usually seen in patients under the age of 50 and it is associated with dietary characteristics and eating habits that contribute to bacterial dysbiosis in the gastrointestinal tract (GIT) and colon cancer.3
CRC is a type of malignancy that affects the large intestine and rectum regions.4 It is caused by the deactivation of the p53 pathway or adenomatous polyposis coli gene, the accumulation of mutations such as in K-ras, the transforming growth factor-beta pathway and the genesis of small polyps. The American Joint Committee on Cancer divided CRC into five phases with different treatment strategies for each stage.5 Stage 0, the presence of abnormal colon cells or polyps on the mucosa, is completely curable with a surgical resection when found early enough. Similarly, surgical excision is the conventional treatment for stages I–II in most cases but with a 5-year survival rate of 37–74%. However, the survival rate drops to 6% in advanced stages of CRC6 and adjuvant pharmaceutical therapies such as chemotherapy are usually recommended following surgical resection.7 In stages III–IV, adjuvant chemotherapy like oxaliplatin, 5-fluorouracil, cisplatin, doxorubicin and other emerging medicines are used. However, the administration of these medicines, has been linked to adverse effects, such as vomiting, hair loss and nausea and not demonstrated the expected efficacy.8 Variety of drawbacks also associated with anticancer drugs, such as hydrophobicity, low water solubility, inadequate biodistribution, and susceptibility to multiple drug resistance.9 This has urged the need for innovative approaches to enhance the physicochemical and pharmacodynamic properties of standard chemotherapeutics, or achieve target-specific delivery to optimise treatment effectiveness while reducing off-target side effects.10
Nanoparticles are among the most promising treatment modalities for cancers including CRC. Nanoparticles taken intravenously can circulate for extended period of time with reduced clearance rates from the renal system due to their ultra-small size and superior drug loading and encapsulation efficacy.11 Furthermore, since nanoparticles allow for continuous or controlled release, the physical encapsulation of anticancer drugs improves their pharmacokinetic properties while reducing the number of dosages administered. This controlled release can circumvent multiple drug resistance since the cells actively ingest bigger amounts of the drug intracellularly.12–14 Nanoparticles surface can be further modified with targeting ligands to achieve drug targeting by increasing the selectivity towards the desired cells/tissues while reducing the availability of the anticancer drug at the normal tissues.15 In addition, amphiphilic lipid-based nanoparticles, such as micelles, possess a hydrophilic surface and a hydrophobic core and able to increase the solubility of hydrophobic drugs.16
Oral drug delivery is the most preferred and convenient route for drug administration and can be utilised for both systemic or local drug delivery.17 The high surface area (>300 m2) of gastrointestinal track coated with a viscous mucosal layer facilitates drug adhesion and absorption.18 Nevertheless, oral drug delivery can be challenging due to the complexity of GIT and obstacles that impact drug delivery like poor drug solubility, stability, and permeability.17 Moreover, oral drugs may also have an unpleasant taste, induce gastric irritation, and be subjected to intestinal and hepatic barriers metabolism.19,20 Oral route can be utilised to target a specific location in the GIT following proper design of the drug or the delivery carrier.21 Several studies utilised oral route to target CRC throughout the rational design of cancer target discovery and development (CTDD).22
Numerous studies demonstrated the suitability of colon as a site for drugs absorption as it has less degradation and thus contributing to a greater drug bioavailability.23 Special considerations are involved while designing CTDD as the drug need to be protected from gastric pH and enzymes. The colon’s physiology differs greatly from that of the rest of the GIT. CTDD is affected by the inconsistency of colonic pH, transit time, and fluid volume, which are highly variable depending on the colon part, food, and metabolic enzymes.24 Other important factors impacting CTDD efficacy include colonic fluid viscosity and the presence of microbial enzymes.25
Nanoparticles for drug delivery are prepared using numerous polymers, which range between natural, synthetic and semisynthetic.26–28 Amongst these polymers, chitosan and its derivatives attracted much attention for oral route of administration owing to their suitable properties. Owing to the presence of amino groups, chitosan carries a cationic charge that is responsible for its permeation enhancing and mucoadhesion effect. It is also biodegradable, biocompatible and non-toxic.29 Chitosan is a linear polysaccharide, comprises of randomly repeated units of N-acetyl-D-glucosamine and D-glucosamine linked by β(1→4) glycosidic bonds.30 Chitosan is a natural polymer obtained from chitin, the second most abundant naturally occurring polymer following cellulose. Both chitin and chitosan are extensively used in several applications such as food technology31 and medical and pharmaceutical applications including wound healing,32 tissue engineering33 and gene delivery.34 Chitosan has been formulated into chitosan nanoparticles (CSNPs) with attracted features especially in ocular and oral delivery. CSNPs are considered a very promising strategy to overcome the low stability and bioavailability of many active ingredients. Chitosan can efficiently bind to the negatively charged mucus membrane, increasing the retention time and enhancing the probability of their cellular uptake. Moreover, chitosan was also used as a coating agent due to its unique properties that enhances the permeation of anti-cancer drugs by transiently opening the tight junction between epithelial cells. Owing to its biodegradability via glycosidic linkage lysis by specified enzymes from the colonic microflora,35 chitosan is very valuable for CRC targeting. Recently, Patil and Killedar developed water-insoluble gallic acid and quercetin into chitosan nanoparticles to assemble a stable and targeted colorectal cancer formulation.36 Kankala et al successfully prepared a versatile drug delivery platform based on nanocarriers by coating the ultrasmall Pt nanoparticles by a composite layer of chitosan over the Zn-doped MSNs to combat MDR in cancer efficaciously.37 In 2017, 5-fluorouracil loaded PLGA-nanoparticles modified with chitosan were developed for target treatment of colorectal cancer. Coated NPs showed significant inhibition of colon cancer cells (HT-29) compared to uncoated NPs or free drug solution.38
Despite the availability of different types and grades of chitosan that differ mainly in the molecular weight and degree of deacetylation, original (un-modified) chitosan may lack the optimal properties for specific applications. Therefore, chemical modifications have been performed on chitosan structure mainly on the functional groups (-NH2 and -OH). Modified chitosan derivatives have shown enhanced properties like bioactivity, biocompatibility and non-toxicity.39,40 These chitosan modifications also facilitated CRC targeting where the drug need to be protected from acidic and enzyme degradation with minimal loss until it reaches the colon.41,42 In 2021, Wang and his team combined of oxaliplatin and resveratrol successfully into N,O-carboxymethyl chitosan nanoparticles as a promise strategy for colorectal cancer therapy. The inhibition efficiency and anti-colon cancer activity of the combined treatment were more stronger than either type of nanoparticle alone or the free drugs.43 In the same year, colon site-specific mucoadhesive system was developed by Samprasita et al composed of a-Mangostin-loaded thiolated chitosan NPs following by genipin (GP) crosslinking and the surface modification by EudragitVR L100.44
This review emphasized on chitosan nanoparticle applications in CRC. We first reviewed CRC pathology and therapy and explained the oral route of drug administration. Then, we explored nanotechnology in drug delivery and targeting before moving to detailed explanation of chitosan and CSNPs and their applications especially in CRC.
Colorectal Cancer
Colorectal cancer is a type of malignancy that affects the large intestine and rectum regions.4 In 2020, the number of death caused by colorectal was 506,449 in Asia, 244,824 in Europe, 63,987 in North America and 69,435 in Latin America and the Caribbean.1 Colorectal cancer statistics are expected to grow with a morbidity estimation of 1.1 million worldwide by 2030.2 Despite continual diagnosis and therapy advancements for both primary and metastatic type of CRC, long-term survival and cure rates are remained low. Males have a greater death rate from CRC than females, and this rate may rise as a result of several stimuli factors including age, unhealthy eating habits, obesity, smoking, alcohol use, high body mass index, sedentary lifestyle, and poor physical activities.45 Adopting a healthy lifestyle appears to be the most important factor in preventing colorectal cancer.
Pathophysiology
Colorectal cancer is the third most prevailing cancer worldwide, usually seen in patients under the age of 50 and it is associated with dietary characteristics and eating habits that contribute to bacterial dysbiosis in the GIT and colon cancer.3 Colorectal cancer is now common in China, India, Singapore, Japan, and South Korea, despite the fact that it was long thought to be a disease only seen in Western nations. It is a multistage disease that can take 10–40 years to evolve from adenoma to carcinoma. Colorectal cancer is caused by the deactivation of the p53 pathway or adenomatous polyposis coli gene, the accumulation of mutations such as in K-ras, the transforming growth factor-beta pathway and the genesis of small polyps. These small polyps are prone to be transformed to large polyps, leading to tumour initiation. A preneoplastic indication of colon tissue is aberrant crypt foci where chemoprevention can be achieved by blocking or delaying the carcinogenesis process.46
T-lymphokine-activated killer cell-originated protein kinase signalling abnormalities promote cancer growth and is abundantly expressed and active in colorectal cancer, where it also stimulates cancer cell survival, inflammation and proliferation. Moreover, T-LAK-derived protein kinase, which interacts with the binding domain of DNA of the tumour suppressor p53 protein and controls the expression of transcription for target p21, has been suggested as a potential therapeutic target to fight colorectal cancer. Also, 3-Deoxysappanchalcone, a compound originated from Caesalpiniasappan L. is a natural component that capable of apoptosis induction via a p53-dependent pathway and hence, inhibiting colon cancer cell proliferation through direct targeting of signaling pathway of T-lymphokine-activated killer cell-originated protein kinase.47 The Tumor, Node, Metastasis (TNM) classification of cancer and the International Union for Cancer Control as well as the Classification of Malignant Tumors provide the foundation for therapeutic decisions. Colorectal cancer is categorised with reference to lymph node involvement (N stage), local invasion depth (T stage), and distant metastases (M stage).48 The laterality of initial tumours is critical for a patient with synchronous metastatic colon cancer. Individuals with right-sided colon cancer had a considerably poorer overall survival rate than patients with left-sided colon cancer.49
Biomarkers of Colorectal Cancer
Despite the accelerated progress of medicine, the prognosis of CRC patients is largely determined by the disease’s stage at the time of diagnosis.50 Considering the fact that early discovery of CRC reduces related morbidity and mortality and that detection of its precursor lesion can even reduce the occurrence, current CRC screening systems still have a number of flaws.51 As a result, it is critical to develop novel non-invasive biomarkers that can detect tumour in the asymptomatic CRC early stages, when the disease is still treatable.
CRC patients, on the other hand, have been treated for decades with relatively reliable diagnostic methods like as colonoscopy and histological study of tumour tissue, as well as imaging modalities such as x-rays, computed tomography, and magnetic resonance imaging. Nevertheless, based on these investigations, clinical illness assessment and tumour stage classification are unable to suggest clinically predictive information and relevant prognostic at the patient level.52 As a result, both clinical and basic scientists are continually searching for more precise individual biomarkers to reduce the severe side effects of chemotherapy, disease relapse, and treatment resistance, which would ultimately improve patients’ longevity and quality of life.
The presence of tumour traces in the blood and other bodily fluids has been recognised as a possible individual biomarker with a diagnostic, prognostic, and predictive value. The ability of detection of so-called circulating tumour cells (CTCs) in the blood samples of patients has been proven in multiple investigations to have a deleterious impact on their survival.53–55 The detection of CTCs from blood is comparable to that of conventional tumour tissue biopsy, hence, the term “liquid biopsy” has been introduced. Liquid biopsy, in contrast to tissue biopsy, has various advantages, including easy and quick procedure, reduced cost, and minimum risk and pain for patients due to its limited invasiveness.
It has recently been discovered that the existence of circulating tumour DNA (ctDNA) is just as important as the presence of CTCs.56 Furthermore, the biological function of microRNAs (miRNAs) in CRC pathogenesis is becoming clearer, and their existence in peripheral blood has been proposed as a major predictive/prognostic biomarker.57 As a result, the concept “liquid biopsy” has been widely known and is now utilised to detect all circulating tumour features, primarily ctDNA, CTCs, circulating miRNAs, as well as others such mRNAs, exosomes, proteins, and others) in peripheral blood or other biological fluids.58
 |
Figure 1 Examples of nanoparticles used in the therapeutic management of colorectal cancer. |
In the context of multiple clinical investigations, the liquid biopsy/circulating biomarker concept is steadily nearing clinical practice Colorectal cancer is typically sporadic, although having a family history of the disease increases your chances of getting it. Prevalent mutations in colorectal cancer includes KRAS, TP53, BRAF, transforming-growth factor-beta receptor 2 (TGFBR2), catenin-beta 1 (CTNNB1), APC, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-alpha (PIK3CA), ERBB2, SMAD4, AT-rich interactive domain 1 A (ARID1A), SRY (sex-determining region Y) box 9 (SOX9) and family with sequence resemblance 123B (FAM123B).59 By interfering with essential signalling pathways, mutations in these genes cause tumorigenesis. The mutations of WNT signalling pathway are seen in the early stages of colorectal cancer, with TP53 mutations appearing later.60 Current colorectal cancer biomarker research aims to target these molecules, as well as a few others, in order to improve cancer management. The gold standard procedure for colon inspection is colonoscopy, which is used for a successful screening programme. However, the discomfort during the examination, high cost and the examination recommended interval of 10 years are the main limitations of this technique. Non-invasive screening fecal tests including fecal immunochemical test (FIT), guaiac fecal occult blood test (gFOBT) and fecal DNA tests are able to detect blood in stool in high sensitivity manner.61 The FIT method uses antibodies, whereas the gFOBT method uses chemicals; however, the faecal DNA test examines colorectal cancer-related DNA biomarkers in faeces from cells released from the colon and rectal lining.
