Back to Journals » International Medical Case Reports Journal » Volume 17
Corticosteroid Therapy in Acute and Subacute Arachnoiditis – A Case Series
Authors Her YF , McWilliams RT, Ovrom EA, Watson JC
Received 4 November 2023
Accepted for publication 20 March 2024
Published 26 March 2024 Volume 2024:17 Pages 235—240
DOI https://doi.org/10.2147/IMCRJ.S445705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Yeng F Her,1 Ryan T McWilliams,2 Erik A Ovrom,3 James C Watson1
1Department of Anesthesiology and Perioperative Medicine, Mayo Clinic Hospital, Rochester, MN, USA; 2Family Medicine, Alaska Native Medical Center, Anchorage, AK, USA; 3Mayo Clinic Alix School of Medicine, Mayo Clinic Hospital, Rochester, MN, USA
Correspondence: Yeng F Her, Department of Anesthesiology and Perioperative Medicine, Mayo Clinic Hospital, Rochester, MN, USA, Email [email protected]
Abstract: Arachnoiditis is difficult to treat. Patients are often left frustrated after many failed trials of conservative therapies without symptom resolution. Surgery may provide symptom relief for a short period of time, but their pain often returned. Herein, we present three cases of acute arachnoiditis following three different pain procedures: epidural blood patch, IDDS implant, and epidural steroid injection. The patients were diagnosed and treated with corticosteroids within 10 days of the procedure. Two patients were treated with the same oral steroid regiment, while the third patient was treated with both oral and IV steroid. All three patients had good outcomes at the completion of their steroid therapy. This case series may provide insight into treating acute and subacute arachnoiditis from pain interventions.
Keywords: arachnoiditis, epidural blood patch, intrathecal pain pump, epidural steroid injection, corticosteroid therapy
Introduction
Arachnoiditis is a rare condition caused by inflammation of the arachnoid mater from spinal surgery, neoplasm, lumbar puncture, infection of the spine, or intrathecal injections into the subdural space with sulfite-containing preservatives, blood, or local anesthetics.1,2 This inflammatory process produces fibrinous and oligo-cellular exudates that can precipitate clumping of the nerve roots, impair blood supply, decrease CSF flow, nerve damage, and possible tethered cord.3 Common clinical symptoms of arachnoiditis are headache, back pain, radiculitis, paresthesia, and/or loss of motor function.4 On MRI, it is often detected as loculated arachnoid cysts, clumping of roots often adherent to the dura, and of an “empty sac” appearance.5 Arachnoiditis is diagnosed based on the patient’s history, clinical symptoms, and MRI findings.
Arachnoiditis is challenging to treat. Surgical interventions, including cyst fenestration, shunt placement, laminectomy, lysis of adhesion, and duraplasty, may result in immediate improvement, but often followed by worsening of symptoms.6 Conservative treatment with different regimens of corticosteroid had mixed results.7–12 Chronic arachnoiditis cases did not improve. In contrast, two acute arachnoiditis patients had positive outcomes. This led to the hypothesis that steroid treatment may be effective in the early stages of arachnoiditis to interrupt the development of adhesion and its sequelae.
We present three cases of arachnoiditis following three different spinal procedures. Two patients were treated with the same regimen of oral Prednisone 60 mg daily with an 18-day taper and had complete resolution of symptoms at follow-up. The third patient was treated with two separate oral Prednisone 40 mg daily with a 12-day taper followed by high-dose methylprednisolone infusion. At follow-up, the patient reported significant pain improvement.
Materials and Methods
We conducted a retrospective review of three patients with acute arachnoiditis following three different procedures (Table 1). Two patients were from Mayo Clinic in Rochester, MN. The third patient was from Alaska Native Medical Center, Anchorage, AK. Mayo Clinic in Rochester, MN, was consulted to assist with the management of this patient. Written informed consents were obtained from the patients for research authorization to review medical records and for publication.
 |
Table 1 Cases of Acute Arachnoiditis |
Results
Case 1
A 74-year-old Caucasian male with a 7-year history of chronic daily headache following a lumbar puncture was referred to our clinic for consideration of an epidural blood patch. The headache was characterized by constant dull pain in the forehead that worsened from supine to standing, bending at the waist, sneezing, or coughing. It improved with lying down. In the past, he was headache free for several months following three separate fluoroscopic guided epidural blood patches.