Need for Novel Oral Drug Delivery Systems
Colorectal polyps, an abnormal noncancerous growth in the inner lining of the intestine, are the most common cause of CRC. However, only about 10% of polyps have been seen to progress to aggressive malignancy. This change is very slow, taking more than 10 to 20 years to complete, and it is more likely as polyps become larger.62 Later, malignant cells infiltrate muscle and lymph nodes prior migrating to other tissues, such as the lungs and liver. CRC is divided into five phases by the American Joint Committee on Cancer, where the treatment strategy is highly related to the corresponding stage.5 Stage 0 is characterised by the presence of abnormal colon cells or polyps on the mucosa when found early enough, and it is completely curable following a surgical resection. In most cases, surgical excision is the conventional treatment for stages I–II, with a 5-year survival rate of 37–74%. Unfortunately, the survival rate in advanced stages of CRC drops to 6% due to the considerable risk of recurrence and metastasis to distant organs.6 As a result, adjuvant pharmaceutical therapies such as chemotherapy are usually recommended in the advanced stages of CRC to lower the risk of recurrence and metastasis following surgical resection.7
In stages III–IV, adjuvant chemotherapy like oxaliplatin, 5-fluorouracil, cisplatin, doxorubicin and other medicines are used to suppress tumour growth and extend survival. Mere administration of these medicines, however, has not demonstrated the expected efficacy at these stages and had been linked to a number of dose-dependent adverse effects, such as vomiting, hair loss and nausea.8 Chemotherapeutics are given in multi-cycles to reduce their adverse effects, while varying the doses, frequency, and duration of each cycle based on the individual’s condition. Anticancer drugs also have a variety of drawbacks, including the hydrophobicity, low water solubility, inadequate biodistribution, and susceptibility to multiple drug resistance.9 This has urged researchers to conduct innovative approaches such as using nanotechnology-based drug carrier system to enhance the physicochemical and pharmacodynamic properties of standard chemotherapeutics, or achieving target-specific delivery of cytotoxic agents to various types of tumour tissues to optimise treatment effectiveness while reducing off-target effects.10
Nanoparticles are among the most promising treatment modalities for CRC and other cancers, as their properties are considered as a benefit in addressing the systemic drug delivery issues that diagnostics and anticancer drugs encounter. The surface area-to-volume ratio of nanoparticles varies between 1 and 100 nm, allowing for greater drug loading through encapsulation inside the core and adsorption on the surfaces of the nanocarrier. Nanoparticles can circulate for extended periods of time and have reduced clearance rates from the renal system due to their ultra-small size and superior drug loading and encapsulation efficacy.11 Furthermore, since nanoparticles allow for continuous or controlled release, the physical confinement of diagnostics agents and anticancer drugs improves their pharmacokinetic properties while reducing the number of dosages administered. This controlled release can circumvent multiple drug resistance since the cells actively ingest bigger amounts of the drug intracellularly. Furthermore, the nanoparticles facilitates the anticancer mobility in the systemic circulation, increases cellular uptake and offers benefits over the bulk equivalent.63
Another advantage of using nanoparticles is the ability to customise their surface with targeting ligands (peptides or small-molecule ligands) to increase their selectivity towards the desired cells/tissues while reducing the availability of the chemotherapeutics at the normal tissues. Furthermore, amphiphilic lipid-based nanoparticles, such as micelles, possess a hydrophilic surface and a hydrophobic core and able to increase the solubility of hydrophobic drugs. In addition, quantum dots, nanoshells, nanosomes, and paramagnetic nanoparticles have structural properties that can be exploited for imaging and diagnostic purposes. Figure 1 illustrates different types of nanoparticles employed for colorectal cancer targeting.
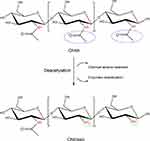 |
Figure 2 Deacetylation of chitin into chitosan. |
Emerging Oral Therapeutic Agents for Treating Colorectal Cancer (Synthetic & Natural)
The main treatment for stage 0 (the early stage) and stages I to III of non-metastatic CRC is radiotherapy with/without chemotherapy whereas chemotherapy is the option for stage IV (metastatic CRC (mCRC)). Several therapeutic agents were emerged as potential therapy for CRC with some gained regulatory approvals. They include synthetic and natural materials as small molecules or macromolecules. In this regard, we discuss the orally administered emerging therapeutic agents for CRC and divided them into synthetic and natural.
Emerging Synthetic Therapeutic Agents
KRAS (Kirsten rat sarcoma virus) gene is a member in tyrosine kinase signaling pathways. It is one of the important identified oncogenes whereby downregulating these pathways becomes a target for tyrosine kinase inhibitors (TKIs).64 Targeting growth factor receptors is one of the important mechanisms for the emerging therapeutic agents against CRC. These growth factors include platelet-derived growth factor receptors (PDGFRs), fibroblast growth factor receptors (FGFRs), vascular endothelial growth factor receptors (VEGFRs) and others.
Tyrosine Kinase Inhibitors Targeting VEGF or KIT
Nintedanib is a TKI that inhibits some of Src tyrosine kinases and targets several growth factor receptors including PDGFRs α and β, FGFRs 1–3 and VEGFRs 1–3.65 Nintedanib is approved in Europe and the USA, with 150 mg twice daily as a standard adult dose.64
Regorafenib is a multi-TKI with activities against Ret, Kit, PDGFR, and Raf kinases. It also inhibits VEGFR 2 and VEGFR 3, which is responsible for its antiangiogenic activity.66 Regorafenib gained FDA and EMA approval for the treatment of mCRC.64
Axitinib is an antiangiogenic agent inhibiting VEGF and PDGF. Axitinib is an effective agent against mCRC.66
Apatinib (rivoceranib) is a TKI and VEGFR 2 inhibitor showing antiangiogenic and antineoplastic effects. Apatinib decreases tumor microvessel density and inhibits VEGF-stimulated endothelial cell migration and proliferation.66 In a study involved patients undergone at least two standard treatments, Apatinib 500 mg once daily was effective against refractory mCRC. However, the dose was then reduced to avoid adverse events.66 In VEGFA-overexpressed tumors, Apatinib decreases the hyperangiogenesis and hypoxia inside the tumor microenvironment, converting it from an immunosuppressive into an immunostimulatory one.66
Famitinib is a TKI that inhibits VEGFRs 2 and 3. In a clinical trial, Famitinib increased the progression-free survival in patients with refractory mCRC compared to the placebo. It showed an acceptable safety profile but the overall survival did not increase.64
Surufatinib (Sulfatinib) is a TKI exhibiting activities on anti-angiogenesis and regulating cancer immunity. Surufatinib inhibits VEGFRs 1–3, FGFR1 and colony stimulating factor 1 receptor (CSF1R). Surufatinib safety and efficacy is being investigated in a clinical trial (NCT04653480) in second-line RAS/BRAF mutant and microsatellite stable CRC.64
Masitinib is a TKI with a specific KIT targeting, which is mutated or overexpressed in many cancers. Activated c-KIT receptor and tryptase have important role in the cancer tissue angiogenesis, which help the cancer cell in the invasion and metastasis.66
BRAF Inhibitors
BRAF is an enzyme in the RAF/MEK/ERK signaling pathway. BRAFV600E mutation is upregulated in many human cancers. In CRC, around 15% of the patients have mutated BRAF. BRAF inhibition showed in success in BRAF-mutant metastatic melanoma, therefore it can be also employed in mCRC treatment.64 Vemurafenib and Encorafenib are among the inhibitors of BRAFV600E-mutated kinase. Encorafenib showed modest antitumor activity in patients with refractory mCRC bearing BRAFV600E mutation.66
MEK Inhibitors
MEK1 and MEK2 are dual-specificity Thr/Tyr kinases. Binimetinib is a mitogen-activated inhibitor of both enzymes while Cobimetinib is a specific inhibitor of MEK1. In vitro, Cobimetinib induces the apoptosis of HCT-116 CRC cells and inhibits cell proliferation.64
STAT3 Inhibitors
In colon cancer and other cancers, a signal transducer and activator of transcription factor 3 (STAT3) is active and have a significant role in cancer progression. In a clinical trial Phase III, Napabucasin as an inhibitor of STAT3 increased the survival of patients with mCRC compared with placebo.66
PD1/PDL-1 Inhibitors
Programmed cell death 1 (PD-1) receptor is found on the immune cell surface including T cells. PD-1 ligand (PDL-1) was detected in several cancers and when binds to PD-1, it inhibits the activation of T cells. Therefore, interfering with the PD-1/PDL1 pathway restores T cell function and this leads to an improvement of the immune response against cancer.66 Combination therapy of capecitabine and oxaliplatin together with immune checkpoint blockers anti-PD-1 and anti-PDL1 was explored in murine syngeneic preclinical models that are similar to the tumor type in patients.66
PDZ Domains Inhibitors
PDZ domains represents the most abundant protein–protein interaction domains of the human proteome. PDZ is composed of 266 different domains located in 150 proteins. PDZ aberrant expression was found in CRC and other cancers.64
By themselves, these domains do not have any enzymatic activity but they orchestrate several pathways. Therefore, inhibition of PDZ domains results in multiple enzyme inhibition simultaneously.
NHERF1 (Na+/H+ exchanger 3 regulating factor 1) is a PDZ containing protein overexpressed in the cytoplasm and nuclei of CRC cells. Therefore, a new class of anticancer drugs can be utilized based on the inhibition of NHERF1. Several small molecules were designed employing molecular and chemical approaches then evaluated as NHERF1 inhibitors.66
Cancer Stem Cells
Cancer stem cells (CSCs) are resistant to the radiation and chemotherapeutic agents. They involve in the self-renewal of many cancers. Colorectal CSCs and other solid tumor stem cells have an insistent activation of several signal transduction pathways, whereby Wnt/β-catenin pathway plays an important role in stemness maintenance and drug resistance of colorectal CSCs.66 CD44 is a well-documented surface marker involved in the proliferation of cancer cells. It has an important role in regulating CSC stemness properties. CD44 was found to regulate the in vitro and in vivo growth of colon cancer xenografts in animals.66 Therefore, CD44 can be utilized in CRC treatment options.
The anti-diabetes drug Metformin downregulates CD44 in primary oral cancer cells.66 Combined with doxorubicin, Metformin inhibits both cancer stem cells and non-stem cancer cells in culture. Therefore, a new use of Metformin may emerge in the anticancer treatment plans.64
The antibiotic Salinomycin inhibits CSCs in different human cancers. The mechanism is suggested to be by interfering with ABC drug transporters and CSC pathways including the Wnt/β-catenin signaling pathway. Salinomycin derivative with cyclopropylamine at position C20 inhibits HMLER CD24-low/CD44-high cells. With high selectivity against CSCs, Salinomycin and its derivative could have the potential to prevent cancer resistance.66
The COX2 inhibitor celecoxib is used as nonsteroidal anti-inflammatory drug. Synergistically, celecoxib and the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin, may eradicate CSCs with CD44 overexpression.66
γ-secretase inhibitors like MK-0752 are being investigated in various CSCs as modulators of the Notch signaling pathway. MK-0752 and ridaforolimus (MK-8669) as a combination are being clinically investigated (phase 1 clinical trial) in patients suffering from advanced and refractory pancreatic and colorectal cancer.66
Emerging Natural Therapeutic Agents
Natural products serve as a main source of new anticancer drugs. It is estimated that about half of the anticancer drugs currently in use are derived from natural products whether directly or not directly. Anticancer natural products fall into several categories based on their structure including alkaloids, polysaccharides, polyphenols, diterpenoid and unsaturated fatty acids.67 The use of chemotherapy for the treatment of CRC is commonly associated with side effects and drug resistance. Therefore, there is a need to find new and more effective treatments for CRC. Several natural products exhibit anti-CRC effects.68
Alkaloids
Irinotecan (CPT-11) is a water-soluble camptothecin derivative. Its mechanism involves the inhibition of DNA topoisomerase I, blocking DNA replication and inhibiting RNA synthesis.67 Irinotecan has a proven anti-cancer effect against mCRC. Irinotecan has been used for standard CRC treatment. In combination with 5-fluorouracil/formyltetrahydrofolate (CF), it has better anti-cancer efficacy against the advanced mCRC.68
SN38 is the active analogue of irinotecan. It inhibits the growth of several cancer cells.67 Its mechanism involves anti-angiogenic activity due to the inhibition of VEGF and hypoxia-inducible factor 1 alpha expression. SN38 inhibits both the endothelial cell proliferation or lumen formation and the angiogenic activity of glioma cells.
Despite of the variable anti-tumor activities of CPT-11 and SN38, they both have significant toxicity including cholinergic syndrome, neutropenia and delayed diarrhea. More research is being performed to improve their efficacy and safety through chemical structure modification and combination chemotherapy.68
Polysaccharides
Fucoidan is a sulfated polysaccharide isolated from the seaweed Sargassum hemiphyllum. It has been shown to induce apoptosis in HT-29 and HCT-116 cells.69 Double-blind, randomized clinical trials was conducted in Taiwan and demonstrated that low-molecular-weight fucoidan (0.8 KDa) has significant activity against CRC in humans in combination with chemotherapy, with relatively few side effects.67
Polyphenols
Resveratrol is a polyphenolic compound with significant inhibitory effects against wide range of cancers. Resveratrol induces apoptosis in CRC by inducing Fas redistribution and inhibiting Wnt signaling.70 The grape seed extract, a rich source of Resveratrol, was shown to inhibit PI3K pathway and induce apoptosis in CaCo-2 cells.71
Curcumin is a polyphenol isolated from Curcuma longa with multiple effects against malignancy. Curcumin exhibits anti-angiogenic properties, and increases the expression of the p53 and cip1/p21. It is able to induce the apoptosis of CRC.72
Terpenoids
Andrographolide is the major bioactive component of Andrographis paniculata. This plant has been used as a traditional herbal medicine in Asia for thousands of years. Andrographolide exhibits anti-cancer effects due to its DNA-binding activity and inhibition of NF-κB activation.68 The combination of andrographolide and 5-fluorouracil induces the apoptosis of HCT-116 cells as a result of andrographolide binding and upregulation of BAX protein expression. The safety and efficacy of the combination treatment of andrographolides and capecitabine was shown in elderly patients with locally advanced CRC.73
Unsaturated Fatty Acids
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are polyunsaturated fatty acids (PUFAs) with significant inhibitory effect against angiogenic factors like platelet growth factor, VEGF, and endothelial cell growth factor. Therefore, they are effective against CRC, and other cancers.74 DHA and EPA as free acids or in the form of phospholipids inhibits the growth of CRC cells, and the studies have shown the strong growth-inhibitory effects of PUFAs in the HT-29 cell line more than in the Caco-2 and DLD-1 cell lines.68,75
Oral Route of Drug Delivery
Oral drug delivery has been recognised as the most attractive approach of drug administration among various others and can be utilised for systemic or local drug delivery as well as enhance the drug solubility and dissolution of low solubility drugs.17 It is the most preferred way by patients, ease of administration, convenience for self-administration, noninvasive, and feasibility for the formulations.19,20 Furthermore, a high surface area (>300 m2) of gastrointestinal track coated with a viscous mucosal layer facilitates drug adhesion and absorption.18 Nevertheless, oral drug delivery can be challenging due to the complexity of GIT, even among healthy people, which poses some obstacles that impact delivery such as poor drug solubility, stability, and permeability.17 Drugs that are administered through the oral route have a slower absorption as they must be solubilised in the gastric fluid to be absorbed.20 The main factors affecting GIT absorption of the drug is drug physicochemical characteristics (polymorphism, solubility, partition coefficient, and pH-pKa), biological factors (blood flow at absorption site, mucus, absorption surface area, ionic concentration, enzymes, and microbiome) as well as maintenance of sync condition (drug concentration at the absorption site).76 Moreover, oral drugs may also have an unpleasant taste, induce gastric irritation, and/or be subjected to intestinal and hepatic barriers.19,20
The rectal route of administration is an alternative route of drug delivery that has some disadvantages in comparison to the oral route. The low surface area and rectal fluids can lead to irregular or incomplete absorption of the drug. Table 1 shows a comparison between characteristics of different parts of GIT.18 Different segments of GIT vary anatomically and physiologically and impact the extent of dissolution and absorption of the drugs. Hence, the basic characteristics of the GIT should be considered during the formulation design of oral drug delivery system.
 |
Table 1 Characteristics of Different Parts of the GIT |
Factors Influencing Gastrointestinal Tract
Regardless of absorption mode, each digested substance will encounter three major biological environments along with the gastrointestinal tract including the lumen (internal space), mucus, and tissue.
Lumen
pH
The pH is the first biological barrier to any orally delivered drug, which denature/depurination most of the administered drugs and substantially reduces their effectiveness. The pH of GIT varies based on GIT part and fed state to unfed state. The pH inside the gastric environment is extremely acidic (pH 1–2) in unfed state and it could rise to pH 5 in fed state. The pH increases rapidly in duodenum (pH 6) and rises along the small intestine to reach pH 7.4 at the terminal ileum.18 Cecum pH decreases just below pH 6 and again increases in the colon and reaches pH 6.7 at the rectum.77 Nevertheless, the pH ranges can vary with factors like dietary intake, microbial metabolism, and diurnal cycle.78 gastrointestinal pH changes affect the extent of drug molecules ionisation, subsequently impact drug absorption.79,80
Enzyme
Enzymatic degradation of dosage forms and drugs can occur throughout the GIT and hence can affect their stability. The major site of the enzymes that are involved in food digestion is mouth (salivary amylase), stomach (pepsin).81 Besides that, pancreatic enzymes secreted into the intestinal lumen including trypsin, amylase, lipase, and peptidases. Those pancreatic enzymes are not considered as the main challenge, although they are considered as a biological barrier. These enzymes are especially abundant in duodenum, and their concentrations are reduced significantly in the later intestinal parts. duodenum transit time is relatively short (10% of the whole small intestine residence time) and is not enough for the enzymatic degradation of the drugs. Duodenal pH is lower compared to the later small intestine parts, and thence the unwanted drug release inside the duodenum can be prevented via increasing delivery carriers pKa.
Microbiome
The intestinal microbiome has over 500 bacterial species,82 and they are important for digestion and metabolism of the ingested food as well as for intestinal health.83 The colon contains the bulk of the intestinal microbiome and they ferment carbohydrates as the main source of nutrition.83 The composition of the microbiome varies between individuals based on genetic and environmental factors.84 Nevertheless, the dominant species appear to be consistent and represent the majority of the colonic flora.82 These bacteria secrete many enzymes such as azoreductase, α-arabinosidase, β-galactosidase, β-glucuronidase, β-xylosidase, deaminase, nitroreductase, urea hydroxylase.85 These enzymes are exploited for CTDD.86
The density of the microbiome inside the small intestinal is lower compared to that of the large intestine. This is due to the intestinal fluid volume, rapid luminal flow, and bactericidal compounds secretion in this part of the GIT.87 Small intestine microbiome composition can highly fluctuate over a short period as it is affected by dietary intake variations.88 The colonic microbiome is claimed to play a crucial role in the regulation of metabolic function.87 Small intestinal microbiota influences oral drug absorption have not yet been elucidated.
Mechanical Degradation
Mechanical degradation inside the lumen including the peristalsis of the GIT muscles, the shear stresses by gastric juice flow rate within the lumen as well as the osmolarity along the GIT are factors that reduce the drug efficiency. Flowing gastric juice could reduce the contact time between the epithelial layer and drugs, hence impeding their absorption. Biologics and microencapsulation are the major elements sensitive to mechanical destruction through mechanical stresses.
Transit Time
Gastrointestinal transit time is the key factor for drugs that have region-specific targeting or absorption characteristics. Gastric transit time varies from several minutes to hours, depending on several factors, such as age, gender, body posture, osmotic pressure, dietary intake, physical activity, etc.89 The transit time in gastric can vary from 0 to 6 h based on fast/fed state,81 while small intestine transit time is considered constant at approximately 4 h.90 On the other hand, colon transit time is extremely variable from 6 to 70 h.91 Gender is highly affecting the colonic transit time and females have significantly longer colonic transit times compared to males.92
Mucus
Mucus is the second barrier for effective oral drug delivery. Mucus facilitates the digested moiety passage and protects the underlying epithelium from mechanical stress and pathogens by capturing any foreign moieties (especially hydrophobic molecules). Although mouth and oesophagus lack a distinct mucus layer, they are washed by salivary gland mucus.93 The small intestine has a loose and unattached mucosal layer, while the stomach and colon have a two-layered mucus system composed of an outer loosely adherent layer and an inner firmly adherent layer.93 It is claimed that mucus is the primary barrier against oral drug bioavailability due to the difficulty to diffuse into the mucus layer. While the inner mucus layer facilitates the absorption and improves the drug uptake efficiency, justifying the dual role of mucus in the absorption/desorption of oral administrated drugs. Goblet cells are continuously secreted mucus; consequently, it is cleared in each part of the GIT (50–270 min).20 This defines the final mucus layer thickness (Table 1).
Fluid Volume
Controlling fluidity is crucial for gastrointestinal function.81 The fluid environment allows digestive enzymes to contact with food, and this aids in transit the intestinal contents along the GIT without damaging the epithelial lining and it facilitates the nutrients and drugs solubility and absorption.81 The daily water balance in a healthy adult human GIT is determined by the secretion from saliva (1.5 L), gastric juice (2.5 L), pancreatic, and other intestinal components (~2.5 L), and absorption from the small and large intestine (7 L and 1.9 L, respectively).81 The pH is influenced by fluid-to-matter ratios, which can potentially alter drug absorption and delivery, especially in lower GIT. For instance, diet can have a big impact on the GIT free fluid volume, bile salts, and digestive enzyme levels.81 Moreover, intestinal fluid secretion affects the viscosity of the mucous-gel layer,81,93 which can alter the drug’s cellular uptake at the site of action. Increased fluid secretion and reduced fluid reabsorption dilute the digestive enzymes and affect the intestinal microbiota which in turn alter the digestion of carbohydrates94 and contribute to changes in intestinal transit time.95 Variations in intestinal fluid volume can impact the way drugs are processed in the GIT.
Tissue
The drug molecule properties determine where it will be absorbed and how it will cross the intestinal epithelial cells. Drugs may be absorbed in four ways: transcellular (transcytosis of the drug by crossing epithelial cells membrane), paracellular (permits only small hydrophilic molecules diffusion through epithelial cell spaces), facilitated transport, and carrier-mediated transcellular.96 Tight junctions, that affect the paracellular absorption pathway for orally delivered drugs, are the primary extracellular biological barrier against oral administration.97
The size and chemistry of the drug affect their transport through tissue. Generally, hydrophilic small drugs prefer paracellular routes, while hydrophobic drug molecules prefer to be absorbed via transcellular routes.98,99 Passive drug diffusion via intercellular pathways is only conceivable for substances as small as 0.5–3 nm, which is too small for most drug molecules to be delivered.100,101
Approaches for Oral Colon Delivery in Colorectal Cancer
Numerous studies demonstrated that the colon is a suitable site for drugs absorption as it has less reactive enzymes against drugs, CTDD protects the drug from hydrolysis by enzymes and decreases their degradation in the duodenum and jejunum, subsequently greater drug systemic bioavailability23 and prolonged colonic residence time of the drug is accompanied by the synergistic effect of absorption enhancers on the drug absorption.102 The goal of developing oral CTDD systems is to avoid or limit the release of drugs in the upper GIT while allowing explicit drug release in the colorectal area for local and/or systemic delivery. To accomplish this, the physiological parameters that affect colonic drug delivery must be taken into consideration. CTDD can be characterised as either prodrug, pH-dependent systems, time-dependent systems, or ligand/receptor-mediated systems. Each of these formulation methods has its own set of benefits and drawbacks. A combination of two or more formulation systems has been examined to overcome the limits of a single system and to maximise CTDD.
Nanotechnology has significantly advanced current drug formulations for improved absorption and site-specificity. The use of nanosystems facilitates the delivery of active compounds to cancer tissues in the colon. The usage of nanoparticles in drug formulations has a lower dose requirement compared to the amount needed for conventional dosing. Nanoparticles can be tailored with various charges to treat colonic diseases. Some examples of known polycation nanoparticles can interact with inflamed tissues of the intestine, while polyanion nanoparticles interact with cationic components (cytokines) in colorectal cancer. Nanoparticles are also prone to be uptaken by macrophages at the inflamed areas of the colon, thus allowing the drug to remain in the colon for a longer time.
Moreover, nanometre-scale sizes of nanoparticles contribute to their efficacy as drug delivery systems. Nanosized particles possess increased epithelial permeability and retention, that promotes selective delivery into inflamed tissues,103 prevents removal through diarrhoea,104 and enhances uptake by immune cells accumulated in the inflamed colon portions. Bioadhesion is the attachment of anionic nanoparticles to inflamed tissues by electrostatic interactions with positively charged proteins like eosinophil and transferrin.105 Cationic nanocarriers are used in the nanoparticles designed as a mucoadhesive system, which is attached to the anionic intestinal mucosa.106 This cationic delivery system can be used in GIT targeting as it enhances the drug cellular uptake into the mucosal surface. pH-dependent nanocarriers have been also used for CTDD. The ideal polymer should withstand gastric acidic pH and proximal small intestine while being dissolved in pH of the terminal ileum and colon. Thus, pH-dependent drug delivery systems with dissolution threshold of pH 6.0–7.0 are expected to delay drug dissolution and prevent unwanted drug release in the upper GIT before reaching the colon.107 This makes the delayed-release profile of the drug formulation.
Nanoparticles with different polymers that are capable of entrapping drugs into their matrix until further release in the colon, have been designed. Polymeric nanoparticles selectively release drugs through the enzymatic activity of colonic bacteria or by manipulating colon transit time for time-dependent release nanoparticles.
Oral drug administration in the form of a prodrug can enhance its bioavailability and prevent early degradation in the upper GIT. Colon-targeted prodrug is the inactive derivative of a drug molecule that becomes an active ingredient upon enzymatic hydrolysis in the colon. One strategy is disulfide bond conjugates formation between the prodrug and the carrier. The disulfide bond is stable in the upper GIT and can be disintegrated via extracellular bacterial reduction process or intracellular enzymes. This results in the carrier breakage and prodrug release into the colon.