Prior to referral to our practice, the patient was extensively evaluated by neurology. A recent contrast MRI Brain did not show signs of intracranial hypotension; however, a digital subtraction myelogram of the cervical, thoracic, and lumbar spine showed nonspecific moderate accumulation of contrast in both renal collecting systems despite no evidence of cerebral spinal fluid (CSF) leak or CSF-venous fistula. The evaluating neurologist was concerned for an occult spontaneous spine CFS leak. Based on the clinical symptoms and imaging features described, an epidural blood patch was pursued. The patient received 34 mL of autologous blood at T8-T9 (27 mL) and L2-L3 (7 mL) and reported complete resolution of the headache following the procedure. However, on post-procedure-day 1, he developed pressure pain in the lumbar and sacrum areas with numbness and tingling radiating to the calves and hamstrings. On post-procedure-day 7, the pain worsened, and he was evaluated at a local Emergency Department. Lumbar spine MRI showed a new T1 hypointense, T2 hyperintense, enhancing lesion surrounding the distal filum terminate and the distal intrathecal sacral nerves at the level of S1-S2 (Figure 1).
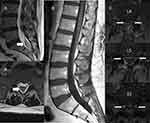 |
Figure 1 Arachnoiditis post pain interventional procedures (A) Case 1; (B) Case 2; (C) Case 3. Arrows indicate arachnoiditis. |
The patient was immediately seen in our clinic for follow-up for concern of acute arachnoiditis from an inadvertent intrathecal injection of autologous blood. In clinic, he denied saddle anesthesia or bowel and bladder changes. On physical exam, it was painful for the patient to stand from a seated position. He did not have ataxia gait. Sensation to light touch was normal throughout the bilateral lower limbs. There was no motor weakness. Straight leg raised was positive for radicular pain in the right lower extremity. We started Prednisone 60 mg daily with 10 mg taper every 3 days and Pregabalin 75 mg BID with titration to 150 mg after 1 week. On post-procedure-day 17, the patient reported complete resolution of back and leg pain. At two months follow-up, the patient was headache free with no back or leg pain.
Case 2
A 43-year-old Caucasian male with a history of spastic diplegic cerebral palsy presented to our clinic for evaluation for an intrathecal pump implant. Prior to the referral, the patient responded well to an intrathecal baclofen test dose with reduced spasticity and pain in the lower extremities and improved ability to stand and ambulate. We proceeded with a Medtronic SynchroMed II 40cc intrathecal drug delivery system (IDDS) implant with the intrathecal access at L2-L3 interspace and advancement of the intrathecal catheter to the level of T9 without difficulty. A pocket site for the pump was created in the right abdomen. In the post-operative period, the patient reported improvement in spasticity. He was able to walk in his room without pain and was discharged home on post-operative-day 1.
On post-operative-day 2, he presented to the Emergency Department with patchy allodynia and burning pain in the buttock and legs. There was no hardware malfunction on interrogation of the IDDS. C-Reactive Protein and Sedimentation Rate were elevated at 14.9 mg/L (Ref range: <8 mg/L) and 26 mm/h (Ref range: 2–12 mm/h), respectively. WBC was 10. CSF was slightly bloody with erythrocytes at 73 and elevated total protein at 73 mg/dL (Ref range: 0–35 mg/dL). Xanthochromia was not checked. There was no growth in the CSF or blood cultures. No organisms were present in the CSF gram stain. Thoracic and lumbar CTs and contrast MRIs showed no evidence of intrathecal, subdural, or epidural hematoma (Figure 1). No evidence of post procedural complications. Contrast MRI lumbar plexus showed no lumbosacral abnormality, enhancement of nerve roots, or denervation changes. The location of the catheter tip had not change. Based on the patient’s history and clinical symptoms, we diagnosed him with idiopathic arachnoiditis.