For a more successful local therapy of colorectal cancer with less toxic side effects, ligand/receptor-mediated drug delivery systems can improve target specificity through the interaction of specific receptors expressed at disease locations and the targeted ligands on the carrier surface have been investigated. It can be also combined with other delivery strategies to improve its GIT stability and site-specificity. Folic acid is a receptor-selective targeting ligand that targets folic acid receptors overexpressed in many types of tumours including colorectal cancer.108 Folate receptor functionalised nanoparticles can enhance selective drug uptake into cancer cells. Folic acid functionalised liposomes enhanced the cytotoxicity of daunorubicin via facilitating folate receptor-mediated drug uptake.109 5-fluorouracil loaded Folic acid conjugated liposomes enhance the selective drug delivery to cancer cells as it showed higher anti-cancer activity and significantly decrease the volume of the tumour when compared to free 5-fluorouracil.110 Hyaluronic acid is a polysaccharide that has a high affinity for CD44 receptors, which is overexpressed in many tumours including colorectal cancer.111 Previous studies have examined the effectiveness of hyaluronic acid nanoparticles as CTDD for colorectal cancer.112,113 Hyaluronic acid-functionalised camptothecin/curcumin-loaded polymeric nanoparticles (HA-CPT/CUR-NPs) showed significant targeting capability towards Colon-26 cancer cells.113 Peptide is gaining popularity as a possible ligand for targeted drug delivery. Peptides have several pros, such as simplicity, low cost, chemical variety, biocompatibility, and stimulus responsiveness.114,115 Moreover, peptide ligands show higher binding affinity and specificity with receptors compared to small molecule ligands.108,116 In addition, the metabolic instability caused by proteases can be addressed by peptide sequence alteration, encouraging the use of peptide ligands in targeted drug delivery systems. Peptide functionalised nanoparticles are mainly being investigated as a viable strategy for tumour-targeted drug delivery. For instance, TWYKIAFQRNRK, TK peptide, has been investigated for CTDD of anticancer drugs. TK peptide has a high affinity to α6β1 integrin, that is upregulated in colorectal cancer. Hence, TK peptide-functionalised PEG-PLA micelles loaded with doxorubicin showed significantly stronger toxicity and more effectively penetrated the tumour spheroids, indicating TK peptide as a potential targeting ligand for CTDD.117
Polysaccharide as CTDD
Natural polysaccharides like pectin, chitosan, xylan, and guar gum have been used in CTDD systems, as they can remain intact in the upper GIT, but are degraded by microflora to release the drug.118 Moreover, polysaccharide structural modifications or derivatives can enhance drug release behaviour, site-specificity, and stability.119 Polysaccharide mucoadhesiveness may be beneficial for drug absorption through extended contact between drug delivery carriers and the mucosal surface. Polysaccharide-based drug delivery systems provide additional advantages such as low toxicity, low cost, immunogenicity, biodegradability, and biocompatibility.120 As a result, the microbiota-triggered polysaccharide-based system offers a viable approach for CTDD. Nevertheless, polysaccharide-based delivery systems have certain potential limitations, such as variable polysaccharide chemistry and a wide range of molecular weights.120 Moreover, the rate of drug release is affected by the type and concentration of polysaccharides.
Chitosan and Its Physicochemical Properties
Chitosan is a linear polysaccharide, comprises of randomly repeated units of N-acetyl-D-glucosamine and D-glucosamine linked by β(1→4) glycosidic bonds.121 Chitosan is the most valuable derivative of chitin, the second most abundant naturally occurring polymer following cellulose.122 Chitin can be found in the exoskeleton of insects and crustaceans, in addition to the cell walls of certain fungi.123 Chitosan, the partially deacetylated form of chitin, can be obtained by chitin deacetylase enzyme or chemical alkaline treatment using concentrating NaOH as shown in (Figure 2).124,125 Chitin polymer can be called chitosan when the degree of deacetylation (DD) exceeds 50%.126 Both chitin and chitosan have attracted tremendous attention in various fields, such as food technology,31 textile industry,127 and diverse medical and pharmaceutical applications including wound healing,32 tissue engineering33 and gene delivery.34
 |
Figure 3 Schematic illustration of the interaction between chitosan loaded nanoparticles with the mucus layer. |
Unlike chitin, chitosan is readily soluble at pH below its pKa (pKa≈6.5) ascribing to the protonation of its amino group (-NH2) at C-2 in each repeating D-glucosamine unit turning the chitosan into a cationic polymer.30 Besides this cationic nature that is responsible for chitosan permeation enhancing and mucoadhesion effects, being also biodegradable, biocompatible and non-toxic enable chitosan to be a polymer of choice for various drug delivery applications.29
Chitosan has become of great interest to be formulated into CSNPs. CSNPs are considered a very promising strategy to overcome the low stability and bioavailability of many active ingredients by combining the favourable natural characteristics of both the polymer itself and nanotechnology. Chitosan can efficiently bind to the negatively charged mucus membrane, increase the retention time and enhance the probability of their cellular uptake. Moreover, chitosan is known for transiently opening the tight junction between epithelial cells, which allows the permeation of the hydrophilic loaded drug.128
Interaction Between Chitosan and Colon Mucosa
Mucus sticks to most particles, it is as a tenacious barrier prevents their penetration to the epithelial surface. Mucus layer is continuously secreted and degraded or digested, thus rightly called dynamic.129 Mucins are consisted of highly glycosylated proteins (>106 Da) that are the main components of mucus covering epithelia. They are involved with several functions such as forming a viscous matrix that controls the adhesion of the mucosal layer to the cells as well as drug targeting and pathogens penetration.129,130 Thus, the development of novel systems for transmucosal drug delivery requires the conceptualization of targeting strategy. Chitosan interacts with mucins as well as it absorbs and changes the rheology of mucus. The interaction between mucins and chitosan depends mainly on electrostatic attraction, hydrophobic interactions and hydrogen bonding that determines the fate and behaviour of chitosan-based drug delivery systems. Thus, CSNPs could go through the mucus and reach the cell surface or be shed with the mucus. Several intrinsic factors have effects on the interaction between chitosan nanocarriers and mucins specially that related to the nature of the polymer such as the molecular weight and surface charge of CSNPs.131 The value of pH of the media is one of the environment factors, the pK0 of chitosan is around 6.5, thus the amine group will be protonated at pH lower than the pK0.132 On the other hand, mucins are glycoproteins with pI between 2 and 3, therefore they will be charged negatively in medium pH > pI, promoting electrostatic interaction between mucins and chitosan especially at pH between 2.4 and 6.3.131 Moreover, the negative charge of the sialic acid, sulfate groups on the oligosaccharide chains of mucins and surrounding anions aid gel formation as well as they enhance the association in the mucus layer and subsequent internalization via endocytosis of CSNPs.133 The positive surface charge of CSNPs may improve also the drug penetration by opening tight junctions of epithelial cell layers as shown in (Figure 3).134 In fact, the interaction between mucins and chitosan is still difficult to understand and predict. It’s worth noting that some studies have found that interacting chitosan with mucins in vivo changes the biopolymer attachment to mucosal surfaces.135,136
 |
Figure 4 Synthesis of N,N,N-trimethyl chitosan. |
Nanotechnology in Drug Targeted Delivery System
Any drug delivery system can carry therapeutic agents, only if it was able to reach the site of action from the site of administration crossing all the biological barriers. Besides, most of the active pharmaceutical ingredients (API) are often likely to exhibit limited bioavailability, poor water solubility and inadvertent serious side effects.137 Therefore, it is a major challenge to deliver therapeutic agents to specific organ or tissue for the treatment of certain disease. In order to overcome those drawbacks, nanotechnology has been widely embraced to develop effective targeting drug loading systems.138 Table 2 summarizes general advantages and potential limitation of nanotechnology in drug delivery.139
 |
Table 2 Advantages and Potential Limitations of Nanotechnology in Drug Delivery |
Equipping nanotechnology with existing cancer therapies to enhance drug efficiency and limit its systematic toxicity, known as cancer nanotechnology.140 Nanocarriers for cancer treatment are aimed to deliver the loaded-molecules to and around the targeted pathologic tissue, killing cancer cells without harming the normal ones.141 Compared to microparticles, nanoparticles have larger surface area with tremendous modifiable options enabling the delivery of the anti-tumour drug to the targeted location with less probability of opsonisation. Additionally, nanoparticles facilitate the drug diffusion and accumulation in tumour cell, through enhanced permeability and retention (EPR) effect.142 Various types of materials have been used to fabricate nanoparticles for colorectal cancer treatment including liposomal,143 polymeric, inorganic,144 mesoporous silica nanoparticles.145 Among the forenamed nanoparticles, polymeric nanoparticles have numerous advantages due to their ease of synthesis and modification, ability of loading and protecting the loaded-molecules from degradation by surrounding environment, high in vivo and in vitro stability profiles and their potential use for targeted controlled releases.146 Chitosan based nanoparticles stand out among polymeric nanoparticles by being efficient, readily available, environment friendly, non-immunogenic, biocompatible and biodegradable nanoparticles.147 It has been reported that chitosan demonstrates an anticancer activity through antioxidant defence mechanism, cellular enzymatic, immunoenhancing, cellular enzymatic, antiangiogenic and apoptotic pathways.40 Furthermore, CSNPs permeate through cancer cell membranes selectively, enhance the drug potency and bioavailability in the cancer cells while being non-toxic to normal cells.40 Nanoparticles can target the cancer cells by two distinguished approaches, namely, passive and active targeting approaches (Table 3). CSNPs can combine both approaches, the passive targeting through EPR effect, and the active targeting through ligand conjugations.148
 |
Table 3 Main Differences Between Passive and Active Targeting |
Passive Targeting
Passive targeting is mainly associated with EPR effect, where the substrates get accumulated at the target site due to the incomplete lymphatic system and newly formed leaky vasculature surrounding tumour stroma.149 Passive targeting approach relies on the physicochemical characteristics of the formulated nanocarrier including particle size (PS), surface charge, morphology, circulating time, and surface chemistry as well as the tumour biology characteristic such as leakiness and vascularity.148 Without any specific ligand targeting, nanoparticles can extravasate from leaky tumour vessels, reaching the cancer cells and can increase the concentration of the drugs acting on these cells for days or even weeks as a result of the sluggish lymphatic drainage.150 The PS has a notable influence on the drug extravasation, since the vascular cut-off pore sizes in cancer and healthy cells are different. While only particles smaller than 2 nm are permeable through normal cells, the pores size of most tumours are from 10 to 1000 nm.151 For efficient permeation of nanoparticles through the fenestrations of the endothelial tissue, the PS should be less than 400 nm.152 The physicochemical properties of the polymeric nanoparticles can be easily modified by altering the formulation composition or by the synthetic method. In case of CSNPs, they accumulate in the low-pH tumour microenvironment, which leads to protonating of chitosan, swelling of the nanoparticle and releasing of the drug in the targeted cancer cells.40
Active Targeting
In order to improve nanoparticles selective accumulation in the tumour tissue, active targeting was developed as a complementary strategy to the passive EPR-based targeting approach.149 In active targeting, high affinity targeting molecules with innate ability can be conjugated on the surface of nanodelivery systems in order to attach to specific receptor expressed at the target site.153 Several types of ligand have been utilized including protein, monoclonal antibodies, folic acid, aptamers, small molecules, nucleic acid and polysaccharides.154 Tumour targeting ligands on the nanocarriers can specifically bind to tumour cells that express unique biomarkers distinguishing them from the surrounding normal healthy cells.155 The binding between targeting molecules and receptors takes place via endosome-depending mechanism, in which cancer cells can be directly killed.156 Table 4 demonstrates both active targeting (targeting molecule and its targeted receptor), the effect of passive targeting (EPR effect) which can be seen from the physicochemical characteristics of the prepared nanoparticles, and their impact on the colon cancer cell lines.
 |  |  |
Table 4 Chitosan-Based Nanoparticles Studies of Colon Targeted Drug Delivery System |
The Role of Chitosan and Its Derivatives for Colorectal Cancer Targeted Drug Delivery
Chitosan polymer has received immense attention in developing nanocarriers as drug delivery systems for anticancer agents.157 In terms of colorectal cancer targeting, chitosan can form complexes with anionic drugs, reduce premature release of entrapped drugs at the upper GIT and release the encapsulant at colon part of body due to its biodegradability via glycosidic linkage lysis by means of the specified enzyme of the colonic microflora.35 Drug release from chitosan matrix is related to matrix swelling behaviour and chitosan-drug interaction that are affected by the chitosan molecular weight and the pH of the medium. Also, drug release is still affected by its solubility and its molecular weight, as well as the release profile is influenced by polymer-drug ratio. Drug release from CSNPs can occur via different mechanisms: diffusion through the swollen matrix, release from the surface of nanoparticles and release due to polymer erosion. However, in most of the conditions, the release occurs as a result of the combination of than one mechanism.14,158 Nevertheless, pure chitosan’s utility and applications are limited due to its insolubility in water and also most organic solvents.159 Different functional groups such as -NH2 and -OH are reactive groups on the chitosan molecule that are involved in chemical modification. Modified chitosan derivatives have displayed superior inherent properties such as bioactivity, biocompatibility, bioactivity and non-toxicity as well as several bioactive effects like antibacterial, antiviral, anticancer effect.39,40 The functional groups C2–NH2 and C6–OH readily involve in chemical modifications in order to introduce new chemical moieties. These chitosan modifications also facilitate in drug delivery to the colon, where therapeutic agents need to be protected from acidic pH of the stomach and enzymes degradation with minimal drug lose until they reach the colon.41,42 Chitosan has three different types of reactive functional groups: a primary hydroxyl group at the C-6, an amino group at the C-6 and a secondary hydroxyl group at the C-3. The most common method for chemical modifications of chitosan is N-substitution, in which the amino group (–NH2) of chitosan serves as the functional group that reacts.30
Chitosan Derivatives with Functional Modifications
Trimethyl Chitosan
Trimethyl Chitosan (TMC) is among of the highly desirable quaternized chitosan derivatives that first synthesized by Muzzarelli and Tanfani.160 Typically, TMC is synthesized through reaction of chitosan with methyl iodide in strong alkaline environment at elevated temperature. The iodide ion is then substituted by chloride by means of an ion exchange process as shown in (Figure 4).161,162 N-Methylated chitosan with the hydrophobic groups N(CH3)2 and the hydrophilic groups N+(CH3)3 is amphiphilic, thus it can be self-assembled to vesicles and also is considered as a suitable derivative for nanoparticles preparation.161 TMC has been proven to be useful in enhancing the paracellular transport of targeted drug delivery systems through increasing the retention time of encapsulant on the mucosal surface as well as binding the protonated amino groups on the C-2 position with negatively-charged mucus and by opening the tight junctions of epithelial cells.163,164 In addition, the degree of quarternization increases the permeation-enhancing effect. Several studies have applied TMC due to its solubility in a wide range of pH (1–9), thus the advantageous properties of the polymer are maintained regardless of the varying pH values along GIT.165
 |
Figure 5 Synthesis of N,O-carboxymethyl chitosan. |
Carboxymethyl Chitosan
Besides TMC, chitosan solubility can be also enhanced through another common modification method; carboxymethylation. Carboxymethyl chitosan is developed by introducing a carboxymethyl group in the structure of chitosan as a hydrophilic modification. It is synthesised by carboxymethylation of the amine and hydroxyl moieties of chitosan. This modification increases chitosan solubility in neutral and basic solutions without negatively affecting other important characteristics.166 Carboxymethylation reaction takes place specially either at C-6 hydroxyl groups or at the NH2 moiety that produces water-soluble N,O–carboxymethyl chitosan compounds and comprises either a primary amino group (–NH2) or as secondary amine (–NH–CH2COOH) as shown in (Figure 5).167,168 Carboxymethyl chitosan have been synthesised with different degrees of carboxymethyl substitution and molecular weights to prepare nanoparticles by means of ionotropic gelation with calcium ions for targeted anticancer drug delivery.43,169,170
 |
Figure 6 Chemical structure of N-succinyl-chitosan. |
N-Succinyl-Chitosan
N-succinyl-chitosan, which is an acyl chitosan derivative obtained by introduction of succinyl groups into chitosan N-terminal of the glucosamine units. Polyion complexes can be generated between the NH3+ and -COOH- groups in the succinyl chitosan as shown in (Figure 6).171,172 The degree of succinylation can be easily modulated by adjusting reaction conditions through succinic anhydride.173,174 N-succinyl-chitosan exhibits a good ability to react with several kinds of agents due to the reactive functional –NH2 and –COOH groups.175–177 Furthermore, this derivative has shown a good water solubility in a wide pH-range with biocompatibility in vitro and in vivo, low toxicity, long-term retention in the systemic circulation and high accumulation in tumour tissue.173,178
 |
Figure 7 Possible chemical structure of hyaluronic acid (HA)-conjugated chitosan (CS). Notes: Adapted from: Sionkowska A, Gadomska M, Musiał K, Piatek J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Mol. 2020;25: 4035. doi.org/10.3390/molecules25184035.185 © 2020 by the authors. Licensee MDPI, Basel, Switzerland. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). |
Hyaluronic Acid-Conjugated Chitosan
Hyaluronic acid, a linear glycosaminoglycan consists of alternating β-1,4 linked d-glucuronic acid (GlcUA) and β-1,3 linked N-acetyl-d-glucosamine (GlcN Ac) units. It has been experimentally shown its ability to be as a tumor-targeting moiety through virtue of its high affinity for the cell surface receptors CD44 and RHAMM which, in a cancer stage-specific manner, overexpressed in colorectal carcinoma.179–181 These lead to enhance the antitumor efficiency of antitumor agents and to increase drug accumulation in tumor cells by facilitating targeted drug delivery via CD44 and RHAMM.182,183 The deposition of hyaluronic acid preferentially on the chitosan-based nanoparticles’ surface can improve cells targeting with overexpressed receptors, enhance in vivo stability and prolong circulation times of therapeutic agents.182,184 Figure 7 represents the possible chemical structure of hyaluronic acid-conjugated chitosan.185
 |
Figure 8 Chemical structure of PEG-conjugated chitosan. |
PEG-Conjugated Chitosan
Polyethylene glycol (PEG) has gained wide recognition as an ideal graft-forming polymer because of its solubility in aqueous and organic solvents as well as having weak immunogenicity, low toxicity, good biodegradability, and biocompatibility. PEG is among of the few synthetic polymers that has been approved for using in biomedical field.186 PEGylation of chitosan via hydroxyl groups was first synthesised by Gorochovceva and Makuska as shown in (Figure 8).168,187 PEGylation is a suitable method for introducing new physicochemical properties to chitosan such as the solubility at wide range of pH values.188 It is also a critical way in developing CSNPs as biomedical carriers and can improve the colloid stability of the particle system.189 Several synthetic strategies were developed for chemical conjugation of chitosan with PEG in previous studies. Among of the desirable approaches, the substitution of amino group of chitosan through n-succinimidyl ester (NHS) derivative (known as NHS-amino coupling) of PEG is considered as a simple method and does not require using complex reaction pathways or equipments.188 PEG chains prevent the adhesion of opsonins existing in the blood by generating a barrier layer surrounding the particles that can be “invisible” to phagocytic cells. Due to these benefits, chitosan-g-PEG was applied as a potential copolymer for colon cancer drug delivery.190
 |
Figure 9 Chemical structure of folic acid-conjugated chitosan. |
Folic Acid-Conjugated Chitosan
Folate receptors are considered as one of the most expressed among receptors on many cancer cells types, besides, their expression levels are limited in normal cells. The cell lines HT-29 and HCT-116 have over levels of folate receptors that can be led to easier interaction with folate targeted nanoparticles; more release and more uptake of folic acid conjugates at colorectal cancer cells.145,191 Folic acid is a stable, inexpensive and has a high affinity for the folate receptors.192 Folic acid can conjugate covalently to chitosan molecules via folate’s γ-carboxyl moiety.152 In particular, the conjugates enter the cells through endocytosis via folate receptor and are then transported to cell cytoplasm via by vesicular trafficking. Later, the unligated folate receptor return to the cell surface once more to transport more folic acid conjugates.193 Folic acid-conjugated CSNPs were used in several studies to improve drug uptake and drug accumulation into colorectal cancer cells. Figure 9194 represents the chemical structure of folic acid-conjugated chitosan. Table 5 represents the most recent examples of chitosan and chitosan derivatives as nanoparticles of anticancer drugs for colorectal cancer treatment.
 |  |  |
Table 5 Summary of Recent Studies of Chitosan and Chitosan Derivatives as Anticolorectal Cancer Drug Nanocarrier |
Chitosan Nanoparticles for siRNA Delivery
siRNA, sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA molecules, usually 20–25 nucleotides in length with 3’ overhanging nucleotides on either end that play a variety of roles in biology.195 SiRNA was used for several purposes including suppression proliferation of colorectal cancer, induction of cell apoptosis as well as prevention of colorectal cancer metastasis due to its ability to bind to the corresponding mRNA and further induce the mRNAs cleavage.196 However, the targeted delivery of siRNA has faced several challenges, including low intracellular uptake and rapid degradation. The positively charged of chitosan and its mucopermeable and mucoadhesive properties have led to the extensive use it for encapsulate the negatively charged siRNA by the electrostatic interaction, forming positively charged stable polyplexes.184 In addition, in order to enhance the efficiency of suppressors of cellular resistance several studies combined therapeutic agents and siRNA within one nanocarrier-based delivery system.197 Table 6 summarises the recent studies of chitosan and chitosan derivatives nanoparticles based-siRNA delivery.
 |
Table 6 Summary of Recent Studies of Chitosan and Chitosan Derivatives Nanoparticles for siRNA Delivery in Colorectal Cancer Therapy |
Future Perspective
Due to its physicochemical properties, chitosan and its derivatives nanoparticles confirmed their efficacy for enhancing drug stability and targeting treatments of colorectal cancer that are administrated orally. We anticipate more research in the next years aim to improve the chitosan characteristics by physical and chemical modifications in order to increase the specific drug accumulation within the colorectal cancer cells. From the aspect of the adverse effects of anticancer treatment, the toxicity and immunogenicity of the chitosan nanocarriers towards more normal cell lines need to be studied particularly to investigate their long-term effects.
Conclusion
Over the last few decades, various efforts were devoted in nanomedicine science to develop novel strategies of smart targeted drug delivery systems. In this approach, targeting cancerous tissues of colorectal with minimum side effects and cellular toxicity is the ultimate goal. The delivery systems based on chitosan nanocarriers have shown high ability to carry therapeutic agents to specific region of the body according to the polymer characteristics. The above-discussed applications of chitosan and its derivatives-based oral nanocarrier systems for colorectal cancer demonstrated their biocompatibility and versatility. CSNPs control drug delivery by means of their pH-sensitivity, surface flexibility, mucoadhesive and penetration properties. Chitosan modification further enhances the efficiency of anticancer drugs by increasing their retention time, accumulation, cellular uptake and cytotoxicity to tumor cells. Studies in colon tumor HT-29, Caco-2, HCT-116, SW480, CT26 and MCF-7 cell lines have shown high anticancer activity as well as significant decrease in tumor volume. In summary, oral chitosan-based nanocarriers conjugated/or loaded with therapeutic agents or targeting ligands represent a great promise in developing colon targeted drug delivery systems with low toxicity level and less monitoring of treatment.
Further understanding of the factors influencing nanoparticle characterization and uptake will help researchers to identify the best approach for targeting oral drug to the colon cancer. While many studies have been done in recent years to develop a successful system for oral drug and gene delivery, the approaches have yet to progress beyond animal studies and establish relevant efficacy in humans. However, the major advantages of chitosan, including mucoadhesion effects, non-toxic, ease of modification, ease of protein or DNA complex formation, biodegradability, and low cost explain the continuing improving of these promising carriers for efficient drug delivery to colon cancer. Therefore, it is envisaged that the use of chitosan in cancer-targeted drug delivery systems will improve the prospects of biomedical and biotechnology applications in the future.
Acknowledgment
The authors wish to thank Universiti Sultan Zainal Abidin for the support.
Funding
This research was funded by Universiti Sultan Zainal Abidin through UniSZA/2021/DPU2.0/05 grant (grant number R0332).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Cancer Today. World Health Organization; 2021.
2. Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi:10.1136/gutjnl-2015-310912
3. Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest Res. 2019;17:317–329. doi:10.5217/ir.2019.00021
4. Sihvola S, Kuosmanen L, Kvist T. Resilience and related factors in colorectal cancer patients: a systematic review. Eur J Oncol Nurs. 2022;56:102079. doi:10.1016/j.ejon.2021.102079
5. Yang C, Merlin D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomaterials. 2020;10:1424. doi:10.3390/nano10071424
6. Tian Q, Liu Y, Zhang Y, et al. THBS2 is a biomarker for AJCC stages and a strong prognostic indicator in colorectal cancer. J Buon. 2018;23:1331–1336.
7. Bennedsgaard K, Ventzel L, Themistocleous AC, et al. Long‐term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy. Cancer Med. 2020;9:5114–5123. doi:10.1002/cam4.3129
8. Duran G, Cruz R, Simoes AR, et al. Efficacy and toxicity of adjuvant chemotherapy on colorectal cancer patients: how much influence from the genetics? J Chemother. 2020;32:310–322. doi:10.1080/1120009X.2020.1764281
9. Yang X, Xie Y. Recent advances in polymeric core–shell nanocarriers for targeted delivery of chemotherapeutic drugs. Int J Pharm. 2021;608:121094. doi:10.1016/j.ijpharm.2021.121094
10. Arumov A, Trabolsi A, Schatz JH. Potency meets precision in nano-optimized chemotherapeutics. Trends Biotechnol. 2021;39:974–977. doi:10.1016/j.tibtech.2021.03.004
11. Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi:10.1038/s41573-020-0090-8
12. Kundu M, Chatterjee S, Ghosh N, et al. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater Sci Eng C. 2020;116:111239. doi:10.1016/j.msec.2020.111239
13. Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–2663. doi:10.1021/acs.chemrev.5b00346
14. Kuen CY, Masarudin MJ. Chitosan nanoparticle-based system: a new insight into the promising controlled release system for lung cancer treatment. Molecules. 2022;27:473. doi:10.3390/molecules27020473
15. Jurczyk M, Jelonek K, Musiał-Kulik M, et al. Single- versus dual-targeted nanoparticles with folic acid and biotin for anticancer drug delivery. Pharmaceutics. 2021;13:326. doi:10.3390/pharmaceutics13030326
16. Mohanty A, Uthaman S, Park IK. Utilization of polymer-lipid hybrid nanoparticles for targeted anti-cancer therapy. Molecules. 2020;25:4377. doi:10.3390/molecules25194377
17. Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620–643. doi:10.1177/00970002042006005
18. Helander HF, Fändriks L. Surface area of the digestive tract-revisited. Scand J Gastroenterol. 2014;49:681–689. doi:10.3109/00365521.2014.898326
19. Shreya AB, Raut SY, Managuli RS, Udupa N, Mutalik S, Marosi G. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: recent advances. AAPS PharmSciTech. 2018;20:1–12. doi:10.1208/s12249-018-1201-2
20. Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11:129. doi:10.3390/pharmaceutics11030129
21. El Moukhtari SH, Rodríguez-Nogales C, Blanco-Prieto MJ. Oral lipid nanomedicines: current status and future perspectives in cancer treatment. Adv Drug Deliv Rev. 2021;173:238–251. doi:10.1016/j.addr.2021.03.004
22. Ying K, Bai B, Gao X, et al. Orally administrable therapeutic nanoparticles for the treatment of colorectal cancer. Front Bioeng Biotechnol. 2021;9:480. doi:10.3389/fbioe.2021.670124
23. Prasanth VV, Jayaprakash R, Mathew ST. Colon specific drug delivery systems: a review on various pharmaceutical approaches. J Appl Pharm Sci. 2012;2:163–169.