The patient did not respond to Acetaminophen 1000 mg PO QID, Lidocaine patch, Celecoxib 200 mg daily, Ketamine-Amitriptyline-Lidocaine gel, Pregabalin 300 mg BID, and PO Hydromorphone 2–4 mg q3h PRN. While hospitalized, he could not turn in bed without pain. He needed two assists to get out of bed and walk to his bathroom. Prednisone 60 mg daily with 10 mg taper every 3 days was started. The patient reported immediate improvement in pain and function. He was able to ambulate in the unit hallway five times throughout the day and was discharged home on post-Prednisone Day 3. Post-Prednisone Day 12, the allodynia had resolved, and the patient was off all pain medications except Pregabalin 300 mg BID. At two months follow-up, the patient was weaned off Prednisone and was pain free.
Case 3
A 38-year-old female with a history of diabetes mellitus type 2, post-traumatic stress syndrome, antiphospholipid antibodies, irritable bowel syndrome, fibromyalgia, nonalcoholic fatty liver, early menopause due total abdominal hysterectomy with oophorectomy, and morbid obesity was seen for back pain with radicular symptoms into both legs after a fall. A lumbar MRI revealed central posterior disc protrusion with an annular tear at L5-S1. She was referred for an intralaminar epidural steroid injection (ESI), which provided 3 days of pain relief. However, on the 4th day, the patient developed progressive low back pain with radiation into the ankles. A lumbar MRI obtained 2 weeks after the ESI showed evidence of arachnoiditis with enhancement of the L4 to S1 nerve roots and enlargement of at least 1 nerve root (Figure 1). The patient was started on Oral Prednisone 40 mg with 10 mg taper every three days, which provided about 50% pain relief. Unfortunately, after one week of completing the oral steroid taper, the pain had returned to the same level before the steroid administration. A second oral steroid taper was re-initiated but had no effect on the pain. A repeat lumbar MRI obtained 2.5 months after the ESI showed increased inflammation of the dura mater. At this point, a neurology-pain specialist was consulted. It was recommended that the patient start high-dose steroid infusion for 3 days. Three months after the ESI, the patient received the first IV Methylprednisolone 1000 mg infusion. Unfortunately, the patient only received two doses. The third dose was held due to a COVID-19 infection. After the two IV infusions, the patient noted complete resolution of the back and leg pain for 2 days. By day 5 after the infusion, all her previous symptoms had returned.
After the patient had recovered from COVID-19 infection, she was restarted on the IV Methylprednisolone 1000 mg infusion for 3 days followed by 6 weeks of weekly infusion. At the completion of the last steroid infusion, the patient reported only mild low back pain with radiation to the buttocks. A follow-up lumbar MRI showed improvement of the inflammation around the nerve roots.
Discussion
This case series report the outcomes of three patients who developed spinal arachnoiditis following an inadvertent intrathecal autologous blood injection, IDDS implantation, and ESI. The first two patients were treated with oral Prednisone 60 mg daily with 3-day pulses and tapered over 18 days within 10 days of symptoms presentation. They had complete resolution of symptoms at follow-up. The third patient was treated with two separated oral Prednisone 40 mg daily with a 12-day taper. The symptoms persisted requiring IV Methylprednisolone 1000 mg daily for 3 days followed by 6 weeks of weekly infusion. At follow-up, the patient noted significant pain improvement.
Inadvertent intrathecal injection of autologous blood causing arachnoiditis is well reported.1 Association of arachnoiditis and IDDS has been observed in patients receiving Morphine, Bupivacaine, and/or Baclofen. Ward et al reported a patient developing adhesive arachnoiditis after receiving high doses of Hydromorphone, Bupivacaine, and Baclofen. Huang et al reported a case of toxic myelitis and arachnoiditis after intrathecal delivery of Bupivacaine via an IDDS.13 Compare this to our IDDS implanted patient, the likelihood that our patient developed arachnoiditis from the intrathecal Baclofen is low. A thorough reviewed of other IDDS patients receiving the same batch of Baclofen did not show any reported adverse effects. Even though intrathecal delivery of Baclofen has been reported with granuloma formation resulting in radicular pain and spinal cord/nerve roots compression,14 there was no evidence of granuloma on the MRI scans. We postulate that our patient may have developed arachnoiditis from direct trauma of the dura from the needle or catheter to the subarachnoid space.