24. Coupe AJ, Davis SS, Wilding IR. Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm Res. 1991;8:360–364. doi:10.1023/A:1015849700421
25. Amidon S, Brown JE, Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16:731–741. doi:10.1208/s12249-015-0350-9
26. Dmour I, Taha MO. Natural and semisynthetic polymers in pharmaceutical nanotechnology. In: Organic Materials as Smart Nanocarriers for Drug Delivery. William Andrew Publishing; 2018:35–100. doi:10.1016/B978-0-12-813663-8.00002-6
27. Naskar S, Das SK, Sharma S, Kuotsu K. A review on designing poly (lactic-co-glycolic acid) nanoparticles as drug delivery systems. Pharm Nanotechnol. 2020;9:36–50. doi:10.2174/2211738508666201214103010
28. Choukaife H, Doolaanea AA, Alfatama M. Alginate nanoformulation: influence of process and selected variables. Pharmaceuticals. 2020;13:1–35. doi:10.3390/ph13110335
29. Ravishankar K, Dhamodharan R. Advances in chitosan-based hydrogels: evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React Funct Polym. 2020;149:104517. doi:10.1016/j.reactfunctpolym.2020.104517
30. Seyam S, Nordin NA, Alfatama M. Recent progress of chitosan and chitosan derivatives-based nanoparticles: pharmaceutical perspectives of oral insulin delivery. Pharmaceuticals. 2020;13:307. doi:10.3390/ph13100307
31. Harkin C, Mehlmer N, Woortman DV, Brück TB, Brück WM. Nutritional and additive uses of chitin and chitosan in the food industry. In: Sustainable Agriculture Reviews. Vol. 36. Cham: Springer; 2019:1–43. doi:10.1007/978-3-030-16581-9_1
32. Feng P, Luo Y, Ke C, et al. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. 2021;9:111. doi:10.3389/fbioe.2021.650598
33. Tang G, Tan Z, Zeng W, et al. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front Bioeng Biotechnol. 2020;8:1084. doi:10.3389/fbioe.2020.587658
34. Chuan D, Jin T, Fan R, Zhou L, Guo G. Chitosan for gene delivery: methods for improvement and applications. Adv Colloid Interface Sci. 2019;268:25–38. doi:10.1016/j.cis.2019.03.007
35. Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv. 2012;9:713–729. doi:10.1517/17425247.2012.682148
36. Patil P, Killedar S. Formulation and characterization of gallic acid and quercetin chitosan nanoparticles for sustained release in treating colorectal cancer. J Drug Deliv Sci Technol. 2021;63:102523. doi:10.1016/j.jddst.2021.102523
37. Kankala RK, Liu CG, Yang DY, Wang S, Chen AZ. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem Eng J. 2020;383:123138. doi:10.1016/j.cej.2019.123138
38. Badran MM, Mady MM, Ghannam MM, Shakeel F. Preparation and characterization of polymeric nanoparticles surface modified with chitosan for target treatment of colorectal cancer. Int J Biol Macromol. 2017;95:643–649. doi:10.1016/j.ijbiomac.2016.11.098
39. Ding S, Wang Y, Li J, Chen S. Progress and prospects in chitosan derivatives: modification strategies and medical applications. J Mater Sci Technol. 2021;89:209–224. doi:10.1016/j.jmst.2020.12.008
40. Adhikari HS, Yadav PN. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int J Biomater. 2018;2018. doi:10.1155/2018/2952085
41. Anitha A, Deepa N, Chennazhi KP, Lakshmanan VK, Jayakumar R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim Biophys Acta. 2014;1840:2730–2743. doi:10.1016/j.bbagen.2014.06.004
42. Alkhader E, Roberts CJ, Rosli R, et al. Pharmacokinetic and anti-colon cancer properties of curcumin-containing chitosan-pectinate composite nanoparticles. J Biomater Sci Polym. 2018;29:2281–2298. doi:10.1080/09205063.2018.1541500
43. Wang Y, Ma J, Qiu T, et al. In vitro and in vivo combinatorial anticancer effects of oxaliplatin- and resveratrol-loaded N,O-carboxymethyl chitosan nanoparticles against colorectal cancer. Eur J Pharm Sci. 2021;163:105864. doi:10.1016/j.ejps.2021.105864
44. Samprasit W, Opanasopit P, Chamsai B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible colon-targeted delivery. Pharm Dev Technol. 2021;26:362–372. doi:10.1080/10837450.2021.1873370
45. Haider M, Zaki KZ, El Hamshary MR, et al. Polymeric nanocarriers: a promising tool for early diagnosis and efficient treatment of colorectal cancer. J Adv Res. 2021;39:237–255. doi:10.1016/j.jare.2021.11.008
46. Shwter AN, Abdullah NA, Alshawsh MA, et al. Chemopreventive effect of Phaleria macrocarpa on colorectal cancer aberrant crypt foci in vivo. J Ethnopharmacol. 2016;193:195–206. doi:10.1016/j.jep.2016.08.002
47. Zhao R, Huang H, Choi BY, et al. Cell growth inhibition by 3-deoxysappanchalcone is mediated by directly targeting the TOPK signaling pathway in colon cancer. Phytomedicine. 2019;61:152813. doi:10.1016/j.phymed.2018.12.036
48. Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg. 2002;236:416. doi:10.1097/00000658-200210000-00003
49. Hodges N, Mackenzie H, D’Souza N, Brown G, Miskovic D. Survival outcomes for right-versus left-sided colon cancer and rectal cancer in England: a propensity-score matched population-based cohort study. Eur J Surg Oncol. 2021;48:841–849. doi:10.1016/j.ejso.2021.10.007
50. Norcic G. Liquid biopsy in colorectal cancer-current status and potential clinical applications. Micromachines. 2018;9:300. doi:10.3390/mi9060300
51. Butler TM. Updated Screening Strategies for Colorectal Cancer. Physician Assist Clin. 2021;6:625–635. doi:10.1016/j.cpha.2021.05.007
52. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi:10.3322/caac.21388
53. Qiu Y, Zhang X, Deng X, et al. Circulating tumor cell-associated white blood cell cluster is associated with poor survival of patients with gastric cancer following radical gastrectomy. Eur J Surg Oncol. 2021;48:1039–1045. doi:10.1016/j.ejso.2021.11.115
54. Wislez M, Domblides C, Greillier L, et al. Circulating tumor DNA in advanced non-small-cell lung cancer patients with HIV is associated with shorter overall survival: results from a Phase II trial (IFCT-1001 CHIVA). Lung Cancer. 2021;157:124–130. doi:10.1016/j.lungcan.2021.05.013
55. Hasanain A, Javed AA, van Oosten F, et al. Presence of transitional circulating tumor cells following resection is associated with worse survival in patients with delayed initiation of adjuvant therapy. HPB. 2020;22:S5. doi:10.1016/j.hpb.2020.04.790
56. Chalfin HJ, Glavaris SA, Gorin MA, et al. Circulating tumor cell and circulating tumor DNA assays reveal complementary information for patients with metastatic urothelial cancer. Eur Urol Oncol. 2021;4:310–314. doi:10.1016/j.euo.2019.08.004
57. Ghafouri-Fard S, Hussen BM, Badrlou E, Abak A, Taheri M. MicroRNAs as important contributors in the pathogenesis of colorectal cancer. Biomed Pharmacother. 2021;140:111759. doi:10.1016/j.biopha.2021.111759
58. Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in liquid biopsy – current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–195. doi:10.1016/j.csbj.2018.05.002
59. Pal M, Muinao T, Boruah HPD, Mahindroo N. Current advances in prognostic and diagnostic biomarkers for solid cancers: detection techniques and future challenges. Biomed Pharmacother. 2022;146:112488. doi:10.1016/j.biopha.2021.112488
60. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27.
61. Chiu H-M, Chang L-C, Hsu W-F, Chou C-K, Wu M-S. Non-invasive screening for colorectal cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:953–965. doi:10.1016/j.bpg.2015.09.015
62. Pickhardt PJ, Pooler BD, Kim DH, et al. The natural history of colorectal polyps: overview of predictive static and dynamic features. Gastroenterol Clin. 2018;47:515–536. doi:10.1016/j.gtc.2018.04.004
63. Lu B, Jan Hendriks A, Nolte TM. A generic model based on the properties of nanoparticles and cells for predicting cellular uptake. Colloids Surfaces B Biointerfaces. 2022;209:112155. doi:10.1016/j.colsurfb.2021.112155
64. Nalli M, Puxeddu M, La Regina G, Gianni S, Silvestri R. Emerging therapeutic agents for colorectal cancer. Molecules. 2021;26:7463. doi:10.3390/molecules26247463
65. Hilberg F, Tontsch-Grunt U, Baum A, et al. Triple angiokinase inhibitor nintedanib directly inhibits tumor cell growth and induces tumor shrinkage via blocking oncogenic receptor tyrosine kinases. J Pharmacol Exp Ther. 2018;364:494–503. doi:10.1124/jpet.117.244129
66. Grothey A, Prager G, Yoshino T. The mechanism of action of regorafenib in colorectal cancer: a guide for the community physician. Clin Adv Hematol Oncol. 2019;17:16–19.
67. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi:10.1021/acs.jnatprod.5b01055
68. Huang X, Yang Z-J, Xie Q, et al. Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother. 2019;117:109142. doi:10.1016/j.biopha.2019.109142
69. Kim EJ, Park SY, Lee J-Y, Park JHY. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10:1–11. doi:10.1186/1471-230X-10-96
70. Ji Q, Liu X, Fu X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One. 2013;8:e78700. doi:10.1371/journal.pone.0078700
71. Engelbrecht A-M, Mattheyse M, Ellis B, et al. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007;258:144–153. doi:10.1016/j.canlet.2007.08.020
72. Su -C-C, Lin J-G, Li T-M, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4389.
73. Su M, Qin B, Liu F, Chen Y, Zhang R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des Devel Ther. 2017;11:3333. doi:10.2147/DDDT.S140354
74. Nauroth JM, Liu YC, Van Elswyk M, et al. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids. 2010;45:375–384. doi:10.1007/s11745-010-3406-3
75. Hossain Z, Hosokawa M, Takahashi K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr Cancer. 2008;61:123–130. doi:10.1080/01635580802395725
76. Hua S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract - influence of physiological, pathophysiological and pharmaceutical factors. Front Pharmacol. 2020;11:524. doi:10.3389/fphar.2020.00524
77. Bratten J, Jones MP. New directions in the assessment of gastric function: clinical applications of physiologic measurements. Dig Dis. 2006;24:252–259. doi:10.1159/000092878
78. Ibekwe VC, Fadda HM, McConnell EL, et al. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm Res. 2008;25:1828–1835. doi:10.1007/s11095-008-9580-9
79. Brunton LL, Knollmann BC, Hilal-Dandan R. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill; 2018.
80. Prabu SL, Suriyaprakash TNK, Ruckmani K. Biopharmaceutics and pharmacokinetics. In: Basic Pharmacokinetic Concepts and Some Clinical Applications. IntechOpen; 2015. doi10.5772/61160
81. Reinus JF, Simon D. Gastrointestinal anatomy and physiology: the essentials. In: Gastrointestinal Anatomy and Physiology: The Essentials. Wiley Blackwell; 2014:1–188. doi:10.1002/9781118833001
82. Huttenhower C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214.
83. Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45:S120–S127. doi:10.1097/MCG.0b013e31822fecfe
84. Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi:10.1053/j.gastro.2010.10.036
85. Gately NM, Kennedy JE. The development of a melt-extruded shellac carrier for the targeted delivery of probiotics to the colon. Pharmaceutics. 2017;9:38. doi:10.3390/pharmaceutics9040038
86. Shahdadi Sardo H, Saremnejad F, Bagheri S, et al. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int J Pharm. 2019;558:367–379. doi:10.1016/j.ijpharm.2019.01.022
87. Aidy SE, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol. 2015;32:14–20. doi:10.1016/j.copbio.2014.09.005
88. Booijink GM, El-Aidy S, Rajilić-Stojanović M, et al. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12:3213–3227. doi:10.1111/j.1462-2920.2010.02294.x
89. Kagan L, Hoffman A. Systems for region selective drug delivery in the gastrointestinal tract: biopharmaceutical considerations. Expert Opin Drug Deliv. 2008;5:681–692. doi:10.1517/17425247.5.6.681
90. Hu Z, Mawatari S, Shibata N, et al. Application of a biomagnetic measurement system (BMS) to the evaluation of gastrointestinal transit of intestinal pressure-controlled colon delivery capsules (PCDCs) in human subjects. Pharm Res. 2000;17:160–167. doi:10.1023/A:1007561129221
91. Rao KA, Yazaki E, Evans DF, Carbon R. Objective evaluation of small bowel and colonic transit time using pH telemetry in athletes with gastrointestinal symptoms. Br J Sports Med. 2004;38:482–487. doi:10.1136/bjsm.2003.006825
92. Buhmann S, Kirchhoff C, Ladurner R, et al. Assessment of colonic transit time using MRI: a feasibility study. Eur Radiol. 2006;17:669–674. doi:10.1007/s00330-006-0414-z
93. Johansson MEV, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi:10.1038/nrgastro.2013.35
94. Yang L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J Control Release. 2008;125:77–86. doi:10.1016/j.jconrel.2007.10.026
95. Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi:10.1007/s11894-006-0021-9
96. Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26. doi:10.1155/2019/3702518