Early diagnosis and treatment of arachnoiditis with corticosteroid may prevent its sequelae. A review of the literature shows a case report of a 30-year-old female who developed acute arachnoiditis following repeated epidural blood patch.1 She was treated with IV methylprednisolone 1000 mg at 100 mL/hr inpatient and 80 mg oral Prednisone tapered over 12 days outpatient. At a 1-month follow-up post steroid therapy, the patient reported significant improvement in pain and restoration of motor function. A repeat MRI showed evidence of inflammation resolution. Additionally, in the case report, the authors performed a systematic review of arachnoiditis post epidural blood patch procedure to evaluate efficacy and availability of treatment modalities. They found only chronic arachnoiditis cases following epidural blood patch procedures. The seven patients in these case reports did not improve with steroid therapy. Furthermore, the authors did not provide sufficient information on the dosing, route of administration, or duration of therapy.
Hackert et al reports four cases of arachnoiditis that were treated with high-dose corticosteroid.7 The only patient that experienced improvement was diagnosed a week after becoming symptomatic and went through four rounds of one gram of Methylprednisolone daily for five consecutive days. The three patients that did not improve with corticosteroid had suffered from chronic adhesive arachnoiditis between 1.5 and 3 years with more pronounced MRI findings. Like these two studies, our patients were diagnosed early in the clinical course, treated with steroid and had positive outcomes. The duration of steroid treatment in Hacket et al was 20 days. In two of our patients, they were treated with an 18-day course of steroid. The third patient went through a more extensive steroid regiment with two separate oral steroid tapers followed by two days of high-dose steroid infusion. The symptoms finally improved after a 3-day high-dose steroid infusion followed by 6 weeks of weekly infusion. The commonality in these cases is that they were treated with steroid within 10 days of arachnoiditis symptom presentation. These cases support the hypothesis that high-dose corticosteroid therapy in the initial stages of arachnoiditis may interrupt the development of adhesive arachnoiditis and its complications.
For the patients that do not respond to corticosteroid-therapy, none-steroid oral medications or surgical interventions may be considered. In a systematic review, Villani et al reports that NSAIDs, opioids, and neuropathic pain medications can provide mild symptoms relief.1 Surgical procedures may provide slightly better results than non-surgical treatment. A review of 36 micro lysis operations for symptomatic spinal adhesive arachnoiditis with an average of 4.6 years follow-up showed positive outcomes in 54% of the patients.15 This increased to 80% when mucolysis was performed with spinal fusion. Patients that underwent decompressive laminectomy reported up to 50% pain relief, but that number dropped to 15% after two years.16 As for endoscopic subarachno-epidurostomy, the patients had resolution of symptoms following surgery, but after 2 years of follow-up, that dropped to 40% compared to the preoperative level.17 It is believed that symptoms recurred after scar tissue accumulates around the surgical site. Furthermore, surgery may increase an already inflamed mass resulting in worsening of pain and function. As a last resort, neuromodulation with dorsal root ganglia or spinal cord stimulation may be considered.
A limitation of this cases series is a lack of a control. Due to the rarity of arachnoiditis, it precludes a comparative study.
Conclusion
Arachnoiditis may spontaneously resolves without intervention or continues its progression. This case series and the literature review underscore the importance of early diagnosis of arachnoiditis when it is still amenable to conservative treatment. In cases of corticosteroid-resistant-therapy, conservative treatments with NSAIDs, opioids, and neuropathic pain agents may provide mild symptoms relief. Surgical procedures may provide slightly better results than conservative treatments, but pain recurs in the long term due to accumulation of scar tissue and increased inflammation.