97. Zeeshan A, Farhan M, Siddiqui A. Nanomedicine and drug delivery: a mini review. Int Nano Lett. 2014;4:1–7.
98. Korrapati PS, Karthikeyan K, Satish A, et al. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater Sci Eng C. 2016;67:747–765. doi:10.1016/j.msec.2016.05.074
99. Pavitra E, Dariya B, Srivani G, et al. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Semin Cancer Biol. 2021;69:293–306. doi:10.1016/j.semcancer.2019.06.017
100. Indoria S, Singh V, Hsieh MF. Recent advances in theranostic polymeric nanoparticles for cancer treatment: a review. Int J Pharm. 2020;582:119314. doi:10.1016/j.ijpharm.2020.119314
101. Chandran SP, Natarajan SB, Chandraseharan S, Mohd Shahimi MSB. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J Cancer Res Pract. 2017;4:45–48. doi:10.1016/j.jcrpr.2017.02.002
102. Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:70–78. doi:10.5001/omj.2010.24
103. Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 2012;9:1393–1407. doi:10.1517/17425247.2012.730517
104. Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomed Nanotechnol Biol Med. 2015;11:1117–1132. doi:10.1016/j.nano.2015.02.018
105. Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755–1762. doi:10.1111/j.1572-0241.2002.05837.x
106. Coco R, Plapied L, Pourcelle V, et al. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm. 2013;440:3–12. doi:10.1016/j.ijpharm.2012.07.017
107. Maroni A, Zema L, Loreti G, Palugan L, Gazzaniga A. Film coatings for oral pulsatile release. Int J Pharm. 2013;457:362–371. doi:10.1016/j.ijpharm.2013.03.010
108. Shia J, Klimstra DS, Nitzkorski JR, et al. Immunohistochemical expression of folate receptor α in colorectal carcinoma: patterns and biological significance. Hum Pathol. 2008;39:498–505. doi:10.1016/j.humpath.2007.09.013
109. Xiong S, Yu B, Wu J, Li H, Lee RJ. Preparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMS. Biomed Pharmacother. 2011;65:2–8. doi:10.1016/j.biopha.2010.10.003
110. Handali S, Moghimipour E, Rezaei M, et al. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed Pharmacother. 2018;108:1259–1273. doi:10.1016/j.biopha.2018.09.128
111. Yu M, Jambhrunkar S, Thorn P, et al. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale. 2012;5:178–183. doi:10.1039/C2NR32145A
112. Tesauro D, Accardo A, Diaferia C, et al. Peptide-based drug-delivery systems in biotechnological applications: recent advances and perspectives. Molecules. 2019;24:351. doi:10.3390/molecules24020351
113. Xiao B, Han MK, Viennois E, et al. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. 2015;7:17745–17755. doi:10.1039/C5NR04831A
114. Ghosh D, Peng X, Leal J, Mohanty RP. Peptides as drug delivery vehicles across biological barriers. J Pharm Investig. 2017;48:89–111. doi:10.1007/s40005-017-0374-0
115. Jiang Z, Guan J, Qian J, Zhan C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater Sci. 2019;7:461–471. doi:10.1039/C8BM01340C
116. Al-azzawi S, Masheta D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J Pharm Investig. 2019;49:643–654. doi:10.1007/s40005-018-00424-w
117. Ren Y, Mu Y, Song Y, et al. A new peptide ligand for colon cancer targeted delivery of micelles. Drug Deliv. 2016;23:1763–1772. doi:10.3109/10717544.2015.1077293
118. Nguyen MNU, Van Vo T, Tran PHL, Tran TTD. Zein-based solid dispersion for potential application in targeted delivery. J Pharm Investig. 2017;47:357–364. doi:10.1007/s40005-017-0314-z
119. Zhu J, Zhong L, Chen W, et al. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: a novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019;95:562–570. doi:10.1016/j.foodhyd.2018.04.042
120. Barclay TG, Day CM, Petrovsky N, Garg S. Review of polysaccharide particle-based functional drug delivery. Carbohydr Polym. 2019;221:94–112. doi:10.1016/j.carbpol.2019.05.067
121. Lopes PP, Tanabe EH, Bertuol DA. Chitosan as biomaterial in drug delivery and tissue engineering. In: Handbook of Chitin and Chitosan. Vol. 3. Elsevier; 2020:407–431.
122. Nurhayati Y, Manaf Ali A. Production of Chitosan Oligosaccharides using β-glycosidic degrading enzyme: optimization using response surface methodology. Malaysian J Appl Sci. 2020;5:30–44. doi:10.37231/myjas.2020.5.2.261
123. Aizat MA, Aziz F. Chitosan nanocomposite application in wastewater treatments. In: Nanotechnology in Water and Wastewater Treatment. Elsevier; 2019:243–265. doi:10.1016/B978-0-12-813902-8.00012-5
124. Hosseinnejad M, Jafari SM. Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol. 2016;85:467–475. doi:10.1016/j.ijbiomac.2016.01.022
125. Nilsen-Nygaard J, Strand SP, Vårum KM, Draget KI, Nordgård CT. Chitosan: gels and interfacial properties. Polymers. 2015;7:552–579.
126. Moine L, Canali MM, Porporatto C, Correa SG. Reviewing the biological activity of chitosan in the mucosa: focus on intestinal immunity. Int J Biol Macromol. 2021;189:324–334. doi:10.1016/j.ijbiomac.2021.08.098
127. Grgac SF, Tarbuk A, Dekanic T, Sujka W, Draczynski Z. The chitosan implementation into cotton and polyester/cotton blend fabrics. Materials. 2020;13:1616. doi:10.3390/ma13071616
128. Aibani N, Rai R, Patel P, Cuddihy G, Wasan EK. Chitosan nanoparticles at the biological interface: implications for drug delivery. Pharmaceutics. 2021;13:1686. doi:10.3390/pharmaceutics13101686
129. Petrou G, Crouzier T. Mucins as multifunctional building blocks of biomaterials. Biomater Sci. 2018;6:2282–2297. doi:10.1039/C8BM00471D
130. Lee S, Müller M, Rezwan K, Spencer ND. Porcine Gastric Mucin (PGM) at the Water/Poly(Dimethylsiloxane) (PDMS) interface: influence of pH and ionic strength on its conformation, adsorption, and aqueous lubrication properties. Langmuir. 2005;21:8344–8353. doi:10.1021/la050779w
131. Collado-González M, Espinosa YG, Goycoolea FM. Interaction between chitosan and mucin: fundamentals and applications. Biomimetics. 2019;4:32. doi:10.3390/biomimetics4020032
132. Jayakumar R, Prabaharan M. Chitosan for Biomaterials III: Structure-Property Relationships. Cham: Springer; 2021. doi:10.1007/978-3-030-83807-2
133. Gavini E, Rassu G, Sanna V, et al. Mucoadhesive microspheres for nasal administration of an antiemetic drug, metoclopramide: in-vitro/ex-vivo studies. J Pharm Pharmacol. 2010;57:287–294. doi:10.1211/0022357055623
134. Garg U, Chauhan S, Nagaich U, Jain N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv Pharm Bull. 2019;9:195. doi:10.15171/apb.2019.023
135. Lin C, Kuo TC, Lin JC, Ho YC, Mi FL. Delivery of polysaccharides from Ophiopogon japonicus (OJPs) using OJPs/chitosan/whey protein co-assembled nanoparticles to treat defective intestinal epithelial tight junction barrier. Int J Biol Macromol. 2020;160:558–570. doi:10.1016/j.ijbiomac.2020.05.151
136. Dou T, Wang J, Han C, et al. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int J Biol Macromol. 2019;138:791–799. doi:10.1016/j.ijbiomac.2019.07.168
137. Da Silva FLO, Marques MB, Kato KC, Carneiro G. Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin Drug Discov. 2020;15:853–864. doi:10.1080/17460441.2020.1750591
138. Yao Y, Zhou Y, Liu L, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. doi:10.3389/fmolb.2020.00193
139. De Jong WH, Paul JB. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi:10.2147/IJN.S596
140. Afzal M, Alharbi KS. Nanomedicine in treatment of breast cancer – a challenge to conventional therapy. Semin Cancer Biol. 2021;69:279–292. doi:10.1016/j.semcancer.2019.12.016
141. White BD, Duan C, Townley HE. Nanoparticle activation methods in cancer treatment. Biomolecules. 2019;9:202. doi:10.3390/biom9050202
142. Sutradhar KB, Amin ML. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnol. 2014;2014:1–12. doi:10.1155/2014/939378
143. Sen K, Banerjee S, Mandal M. Dual drug loaded liposome bearing apigenin and 5-fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf B Biointerfaces. 2019;180:9–22. doi:10.1016/j.colsurfb.2019.04.035
144. Vigneshwaran R, Ezhilarasan D, Rajeshkumar S. Inorganic titanium dioxide nanoparticles induces cytotoxicity in colon cancer cells. Inorg Chem Commun. 2021;133:108920. doi:10.1016/j.inoche.2021.108920
145. Abouaitah K, Hassan HA, Swiderska-Sroda A, et al. Targeted nano-drug delivery of colchicine against colon cancer cells by means of mesoporous silica nanoparticles. Cancers. 2020;12:12. doi:10.3390/cancers12010144
146. Zielinska A, Carreiró F, Oliveira AM, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731. doi:10.3390/molecules25163731
147. Begines B, Ortiz T, Pérez-Aranda M, et al. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials. 2020;10:1–41. doi:10.3390/nano10071403
148. Herdiana Y, Wathoni N, Shamsuddin S, Joni IM, Muchtaridi M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers. 2021;13:1717. doi:10.3390/polym13111717
149. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi:10.7150/thno.49577
150. Morshed M, Chowdhury EH. Gene delivery and clinical applications. In: Encyclopedia of Biomedical Engineering. Vol. 1–3. Elsevier; 2019:345–351.
151. Shah MR, Imran M, Ullah S. Nanocarrier-based targeted pulmonary delivery: novel approaches for effective lung cancer treatment. Nanocarriers Cancer Diagnosis Target Chemother. 2019;129–161. doi:10.1016/B978-0-12-816773-1.00006-7
152. Bose A, Wong TW. Oral colon cancer targeting by chitosan nanocomposites. Appl Nanocomposite Mater Drug Deliv. 2018;409–429. doi:10.1016/B978-0-12-813741-3.00018-2
153. Salahpour anarjan F. Active targeting drug delivery nanocarriers: ligands. Nano Struct Nano Objects. 2019;19:100370. doi:10.1016/j.nanoso.2019.100370
154. Sen P, Saha M, Ghosh SS. Nanoparticle mediated alteration of EMT dynamics: an approach to modulate cancer therapeutics. Mater Adv. 2020;1:2614–2630. doi:10.1039/D0MA00455C
155. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. doi:10.1186/s13045-019-0833-3
156. Hwang J, Sullivan MO, Kiick KL. Targeted drug delivery via the use of ECM-mimetic materials. Front Bioeng Biotechnol. 2020;8:69. doi:10.3389/fbioe.2020.00069
157. Senthil Kumar C, Thangam R, Mary SA, et al. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym. 2020;231:115682. doi:10.1016/j.carbpol.2019.115682
158. Safdar R, Omar AA, Arunagiri A, Regupathi I, Thanabalan M. Potential of Chitosan and its derivatives for controlled drug release applications – a review. J Drug Deliv Sci Technol. 2019;49:642–659. doi:10.1016/j.jddst.2018.10.020
159. Qin C, Li H, Xiao Q, et al. Water-solubility of chitosan and its antimicrobial activity. Carbohydr Polym. 2006;63:367–374. doi:10.1016/j.carbpol.2005.09.023
160. Muzzarelli RAA, Tanfani F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr Polym. 1985;5:297–307. doi:10.1016/0144-8617(85)90037-2
161. Jintapattanakit A, Mao S, Kissel T, Junyaprasert VB. Physicochemical properties and biocompatibility of N-trimethyl chitosan: effect of quaternization and dimethylation. Eur J Pharm Biopharm. 2008;70:563–571. doi:10.1016/j.ejpb.2008.06.002
162. Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2008;20:1057–1079. doi:10.1007/s10856-008-3659-z
163. Chen G, Svirskis D, Lu W, et al. N-trimethyl chitosan coated nano-complexes enhance the oral bioavailability and chemotherapeutic effects of gemcitabine. Carbohydr Polym. 2021;273:118592. doi:10.1016/j.carbpol.2021.118592
164. Kotzé AF, Lueßen HL, De leeuw BJ, et al. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J Control Release. 1998;51:35–46. doi:10.1016/S0168-3659(97)00154-5
165. Kotze AF, Thanou MM, Lueßen HL, et al. Effect of the degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur J Pharm Biopharm. 1999;47:269–274. doi:10.1016/S0939-6411(99)00006-5
166. Shariatinia Z. Carboxymethyl chitosan: properties and biomedical applications. Int J Biol Macromol. 2018;120:1406–1419. doi:10.1016/j.ijbiomac.2018.09.131
167. Rahmani S, Mohammadi Z, Amini M, et al. Methylated 4-N,N dimethyl aminobenzyl N,O carboxymethyl chitosan as a new chitosan derivative: synthesis, characterization, cytotoxicity and antibacterial activity. Carbohydr Polym. 2016;149:131–139. doi:10.1016/j.carbpol.2016.04.116
168. Muñoz Ruiz GA, Fabio H, Corrales Z. Chitosan, chitosan derivatives and their biomedical applications. In: Biological Activities and Application of Marine Polysaccharides. IntechOpen; 2017. doi10.5772/66527
169. Moghaddam AS, Khonakdar HA, Sarikhani E, et al. Fabrication of carboxymethyl chitosan nanoparticles to deliver paclitaxel for melanoma treatment. ChemNanoMat. 2020;6:1373–1385. doi:10.1002/cnma.202000229
170. Pan X, Chen J, Yang M, et al. Enzyme/pH dual-responsive polymer prodrug nanoparticles based on 10-hydroxycamptothecin-carboxymethyl chitosan for enhanced drug stability and anticancer efficacy. Eur Polym J. 2019;117:372–381. doi:10.1016/j.eurpolymj.2019.04.050
171. Yan C, Chen D, Gu J, et al. Preparation of N-Succinyl-chitosan and their physical-chemical properties as a novel excipient. Yakugaku Zasshi. 2006;126:789–793. doi:10.1248/yakushi.126.789
172. Raizaday A, Yadav HK, Jayanth A, Kaushi SR, Mathew M, Zachariah AB. Formulation and evaluation of pH sensitive microspheres of N-succinyl chitosan for the treatment of diverticulitis. Cell Chem Technol. 2015;49:41–50.
173. Bashir S, Teo YY, Ramesh S, Ramesh K, Khan AA. N-succinyl chitosan preparation, characterization, properties and biomedical applications: a state of the art review. Rev Chem Eng. 2015;31:563–597.
174. Kato Y, Onishi H, Machida Y. N-succinyl-chitosan as a drug carrier: water-insoluble and water-soluble conjugates. Biomaterials. 2004;25:907–915. doi:10.1016/S0142-9612(03)00598-2
175. Wang Y, Karmakar T, Ghosh N, Basak S, Gopal Sahoo N. Targeting mangiferin loaded N-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis – a case study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022;370:131376. doi:10.1016/j.foodchem.2021.131376
176. Ghaffari SB, Sarrafzadeh MH, Salami M, Khorramizadeh MR. A pH-sensitive delivery system based on N-succinyl chitosan-ZnO nanoparticles for improving antibacterial and anticancer activities of curcumin. Int J Biol Macromol. 2020;151:428–440. doi:10.1016/j.ijbiomac.2020.02.141
177. Bashir S, Teo YY, Naeem S, Ramesh S, Ramesh K. pH responsive N-succinyl chitosan/poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS One. 2017;12:e0179250. doi:10.1371/journal.pone.0179250
178. Cristallini C, Barbani N, Bianchi S. Injectable hydrogel based on dialdehyde galactomannan and N-succinyl chitosan: a suitable platform for cell culture. J Mater Sci Mater Med. 2019;31:1–13. doi:10.1007/s10856-019-6328-5
179. Zhu Y, Kruglikov IL, Akgul Y, Scherer PE. Hyaluronan in adipogenesis, adipose tissue physiology and systemic metabolism. Matrix Biol. 2019;78–79:284–291. doi:10.1016/j.matbio.2018.02.012
180. Luo Z, Dai Y, Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;9:1099–1112. doi:10.1016/j.apsb.2019.06.004
181. Jain A, Jain SK. Optimization of chitosan nanoparticles for colon tumors using experimental design methodology. Artif Cells Nanomedicine Biotechnol. 2015;44:1917–1926. doi:10.3109/21691401.2015.1111236
182. Liang Y, Wang Y, Wang L, et al. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact Mater. 2021;6:433–446. doi:10.1016/j.bioactmat.2020.08.019
183. Chiesa E, Dorati R, Conti B, et al. Hyaluronic acid-decorated chitosan nanoparticles for CD44-targeted delivery of everolimus. Int J Mol Sci. 2018;19:2310. doi:10.3390/ijms19082310
184. Serrano-Sevilla I, Artiga Á, Mitchell SG, De Matteis L, de la Fuente JM. Natural polysaccharides for siRNA delivery: nanocarriers based on chitosan, hyaluronic acid, and their derivatives. Molecules. 2019;24:2570. doi:10.3390/molecules24142570
185. Sionkowska A, Gadomska M, Musiał K, Piatek J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: a review. Molecules. 2020;25:4035.
186. Mohapatra A, Uthaman S, Park I-K. Polyethylene glycol nanoparticles as promising tools for anticancer therapeutics. Tool Anti Cancer Ther. 2019;205–231. doi:10.1016/B978-0-12-816963-6.00010-8
187. Gorochovceva N, Makuška R. Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. Eur Polym J. 2004;40:685–691. doi:10.1016/j.eurpolymj.2003.12.005
188. Casettari L, Vllasaliu D, Castagnino E, et al. PEGylated chitosan derivatives: synthesis, characterizations and pharmaceutical applications. Prog Polym Sci. 2012;37:659–685. doi:10.1016/j.progpolymsci.2011.10.001
189. Bozuyuk U, Gokulu IS, Dogan NO, Kizilel S. A novel method for PEGylation of chitosan nanoparticles through photopolymerization. RSC Adv. 2019;9:14011–14015. doi:10.1039/C9RA00780F
190. Andrade F, Goycoolea F, Chiappetta DA, et al. Chitosan-grafted copolymers and chitosan-ligand conjugates as matrices for pulmonary drug delivery. Int J Carbohydr Chem. 2011;2011:1–14. doi:10.1155/2011/865704
191. Shad PM, Karizi SZ, Javan RS, et al. Folate conjugated hyaluronic acid coated alginate nanogels encapsulated oxaliplatin enhance antitumor and apoptosis efficacy on colorectal cancer cells (HT29 cell line). Toxicol Vitr. 2020;65:104756. doi:10.1016/j.tiv.2019.104756
192. Soleymani J, Hasanzadeh M, Shadjou N, Somi MH, Jouyban A. The role of nanomaterials on the cancer cells sensing based on folate receptor: analytical approach. Trends Anal Chem. 2020;125:115834. doi:10.1016/j.trac.2020.115834
193. Ghaz-Jahanian MA, Abbaspour-Aghdam F, Anarjan N, Berenjian A, Jafarizadeh-Malmiri H. Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Mol Biotechnol. 2015;57:201–218. doi:10.1007/s12033-014-9816-3
194. Cheng L, Ma H, Shao M. Synthesis of folate‑chitosan nanoparticles loaded with ligustrazine to target folate receptor positive cancer cells. Mol Med Rep. 2017;16:1101–1108. doi:10.3892/mmr.2017.6740
195. Xu W, Jiang X, Huang L. RNA interference technology. In: Comprehensive Biotechnology.
196. Mahmoodi Chalbatani G, Dana H, Gharagouzloo E, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomedicine. 2019;14:3111. doi:10.2147/IJN.S200253
197. Sadreddini S, Safaralizadeh R, Baradaran B, et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol Lett. 2017;181:79–86. doi:10.1016/j.imlet.2016.11.013
198. Gomathysankar S, Halim AS, Yaacob NS. Proliferation of keratinocytes induced by adipose-derived stem cells on a chitosan scaffold and its role in wound healing, a review. Arch Plast Surg. 2014;41:452–457. doi:10.5999/aps.2014.41.5.452
199. Sabra R, Billa N, Roberts CJ. An augmented delivery of the anticancer agent, curcumin, to the colon. React Funct Polym. 2018;123:54–60. doi:10.1016/j.reactfunctpolym.2017.12.012
200. Sabra R, Billa N, Roberts CJ. Cetuximab-conjugated chitosan-pectinate (modified) composite nanoparticles for targeting colon cancer. Int J Pharm. 2019;572:118775. doi:10.1016/j.ijpharm.2019.118775
201. Bhattacharya S. Fabrication and characterization of chitosan-based polymeric nanoparticles of Imatinib for colorectal cancer targeting application. Int J Biol Macromol. 2020;151:104–115. doi:10.1016/j.ijbiomac.2020.02.151
202. Liu W, Zhu Y, Wang F, et al. Galactosylated chitosan-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. R Soc Open Sci. 2018;5:181027. doi:10.1098/rsos.181027
203. Chen G, Zhao Y, Xu Y, et al. Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int J Pharm. 2020;589:119763. doi:10.1016/j.ijpharm.2020.119763
204. Gouda M, Ibrahim HIM, Negm A. Chitosan containing nano Zn-organic framework: synthesis, characterization and biological activity. Polym. 2022;14:1276. doi:10.3390/polym14071276
205. Kamel KM, Khalil IA, Rateb ME, Elgendy H, Elhawary S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: extract standardization, nanoparticle optimization, and cytotoxicity evaluation. J Agric Food Chem. 2017;65:7966–7981. doi:10.1021/acs.jafc.7b03093
206. Udompornmongkol P, Chiang BH. Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J Biomater Appl. 2015;30:537–546. doi:10.1177/0885328215594479
207. Yusefi M, Chan H-Y, Teow S-Y, et al. 5-fluorouracil encapsulated chitosan-cellulose fiber bionanocomposites: synthesis, characterization and in vitro analysis towards colorectal cancer cells. Nanomaterials. 2021;11:1691. doi:10.3390/nano11071691
208. Han W, Xie B, Li Y, et al. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics. 2019;9:7458. doi:10.7150/thno.38081
209. Yang SJ, Lin F-H, Tsai K-C, et al. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin ix accumulation in colorectal cancer cells. Bioconjug Chem. 2010;21:679–689. doi:10.1021/bc9004798
210. Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomed Nanotechnol Biol Med. 2010;6:179–190. doi:10.1016/j.nano.2009.03.002
211. Soe ZC, Poudel BK, Nguyen HT, et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J Pharm Sci. 2019;14:40–51. doi:10.1016/j.ajps.2018.09.004
212. Guo XY, Wang P, Du QG, et al. Paclitaxel and gemcitabine combinational drug-loaded mucoadhesive delivery system in the treatment of colon cancers. Drug Res. 2015;65:199–204.
213. Sun L, Li Z, Li Z, et al. Improving antitumor activity with N -trimethyl chitosan entrapping camptothecin in colon cancer and lung cancer. J Nanosci Nanotechnol. 2015;15:6397–6404. doi:10.1166/jnn.2015.10736
214. Abu Elella MH, Mohamed RR, Abdel-Aziz MM, Sabaa MW. Green synthesis of antimicrobial and antitumor N,N,N-trimethyl chitosan chloride/poly (acrylic acid)/silver nanocomposites. Int J Biol Macromol. 2018;111:706–716. doi:10.1016/j.ijbiomac.2018.01.055
215. Rahmati A, Homayouni Tabrizi M, Karimi E, Zarei B. Fabrication and assessment of folic acid conjugated-chitosan modified PLGA nanoparticle for delivery of alpha terpineol in colon cancer. Journal of Biomaterials Science, Polymer Edition. 2022;33:1289–1307. doi:10.1080/09205063.2022.2051693
216. Lazer LM, Kesavan Y, Gor R, et al. Targeting colon cancer stem cells using novel doublecortin like kinase 1 antibody functionalized folic acid conjugated hesperetin encapsulated chitosan nanoparticles. Colloids Surfaces B Biointerfaces. 2022;217:112612. doi:10.1016/j.colsurfb.2022.112612
217. Mary lazer L, Sadhasivam B, Palaniyandi K, et al. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int J Biol Macromol. 2018;107:1988–1998. doi:10.1016/j.ijbiomac.2017.10.064
218. Ullah S, Azad AK, Nawaz A, et al. 5-fluorouracil-loaded folic-acid-fabricated chitosan nanoparticles for site-targeted drug delivery cargo. Polymers. 2022;14:2010. doi:10.3390/polym14102010
219. Anirudhan TS, Sekhar VC, Nair SS. Polyelectrolyte complexes of carboxymethyl chitosan/alginate based drug carrier for targeted and controlled release of dual drug. J Drug Deliv Sci Technol. 2019;51:569–582. doi:10.1016/j.jddst.2019.03.036
220. Bhaskaran NA, Jitta SR, Cheruku S, et al. Orally delivered solid lipid nanoparticles of irinotecan coupled with chitosan surface modification to treat colon cancer: preparation, in-vitro and in-vivo evaluations. Int J Biol Macromol. 2022;211:301–315. doi:10.1016/j.ijbiomac.2022.05.060
221. Samprasit W, Opanasopit P, Chamsai B. Alpha-mangostin and resveratrol, dual-drugs-loaded mucoadhesive thiolated chitosan-based nanoparticles for synergistic activity against colon cancer cells. J Biomed Mater Res Part B Appl Biomater. 2022;110:1221–1233. doi:10.1002/jbm.b.34992
222. Siahmansouri H, Somi MH, Babaloo Z, et al. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT-29 colorectal cancer cell line. J Pharm Pharmacol. 2016;68:1119–1130. doi:10.1111/jphp.12593
223. Yan L, Gao S, Shui S, et al. Small interfering RNA-loaded chitosan hydrochloride/carboxymethyl chitosan nanoparticles for ultrasound-triggered release to hamper colorectal cancer growth in vitro. Int J Biol Macromol. 2020;162:1303–1310. doi:10.1016/j.ijbiomac.2020.06.246
224. Nikkhoo A, Rostami N, Farhadi S, et al. Codelivery of STAT3 siRNA and BV6 by carboxymethyl dextran trimethyl chitosan nanoparticles suppresses cancer cell progression. Int J Pharm. 2020;581:119236. doi:10.1016/j.ijpharm.2020.119236
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