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Villani LA, Digre KB, Cortez MM, Bokat C, Rassner UA, Ozudogru SN. Arachnoiditis, a complication of epidural blood patch for the treatment of low-pressure headache: a case report and systematic review. Headache. 2021;61(2):244–252. doi:10.1111/head.14076
2. Al Maach N, Vogels OJM, Bollen TL, Wessels PH. Arachnoiditis and communicating hydrocephalus as a complication of epidural blood patch. J Neurol. 2010;257(4):672–673. doi:10.1007/s00415-009-5423-9
3. Delamarter RB, Ross JS, Masaryk TJ, Modic MT, Bohlman HH. Diagnosis of lumbar arachnoiditis by magnetic-resonance-imaging. Spine. 1990;15(4):304–310. doi:10.1097/00007632-199004000-00011
4. Aldrete JA. Neurologic deficits and arachnoiditis following neuroaxial anesthesia. Acta Anaesth Scand. 2003;47(1):3–12. doi:10.1034/j.1399-6576.2003.470102.x
5. Anderson TL, Morris JM, Wald JT, Kotsenas AL. Imaging appearance of advanced chronic adhesive arachnoiditis: a retrospective review. Am J Roentgenol. 2017;209(3):648–655. doi:10.2214/Ajr.16.16704
6. Guyer DW, Wiltse LL, Eskay ML, Guyer BH. The long-range prognosis of arachnoiditis. Spine. 1989;14(12):1332–1341. doi:10.1097/00007632-198912000-00010
7. Hackert J, Massmann L, Sure U, et al. Immunotherapies in chronic adhesive arachnoiditis - A case series and literature review. eNeurologicalSci. 2021;24:100350. doi:10.1016/j.ensci.2021.100350
8. Carlsward C, Darvish B, Tunelli J, Irestedt L. Chronic adhesive arachnoiditis after repeat epidural blood patch. Int J Obstet Anesth. 2015;24(3):280–283. doi:10.1016/j.ijoa.2015.04.005
9. Jurga S, Szymanska-Adamcewicz O, Wierzcholowski W, Pilchowska-Ujma E, Urbaniak L. Spinal adhesive arachnoiditis: three case reports and review of literature. Acta Neurol Belg. 2021;121(1):47–53. doi:10.1007/s13760-020-01431-1
10. Krätzig T, Dreimann M, Mende KC, Königs I, Westphal M, Eicker SO. Extensive spinal adhesive arachnoiditis after extradural spinal infection-spinal dura mater is no barrier to inflammation. World Neurosurg. 2018;116:E1194–E1203. doi:10.1016/j.wneu.2018.05.219
11. Mohamed Iqbal I, Morris R, Hersch M. Adhesive arachnoiditis following inadvertent epidural injection of 2% chlorhexidine in 70% alcohol-partial recovery over the ensuing eight years. Anaesth Intensive Care. 2018;46(6):572–574. doi:10.1177/0310057X1804600606
12. Shields LBE, Iyer VG, Zhang YP, Shields CB. Acute cauda equina syndrome following orthopedic procedures as a result of epidural anesthesia. Surg Neurol Int. 2018;9:81. doi:10.4103/sni.sni_492_17
13. Huang M, Dalm B, Simpson RK. Toxic myelitis and arachnoiditis after intrathecal delivery of bupivacaine via an implanted drug delivery system: case report and review of the literature. Cureus. 2018;10(2):e2240. doi:10.7759/cureus.2240
14. Murphy PM, Skouvaklis DE, Amadeo RJ, Haberman C, Brazier DH, Cousins MJ. Intrathecal catheter granuloma associated with isolated baclofen infusion. Anesth Analg. 2006;102(3):848–852. doi:10.1213/01.ane.0000196523.06573.10
15. Shikata J, Yamamuro T, Iida H, Sugimoto M. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine. 1989;14(8):870–875. doi:10.1097/00007632-198908000-00018
16. Esses SI, Morley TP. Spinal arachnoiditis. Can J Neurol Sci. 1983;10(1):2–10. doi:10.1017/s0317167100044486
17. Warnke JP, Mourgela S. [Adhesive lumbar arachnoiditis. Endoscopic subarachnoepidurostomy as a new treatment]. Lumbale adhesive Arachnoiditis. Endoskopische Subarachnoepidurostomie als neue Behandlungsmoglichkeit. Nervenarzt. 2007;78(10):1182–1187. German. doi:10.1007/s00115-007-2289-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
